Abstract
Although immunization during pregnancy can protect mothers and their infants from vaccine-preventable morbidity and mortality, vaccination rates are often poor. We systematically reviewed the literature from inception to July 4, 2023, for randomized and non-randomized quasi-experimental studies estimating the effects of interventions to increase vaccination during pregnancy. Of 9331 studies screened, 36 met inclusion criteria, including 18 demand-side interventions, 11 supply-side interventions, and seven multi-level (demand and supply-side) interventions. Demand-side interventions commonly addressed patient education, showing modest improvement (pooled RR 1.18; 95% CI: 1.04, 1.33; I2 = 63.1%, low certainty). Supply-side interventions commonly implemented Assessment-Feedback-Incentive-eXchange interventions with little improvement (pooled RR 1.13; 95% CI: 0.96, 1.33; I2 = 94.0%, low certainty). Multi-level interventions were modestly effective in increasing vaccination (pooled RR 1.62; 95% CI: 1.09, 2.42; I2 = 97%, very low certainty). Interventions identified in the literature mostly resulted in low to moderate increases in vaccination with likely high heterogeneity and low to very low certainty in the findings.
Similar content being viewed by others
Introduction
Pregnancy and early infancy present life stages susceptible to more severe infection1. Additionally, infants under the age of six months are either too young to receive licensed vaccines (e.g., influenza and COVID-19 vaccines) or are too young to have achieved a protective level of antibodies in response to their first few doses of routine childhood vaccines (e.g., pertussis and tetanus-containing vaccines). Maternal immunization, or vaccination during pregnancy, can prevent severe illness both in the mother and the newborn2,3. Maternal antibodies offer direct protection against illness to the immunized mothers, and passive protection to the newborn through transplacental antibody passage4. In some cases (e.g., respiratory syncytial virus vaccine), maternal immunization may offer the only vaccine strategy to protect infants from disease.
Several vaccines are routinely recommended during pregnancy, including influenza, COVID-19, pertussis and tetanus-containing vaccines, and respiratory syncytial virus (RSV) vaccine. Additional vaccines may be recommended in specific circumstances, such as pneumococcal, meningococcal, Haemophilus influenzae type b, hepatitis A and B, inactivated polio, typhoid, yellow fever, Japanese encephalitis, rabies, anthrax, Ebola, dengue, and smallpox vaccines5,6. Presently, there are additional vaccines under development for future use during pregnancy to prevent maternal-newborn infection, including group B streptococcus5.
Routinely recommended vaccines have been shown to be safe and effective during pregnancy. For example, pertussis vaccination during pregnancy prevents between 69-91% of pertussis infections and up to 94% of pertussis-associated hospitalizations and deaths among infants < 2-months-old2. Influenza vaccination during pregnancy prevents between 30% and 63% of influenza infections among infants <6 -months-old and 31–70% of influenza infections among pregnant persons4.
Despite the benefits of immunization, low vaccination rates during pregnancy are commonly documented in high-income and low and middle-income countries7,8,9,10, indicating that interventions to increase vaccine uptake during pregnancy are needed. Vaccine uptake is driven by a combination of patient and provider factors, including patient awareness and acceptance of vaccines and access to and affordability of vaccines11. Interventions to increase vaccine uptake can therefore address demand-side factors (i.e., patient awareness and acceptance), supply-side factors (i.e., increased access to vaccines by enhanced immunization services), or a combination of both12. This review aimed to systematically describe and assess the effects of interventions designed to increase uptake of recommended vaccines among pregnant persons.
Results
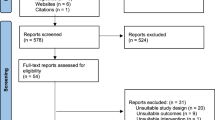
A total of 14,372 studies were identified from database searches, and 11 studies were identified from review of reference lists and clinical trial registries. After removing duplicates, the abstracts and titles of 9331 studies were screened for inclusion, of which 68 studies met inclusion criteria. After reviewing 68 full-text articles, 36 unique studies met inclusion criteria and 32 were suitable for meta-analysis (Fig. 1). A summary of full-text articles excluded from further reviews is provided in Table S1. Eighteen (50%) studies were randomized trials, including 15 randomized controlled trials and three cluster randomized controlled trials. We identified one non-randomized controlled trial13. Seventeen (47%) studies were quasi-experimental, including ten studies using a historical control, six studies using a geographic control, and one study using both a historical and geographic control. Fourteen studies aimed to increase uptake of influenza vaccine, four to increase uptake of pertussis-containing vaccines, and six to increase uptake of influenza and pertussis-containing vaccines. Eleven studies aimed to increase uptake of the tetanus toxoid vaccine, and one study aimed to increase COVID-19 vaccine uptake.
Nineteen (53%) of the 36 included studies were conducted in the United States (Table 1). The remaining studies were conducted in one of 14 other countries; 12 (34%) in a low or middle-income country (LMIC) and 24 (67%) in a high-income country (HIC; Fig. S1). We identified 24 studies evaluating patient-level (demand-side) interventions and 17 studies that evaluated systems or provider-level (supply-side) interventions; 19 studies implemented patient-level interventions only13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,38, 11 studies implemented systems or provider-level interventions exclusively31,32,33,34,35,36,37,38,39,40,41, and six were bundled with interventions that targeting patients and providers or health systems42,43,44,45,46,47.
Risk of bias
Among the 17 randomized clinical trials, eight were deemed at low risk of bias, eight had some concerns, and one was deemed at high risk of bias (Table 2). Among the 15 non-randomized studies, one was deemed to be at low risk of bias, five were at moderate risk of bias, eight were at serious risk of bias, and one was at critical risk of bias (Table 3). The study deemed to be at critical risk of bias was due to self-selection into the intervention and limited comparability between groups13. This study was excluded from meta-analyses.
Patient-level interventions
Many of the interventions aligned well with evidence-based strategies recommended by the Community Preventive Services Task Force12. We identified 18 patient-level, demand-side only studies; three studies investigated the use of patient reminder and recall systems16,20,21, nine investigated the use of clinic-based patient education15,16,17,18,22,23,25,26,46, two studies investigated the use of patient or family incentive rewards through conditional cash transfers27,28, two studies investigated the use of persuasive messaging with patient education14,24, and one study investigated the use of patient-held immunization records38.
Handheld immunization records appeared to increase vaccination rates the most, albeit with low certainty (Table 4), with effect estimates for increasing tetanus toxoid vaccine uptake ranging from 4.85 (95% CI: 3.79, 7.42) to 6.41 (95% CI: 5.36, 7.67). The pooled RR was 5.65 (95% CI: 4.30, 7.42), with possibly substantial heterogeneity (I2 = 69%, tau = 0.03, P = 0.07). These estimates were also derived from multiple sites within a single study, making it difficult to draw firm conclusions. Two studies examined the use of patient incentives via conditional cash transfer programs, where participants received cash stipends in exchange for receiving antenatal care services27,28. In Nigeria, Sato and Fintan (2019) showed that payments in the amount of C300 or C800 (equivalent to $2.00 and $5.33 US dollars, respectively) increased tetanus toxoid vaccination modestly (RR 1.38; 95% CI: 1.28, 1.49 and RR 1.56; 95% CI: 1.46, 1.67, respectively)28. However, a similar conditional cash transfer program in rural India did not observe a significant effect on tetanus toxoid vaccination (RR 1.04; 95% CI: 0.97, 1.12)27. The pooled RR indicated a possible modest effect of conditional cash transfer programs (pooled RR 1.31; 95% CI: 1.03, 1.66) but with possibly considerable heterogeneity (I2 = 97%; tau=0.04; P < 0.001) and low certainty (Table 4).
Other patient-level interventions showed more modest effects on vaccine uptake during pregnancy. Although most studies evaluated the use of patient education strategies, these showed little to moderate improvements in vaccination rates and were entirely implemented in HIC settings. Patient education studies used printed materials (i.e., posters, statements, or pamphlets) or electronic materials (i.e., interactive website, videos, or text messages). One study used brief one-on-one education with a healthcare provider22. Effect estimates ranged from 0.98 (95% CI: 0.84, 1.15)16 to 2.11 (95% CI: 1.22, 3.67)22, with three studies showing a significant improvement in influenza and/or pertussis vaccine uptake17,22,26. The pooled RR indicated a modest effect of patient education on influenza or pertussis vaccine uptake (pooled RR 1.18; 95% CI: 1.04, 1.33), with possibly substantial heterogeneity (I2 = 63%; tau = 0.03; P < 0.01) (Fig. 2) and low certainty (Table 4). Results were similar when we considered any study implementing the effect of patient education, either alone or in combination with other interventions, and pooled RRs by vaccine suggested a possible modest increase in influenza vaccine uptake (pooled RR 1.21; 95% CI: 1.06, 1.37) but no increase in pertussis vaccine uptake (pooled RR 1.00; 95% CI: 0.96, 1.04) (Fig. S2).
Individual and pooled effect estimates for interventions that target patient-level demand-side interventions to increase the uptake of recommended vaccines during pregnancy. Pooled effect estimates were estimated by random effects meta-analysis overall and by intervention. CI indicates 95% confidence intervals, and RR indicates relative risk estimates.
Three studies evaluated the use of text message-based immunization reminders. Effect estimates for the use of text message reminders ranged from 0.90 (95% CI: 0.53, 1.54)20 to 1.06 (95% CI: 0.94, 1.19)21. The pooled RR indicated no effect of SMS-based immunization reminder systems on influenza vaccine uptake (pooled RR 1.01; 95% CI: 0.93, 1.09), with possibly low heterogeneity (I2 < 0.1; tau < 0.01; P = 0.51) and very low certainty (Table 4). Two small US studies (n < 36 per group) evaluated the use of different persuasive messaging using an affective messaging video or iBook14,24. Neither study showed a significant effect on influenza or pertussis vaccine uptake (pooled RR 0.94; 95% CI: 0.42, 2.11; I2 < 0.1; tau < 0.01; P = 0.92), and the evidence in support of a persuasive message was deemed to have very low certainty (Table 4).
Across all studies evaluating patient-level interventions exclusively, the evidence suggested a possible modest effect of patient-level interventions (pooled RR 1.37; 95% CI: 1.11, 1.69) with possibly considerable heterogeneity (I2 = 96%; tau = 0.24; P < 0.001) (Fig. 2). Among the 12 randomized controlled trials, six were deemed to be at low risk of bias, and six had some concerns (Table 2). Among the three quasi-experimental studies, one was at moderate risk, one at serious risk, and one at critical risk of bias (Table 3). Results from the six randomized controlled trials at low risk of bias showed no effect of patient education (n = 5/5)15,18,20,23,25 but increases in tetanus vaccination after cash incentives (n = 1)28.
We identified little evidence for publication bias (Egger statistic = 1.46; P = 0.33). However, examination of funnel plots showed more than expected larger studies with positive findings identified in the literature for patient-level interventions (Fig. S3), including patient education interventions (Fig. S4). Patient-level evaluations were deemed to have low certainty evidence in increasing vaccine uptake during pregnancy (Table 4).
Systems or provider-level interventions
Among the 11 studies of supply-side only interventions, three studies trialed some form of an Assessment-Feedback-Incentives-eXchange (AFIX) quality improvement program to increase influenza (n = 1) or both influenza and pertussis (n = 2) vaccine uptake32,34,46, three trialed the use of performance-based financing to improve antenatal care services (including tetanus toxoid vaccination)36,37,40, two trialed vaccine delivery systems for influenza or pertussis vaccination33,48, one trialed the use of a provider reminder system31, one trialed the use of a mobile health platform for improving the continuum of antenatal care35, and one trialed the use of socially-accountable medical education39.
Results did not strongly support supply-side interventions that were evaluated. Among AFIX interventions, effect estimates ranged from 0.94 (95% CI: 0.88, 1.00)46 to 1.54 (95% CI: 1.39, 1.71)32, with a pooled RR estimate of 1.13 (95% CI: 0.96, 1.33) with possibly considerable heterogeneity (I2 = 94%, tau = 0.03; P < 0.001) (Fig. 3) and very low certainty (Table 4). Effect estimates from the two LMIC-based studies of performance-based financing to increase tetanus toxoid vaccination ranged from 0.85 (95% CI: 0.78, 0.92)36 to 1.08 (95% CI: 0.98, 1.20)36. The pooled RR was 0.95 (95% CI: 0.83, 1.08), with possibly considerable heterogeneity (I2 = 85%; P < 0.001) and very low certainty (Table 4). The single study in India that evaluated the use of a mobile health (mHealth) platform for supporting case management and continuum of care for maternal and child health services showed a modest improvement in tetanus toxoid vaccination (RR 1.07; 95% CI: 1.06, 1.08)35 with very low certainty. The US study that evaluated the use of a healthcare provider reminder via an electronic health record alert, showing a 46% improvement in influenza vaccination rates (RR 1.46; 95% CI: 1.31, 1.63)31 with very low certainty. A study in Canada trialed different vaccine delivery systems and showed that administration of pertussis vaccine in obstetrics clinics or family medicine group practices resulted in higher uptake compared to the pre-intervention standard of vaccine administration in community clinics (RR 1.30; 95% CI: 1.17, 1.44 and RR 1.31; 95% CI: 1.17, 1.45, respectively)33 with very low certainty. Finally, one study in the Philippines investigated the use of a socially accountable medical education program to improve community health services, which admitted students who more accurately reflected the geographical, ethnic, and socio-economic diversity of the school’s reference population and applied an extended community-engaged service learning model with locally relevant curriculum. The socially accountable medical education program showed no effect on tetanus toxoid vaccination (RR 0.97; 95% CI: 0.93, 1.00)39 with very low certainty.
Individual and pooled effect estimates for interventions that target health systems and/or healthcare providers to increase the uptake of recommended vaccines during pregnancy. Pooled effect estimates were estimated by random effects meta-analysis overall and by intervention. AFIX indicates Assessment, Feedback, Incentive, eXchange programs, CI indicates 95% confidence intervals, and RR indicates relative risk estimates.
Results were similar when we considered provider or systems-level interventions when administered alone or in combination with other interventions. Seven studies evaluated provider education, either alone or in combination with patient-level interventions (Fig. S5). Pooled RR showed a modest improvement in influenza vaccine uptake (pooled RR 1.20; 95% CI: 1.02; 1.42) with possibly considerable heterogeneity (I2 = 89%; P < 0.001). A modest effect was similarly observed for pertussis vaccine (pooled RR 1.63; 95% CI: 0.74, 3.57), but with wider confidence intervals and possibly considerable heterogeneity (I2 = 92%; P < 0.001). Three studies examined provider reminders, either alone or in combination with other interventions (Fig. S6), and these showed modest improvement in either tetanus toxoid or influenza vaccine uptake (pooled RR 1.18; 95% CI: 0.97, 1.44) with possibly considerable heterogeneity (I2 = 94%; P < 0.001). Six US studies evaluated the nomination of an immunization champion (i.e., a dedicated member of staff who was compensated for championing immunization services and implementing quality improvement interventions) and enhanced electronic immunization documentation within practices (Fig. S7), showing possible modest improvement in influenza vaccine uptake (pooled RR 1.22; 95% CI: 1.04, 1.43) and pertussis vaccine uptake (pooled RR 1.39; 95% CI: 1.03, 1.57). However, meta-analyses indicated possibly considerable heterogeneity for both (I2 = 87% and I2 = 91%, respectively). Five studies evaluated the use of standing orders and AFIX programs and showed possible modest improvements in influenza vaccination (pooled RR 1.21; 95% CI: 1.03, 1.43) and pertussis vaccination (pooled RR 1.37; 95% CI: 0.99, 2.47), both with possibly considerable heterogeneity (I2 = 90% and I2 = 92%, respectively) (Fig. S8).
Across all studies evaluating provider or systems-level interventions exclusively, the evidence suggested a possible small effect of provider or systems-level interventions (pooled RR 1.09; 95% CI: 1.00, 1.19) with possibly considerable heterogeneity (I2 = 93%; tau=0.03; P < 0.001) (Fig. 3). Among the two randomized controlled trials, one was deemed to be at low risk of bias, and one had some concerns (Table 2). Among the seven quasi-experimental studies, two were at moderate risk, and five were at serious risk of bias (Table 3). Results from the one randomized controlled trial at low risk of bias found no increase in influenza vaccination during pregnancy after an opt-out approach to immunization48.
We observed no statistical evidence of publication bias in systems or provider-level interventions (Egger statistic = 0.28; P = 0.83); however, examination of funnel plots showed more studies with positive findings than would be expected appear in the published literature for provider or systems-level interventions (Fig. S9), including provider education (Fig. S10), provider reminders (Fig. S11), immunization champions and enhanced vaccine documentation (Fig. S12), and standing orders and AFIX programs (Fig. S13).
Patient and provider or systems-level interventions
Six studies were identified that evaluated ‘bundled’ multi-level interventions that addressed both demand and supply-side factors (Fig. 4)42,43,44,45,46,47. Five of these studies were conducted in the US and one in Pakistan. Bundled interventions often incorporated patient-level components like patient education and provider and systems-level components such as AFIX, enhanced vaccine documentation, and standing orders for vaccination. Effect estimates ranged from 1.00 (95% CI: 0.94, 1.06)36 to 5.42 (95% CI: 3.10, 9.48)34. Among the two randomized trials in the US, neither showed a significant effect of multi-level interventions on influenza or pertussis vaccine uptake45,46. Three US studies using a historical control reported a significant increase in influenza vaccine uptake42, pertussis vaccine uptake43, or influenza and pertussis vaccine uptake44. The pooled RR for multi-level interventions was 1.62 (95% CI: 1.09, 2.42) with possible high heterogeneity (I2 = 97%; P < 0.001) (Fig. 4) and very low certainty (Table 4). Among the three randomized controlled trials, one was deemed to be at low risk of bias, one had some concerns, and one was at high risk of bias (Table 2). Among the four quasi-experimental studies, two were at moderate risk of bias, and two were at serious risk of bias (Table 3). Results from the one randomized controlled trial at low risk of bias found no increase in influenza or pertussis vaccination during pregnancy after a multi-level intervention46. We observed no statistical evidence of publication bias (Egger statistic = 3.69; P-value = 0.14); however, the funnel plot indicated some asymmetry in studies with more positive findings published (Fig. S14).
Ongoing studies
We identified seven ongoing registered clinical trials describing interventions to increase vaccination rates in pregnant people (Table S2). Four studies are being conducted in the US, two in Italy, and one in the Netherlands. Two target COVID-19 vaccine uptake, two target both influenza and pertussis vaccine uptake, one targets pertussis vaccine uptake exclusively, and another influenza vaccine uptake exclusively, and one targets respiratory syncytial virus vaccine uptake. Interventions focused on patient education, the AFIX framework, motivational interviewing, or tailored decision aids.
Discussion
This systematic review and meta-analysis synthesizes the existing evidence from completed randomized controlled trials and quasi-experimental studies of interventions to increase vaccine uptake during pregnancy and provides a summary of ongoing studies in this field. Interventions that showed modest improvement in vaccine uptake included patient incentives, patient-held immunization records, provider reminders, and multi-level interventions that address patient, provider, and systems-level factors. However, the number of studies for individual interventions were small, there was considerable heterogeneity in the results of included studies, and with exception to patient incentives, studies deemed to be at low risk of bias did not report significant effects of patient, provider, or patient and provider-level interventions on vaccine uptake during pregnancy. As a result, the evidence from the existing literature provides low to very low certainty in the effects of these interventions. Further research on interventions to increase vaccine uptake during pregnancy is needed.
Several studies supported the effectiveness of demand-side patient-level interventions, but with low certainty. Patient incentives appeared to increase vaccination rates, although only in one of two studies and only in an LMIC context. Although these findings align with the Community Preventive Services Task Force recommendations for strategies to increase vaccination uptake12, there were several notable differences. For example, patient education was the most commonly evaluated demand-side intervention identified in this review, yet we found that seven of the nine studies reported no increase in vaccine uptake during pregnancy associated with patient education15,16,18,23,25,26,46. Furthermore, given the evidence that studies with positive findings were over-represented in the literature, any benefits of patient-level interventions, including patient education, could be overstated. While evidence supports patient education as an intervention to improve vaccine uptake12, prior studies have mostly targeted non-pregnant populations, and it is possible that patient education is less effective for pregnant people. Similarly, although evidence strongly supports reminder/recall strategies to increase vaccination rates12, we observed consistent evidence against any significant effect of reminder/recall interventions on vaccination rates in pregnant people. Overall, these findings indicate that although these strategies may be effective in other patient groups, when administered on their own or bundled with other strategies, they may be less effective in increasing vaccination rates in pregnant people.
Supply-side provider or systems-level interventions were less commonly effective in increasing vaccine uptake, with very low certainty in the evidence in support of the effectiveness of provider or systems-level interventions. Although few studies focused on provider reminders, one study showed modest improvement in vaccination rates following a provider reminder system31. Results for other provider or systems-level interventions were more mixed, and evidence suggesting possible publication bias further reduces certainty in the effects of these types of interventions. Additional studies investigating the effects of provider or systems-level interventions are needed to understand the effects of such interventions with more certainty.
Three of the four studies examining the effect of multi-level bundled interventions that incorporated AFIX, standing orders, immunization champions, and enhanced immunization documentation showed an indication of modest improvement in influenza and pertussis vaccine uptake during pregnancy. However, these were exclusively implemented in one or two US states, meta-analyses showed considerable heterogeneity, and the evidence was deemed to be of very low certainty. Further, because these interventions were often bundled, it is difficult to identify which components effectively increased vaccination uptake. Additional well-designed randomized controlled trials should investigate these strategies. Studies that can isolate and report on the effects of components within bundled interventions would also be informative.
There were several recommended interventions by the Community Preventive Services Task Force that were not identified in the published literature. No study examined community-wide education to improve vaccination rates in pregnancy12, although several ongoing, unpublished studies have been planned to evaluate community education programs. We identified no studies that evaluated the effectiveness of interventions to reduce patient out-of-pocket vaccination costs, administering maternal vaccines in government support programs like Women, Infants, and Children (WIC) clinics or home visits. One study did evaluate an intervention that enhanced the scheduling of existing home visits and showed a 7% relative improvement in tetanus toxoid vaccination35, although home visits were the norm in the study setting and available across treatment groups. These may present additional opportunities to explore to increase vaccination rates during pregnancy.
We noted several important differences in the interventions and study designs implemented in HIC vs LMIC settings. Studies in LMICs often utilized systems-level interventions and targeted uptake of tetanus toxoid vaccine as one of many maternal health care outcomes. In contrast, studies in HICs more commonly focused on vaccination during pregnancy (or the perinatal period) as the primary outcome and most commonly trialed patient-level interventions. We also identified differences in the class of interventions implemented in HIC vs LMIC settings, with three LMIC studies compared with 13 in HIC settings. Patient-level interventions evaluated in LMIC studies included the use of patient incentives (via conditional cash transfers) and patient-held immunization records, which were not investigated in HIC studies. Studies in LMIC settings often evaluated systems-level interventions that aimed to improve the continuum of antenatal care or monetarily incentivize health care system improvements. These interventions were also not identified in any of the HIC studies. In HIC studies, AFIX programs and vaccine delivery systems were more commonly implemented. Six HIC studies investigated the use of AFIX as part of a multi-level, bundled intervention, and just one such study was conducted in an LMIC setting. The differences in interventions investigated are likely due to the unique health care systems, infrastructure, and patient populations across countries. However, failure to examine the effect of different interventions in settings could present missed opportunities to identify effective interventions. No HIC or LMIC study involved community input in the design of the intervention, and future evaluations of additional interventions with community input could ensure interventions are culturally appropriate and offer better success.
This review had several strengths and limitations. Despite the use of an experienced research librarian and an inclusive, peer-reviewed search strategy applied to multiple databases, we failed to identify 11 studies that were only retrievable through reference lists and clinical trial registries. This indicates that screening of additional resources is important for comprehensively identifying all relevant studies on this subject. Furthermore, over half of the studies included in this review were conducted in the United States, and no study evaluated the use of AFIX principles outside of the United States. Additional studies outside of the United States would be useful for understanding the effect of these interventions globally. Finally, we identified substantial heterogeneity when pooling most of the effect estimates from studies. While subgroup analyses that separated interventions by vaccine target (i.e., reducing outcome heterogeneity) reduced some heterogeneity, this did not resolve the issue entirely. It is possible that differences in patient populations, healthcare settings, and intervention components could additionally contribute to statistical heterogeneity; however, the limited number of studies addressing each intervention makes evaluating additional sources of heterogeneity challenging. As additional studies are published, further consideration for these sources of heterogeneity would be useful.
With a RSV vaccine recently introduced for pregnant people and the impending introduction of GBS vaccines, the suboptimal vaccination rates in pregnant people are a growing public health concern. The increasing number of vaccines recommended during pregnancy introduces additional challenges for implementation and may result in increasing maternal vaccine hesitancy. Recent data in the US indicate the problem may be worsening, with declining rates of some vaccines and increasing rates of vaccine hesitancy during pregnancy49,50. Effective strategies to increase vaccination uptake in this priority population are needed to actualize the benefits of maternal vaccination programs and ensure the success of future maternal vaccine programs. While our findings point toward several promising strategies, further research is needed to fully understand the benefits of these strategies in addition to strategies not yet investigated. Evidence-based strategies will be needed to increase and maintain high levels of immunization during pregnancy, and such evidence is needed for programs in both HIC and LMIC settings.
Methods
We conducted a systematic review and meta-analysis of the published literature. The study protocol, including the review question, search strategy, information on the relevant participant, intervention, comparison, and outcome (PICO) criteria for inclusion, types of studies to be included, data elements for extraction and data extraction procedures, risk of bias assessment, and strategies for data synthesis and meta-analysis, was prospectively registered (Prospero #CRD42023441073). We included all randomized controlled trials and quasi-experimental studies that measured the effect of an intervention on the uptake of at least one recommended vaccine during pregnancy. Our primary outcome of interest was uptake of recommended vaccines during pregnancy, as measured by the percent of pregnant participants who received a recommended vaccine. Interventions may target pregnant people directly or target healthcare systems or healthcare providers. Eligible studies included either a standard care comparator or an active comparison group. Because our research question aims to measure the effects of interventions on health care, and randomized trials were feasible to address our research question, in line with Cochrane guidance51, our focus was on randomized trials, including parallel arm, stepped wedge, and cluster randomized trials. We additionally included quasi-experimental design studies (e.g., pre/post, geographic controls), since some systems-level or policy interventions may be difficult to evaluate using a randomized trial. This systematic review therefore excluded non-experimental, observational studies of interventions, review articles, commentaries, and letters to the editor. We did not restrict studies based on language and used online translation services (e.g., translate.google.com) and library services to translate articles not published in English. We did not place any restrictions on the follow-up period in the included studies. We excluded studies that (1) were not a primary study (e.g., conference abstract, review, commentary); (2) did not use an experimental or quasi-experimental study design; (3) did not address vaccine uptake, intent, or hesitancy/acceptance of a recommended vaccine during pregnancy; or (4) did not evaluate the effect of an intervention.
The electronic search strategy was developed in collaboration with a Cochrane Gynecological, Neuro-oncology and Orphan Cancers information specialist (JP, see acknowledgements) and was peer-reviewed by a second independent information specialist (BS, see acknowledgements). Published literature was searched using Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE Ovid, Embase Ovid, and CINAHL EBSCO (Cumulative Index to Nursing and Allied Health Literature). The complete search strategy for each database is included in the Web Supplement (Supplementary Note A–E). In addition, we identified ongoing and unpublished trials using the ISRCTN registry (www.isrctn.com), US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov), World Health Organization International Clinical Trials Registry Platform (ICTRP) Search Portal (apps.who.int/trialsearch), Australian New Zealand Clinical Trials Registry (www.anzctr.org.au), EU Clinical Trials Register (www.clinicaltrialsregister.eu). Reference lists of all included studies and relevant systematic reviews were screened to identify any further relevant studies.
Two reviewers screened titles and abstracts of identified studies to assess eligibility for inclusion. Articles deemed eligible for inclusion based on title and abstract screening were selected for full text review; a random 10% of excluded articles were reviewed and verified by an independent author to ensure accuracy of exclusions. A review of full-text articles was performed by two review authors independently. Disagreements between reviewers were resolved by a third review author. The selection process adhered to PRISMA guidelines for high-quality systematic reviews (Supplementary Note F)52. The research team aims to update the search every five years and update results accordingly, with the next anticipated update in July 2028.
Data extraction
Data extraction was performed using a pre-tested, standardized data extraction form following pilot testing on the first 10% of included citations. Two reviewers independently extracted data (H.U., E.O., S.R.). Disagreements were resolved by a third independent reviewer (A.K.R.). Extracted data elements from each eligible citation included the study design, date and duration of study, number of study centers or locations, study setting, number of participants/clusters randomized, baseline differences between treatment groups, description of intervention(s) and comparison group(s), target of the intervention (i.e., healthcare system, healthcare providers, or pregnant patients), primary and secondary outcomes measured, method of outcome measurement, types of statistical analyses performed, counts of vaccinated participants in the intervention and control group, and relative risks and 95% confidence intervals (where provided).
We applied the framework developed by the Community Preventive Services Task Force for increasing vaccination12 to identify interventions represented in the literature, which includes: (1) those focused on increasing patient demand; (2) those directed at health care providers; and (3) those that enhance access to vaccinations (Fig. 5). Based on this, interventions were classified into patient-level (demand-side interventions), provider or systems-level (supply-side interventions), or a combination of patient, provider, and systems levels (demand- and supply-side interventions).
The Community Preventive Services Task Force framework for increasing vaccination rates recommends using a combination of community-based and healthcare system-based interventions to increase demand and supply of vaccines (SOURCE: https://www.thecommunityguide.org/topics/vaccination.html).
Data synthesis
We considered the intention-to-treat effects of interventions and conducted a separate meta-analysis of treatment effects for demand-side interventions, supply-side interventions, and demand and supply-side interventions. Where provided by the study authors, we used information on relative risk to inform meta-analyses. When relative risks were not reported, we manually calculated these using reported counts. We conducted random effects meta-analysis to estimate the treatment effect of study interventions. We conducted subgroup analyses by the intervention type and recommended vaccine, where possible. The I2 and tau statistics were used to estimate heterogeneity53. We considered <30% to be possibly low, 30–60% to be possibly moderate, 50–90% to be possibly substantial, and 75–100% to be possibly considerable53.
Risk of bias for randomized and cluster randomized control trials was assessed using version 2 of the Cochrane risk-of-bias (RoB 2) tool54. Risk of bias in quasi-experimental studies (non-randomized) was assessed using the ROBINS-I tool55. Two investigators (H.U. and S.R.) independently rated each study. A third investigator (A.K.R.) performed a consensus review, and team discussion occurred where there were conflicts. The likelihood of publication bias was assessed using the Egger statistic and by examining symmetry in funnel plots56,57.
Finally, we evaluated the certainty of the evidence in support of each intervention type using GRADE criteria58. Two investigators (A.K.R., S.R.) independently rated each intervention using GRADE criteria. Where there were conflicts, consensus was reached through consultation with the wider study team.
Patient and public involvement
This study did not include input or participation from patients or the public in the design, conduct, or interpretation of results.
Data availability
The extracted data summarized in this review are available to researchers in an Open Science Framework repository using the following link: https://osf.io/rmxny/?view_only=a81bb3a49669458282d75ee2eccefb65.
Code availability
R code for conducting meta-analyses with the extracted data is available in an Open Science Framework repository here: https://osf.io/rmxny/?view_only=a81bb3a49669458282d75ee2eccefb65.
References
Jones, C. & Heath, P. Antenatal immunization. Hum. Vaccin Immunother. 10, 2118–2122 (2014).
Vygen-Bonnet, S. et al. Safety and effectiveness of acellular pertussis vaccination during pregnancy: a systematic review. BMC Infect. Dis. 20, 136 (2020).
Fu, W. et al. Systematic review of the safety, immunogenicity, and effectiveness of COVID-19 vaccines in pregnant and lactating individuals and their infants. Int. J. Gynaecol. Obstet. https://doi.org/10.1002/ijgo.14008 (2021).
Omer, S. B. Maternal immunization. N. Engl. J. Med 376, 1256–1267 (2017).
Munoz, F. M. & Jamieson, D. J. Maternal immunization. Obstet. Gynecol. 133, 739–753 (2019).
Etti, M. et al. Maternal vaccination: a review of current evidence and recommendations. Am. J. Obstet. Gynecol. 226, 459–474 (2022).
Kilich, E. et al. Factors that influence vaccination decision-making among pregnant women: A systematic review and meta-analysis. PLoS One 15, e0234827 (2020).
Yuen, C. Y. S. & Tarrant, M. Determinants of uptake of influenza vaccination among pregnant women—a systematic review. Vaccine 32, 4602–4613 (2014).
Faria, A. P. V. et al. Tetanus vaccination in pregnant women: a systematic review and meta-analysis of the global literature. Public Health 196, 43–51 (2021).
Galanis, P. et al. Uptake of COVID-19 vaccines among pregnant women: a systematic review and meta-analysis. Vaccines 10, 766 (2022).
Thomson, A., Robinson, K. & Vallée-Tourangeau, G. The 5As: a practical taxonomy for the determinants of vaccine uptake. Vaccine 34, 1018–1024 (2016).
CPSTF. CPSTF Findings for Increasing Vaccination. https://www.thecommunityguide.org/pages/task-force-findings-increasing-vaccination.html (2024).
Momani, A. et al. The effect of COVID-19 vaccine tele-educational program on vaccine hesitancy and receiving the vaccine among women planning for pregnancy, pregnant or breast-feeding mothers. PLoS One 18, e0282627 (2023).
Frew, P. M. et al. A randomized trial of maternal influenza immunization decision-making: a test of persuasive messaging models. Hum. Vaccin Immunother. 12, 1989–1996 (2016).
Goodman, K. et al. Impact of video education on influenza vaccination in pregnancy. J. Reprod. Med. 60, 471–479 (2015).
Jordan, E. T., Bushar, J. A., Kendrick, J. S., Johnson, P. & Wang, J. Encouraging influenza vaccination among Text4baby pregnant women and mothers. Am. J. Prev. Med. 49, 563–572 (2015).
Meharry, P. M., Cusson, R. M., Stiller, R. & Vázquez, M. Maternal influenza vaccination: evaluation of a patient-centered pamphlet designed to increase uptake in pregnancy. Matern. Child Health J. 18, 1205–1214 (2014).
Moniz, M. H., Hasley, S., Meyn, L. A. & Beigi, R. H. Improving influenza vaccination rates in pregnancy through text messaging: a randomized controlled trial. Obstet. Gynecol. 121, 734–740 (2013).
Parsons, J., Griffiths, S. E., Thomas, N. & Atherton, H. How effective are digital interventions in increasing flu vaccination among pregnant women? A systematic review and meta-analysis. J. Public Health. https://doi.org/10.1093/pubmed/fdab220 (2021).
Regan, A. K., Bloomfield, L., Peters, I. & Effler, P. V. Randomized controlled trial of text message reminders for increasing influenza vaccination. Ann. Fam. Med. 15, 507–514 (2017).
Stockwell, M. S. et al. Influenza vaccine text message reminders for urban, low-income pregnant women: a randomized controlled trial. Am. J. Public Health 104, e7–e12 (2014).
Wong, V. W. Y. et al. Brief education to promote maternal influenza vaccine uptake: a randomized controlled trial. Vaccine 34, 5243–5250 (2016).
Yudin, M. H. et al. Text messages for influenza vaccination among pregnant women: a randomized controlled trial. Vaccine 35, 842–848 (2017).
Kriss, J. L. et al. Evaluation of two vaccine education interventions to improve pertussis vaccination among pregnant African American women: a randomized controlled trial. Vaccine 35, 1551–1558 (2017).
Payakachat, N., Hadden, K. B. & Ragland, D. Promoting Tdap immunization in pregnancy: associations between maternal perceptions and vaccination rates. Vaccine 34, 179–186 (2016).
O’Leary, S. T. et al. Efficacy of a web-based intervention to increase uptake of maternal vaccines: an RCT. Am. J. Prev. Med 57, e125–e133 (2019).
Chakrabarti, S., Pan, A. & Singh, P. Maternal and child health benefits of the mamata conditional cash transfer program in Odisha, India. J. Nutr. 151, 2271–2281 (2021).
Sato, R. & Fintan, B. Effect of cash incentives on tetanus toxoid vaccination among rural Nigerian women: a randomized controlled trial. Hum. Vaccin. Immunother. 16, 1181–1188 (2020).
Dudley, M. Z. et al. MomsTalkShots, tailored educational app, improves vaccine attitudes: a randomized controlled trial. BMC Public Health 22, 2134 (2022).
Choudhury, A., Asan, O. & Choudhury, M. M. Mobile health technology to improve maternal health awareness in tribal populations: mobile for mothers. J. Am. Med. Inf. Assoc. 28, 2467–2474 (2021).
Klatt, T. E. & Hopp, E. Effect of a best-practice alert on the rate of influenza vaccination of pregnant women. Obstet. Gynecol. 119, 301–305 (2012).
Mouzoon, M. E. et al. Improving influenza immunization in pregnant women and healthcare workers. Am. J. Manag Care 16, 209–216 (2010).
Li, Y. et al. Coverage for pertussis vaccination during pregnancy with 4 models of vaccine delivery: a quasiexperimental, multicentre observational study. CMAJ Open 10, E56–E63 (2022).
Spina, C. I. et al. Adapting center for disease control and prevention’s immunization quality improvement program to improve maternal vaccination uptake in obstetrics. Vaccine 38, 7963–7969 (2020).
Balakrishnan, R. et al. Continuum of care services for maternal and child health using mobile technology—a health system strengthening strategy in low and middle income countries. BMC Med. Inf. Decis. Mak. 16, 84 (2016).
Bonfrer, I. et al. Introduction of performance-based financing in burundi was associated with improvements in care and quality. Health Aff. 33, 2179–2187 (2014).
Mwase, T. et al. Can combining performance-based financing with equity measures result in greater equity in utilization of maternal care services? Evidence from Burkina Faso. Int J. Health Policy Manag. 11, 308–322 (2022).
Shah, P. M., Selwyn, B. J., Shah, K. & Kumar, V. Evaluation of the home-based maternal record: a WHO collaborative study. Bull. World Health Organ 71, 535–548 (1993).
Woolley, T. et al. Socially accountable medical education strengthens community health services. Med. Educ. 52, 391–403 (2018).
de Walque, D., Robyn, P. J., Saidou, H., Sorgho, G. & Steenland, M. Looking into the performance-based financing black box: evidence from an impact evaluation in the health sector in Cameroon. Health Policy Plan 36, 835–847 (2021).
Zeng, W. et al. Evaluation of results-based financing in the Republic of the Congo: a comparison group pre-post study. Health Policy Plan 33, 392–400 (2018).
Dehlinger, C., Nypaver, C. & Whiteside, J. Use of an evidence-based approach to improve influenza vaccination uptake in pregnancy. J. Midwifery Women’s Health 66, 360–365 (2021).
Mazzoni, S. E. et al. Effect of a multi-modal intervention on immunization rates in obstetrics and gynecology clinics. Am. J. Obstet. Gynecol. 214, 617.e1–7 (2016).
Brewer, S. E., Barnard, J., Pyrzanowski, J., O’Leary, S. T. & Dempsey, A. F. Use of electronic health records to improve maternal vaccination. Women’s Health Issues 29, 341–348 (2019).
Chamberlain, A. T. et al. Improving influenza and Tdap vaccination during pregnancy: a cluster-randomized trial of a multi-component antenatal vaccine promotion package in late influenza season. Vaccine 33, 3571–3579 (2015).
Omer, S. B. et al. Multi-tiered intervention to increase maternal immunization coverage: a randomized, controlled trial. Vaccine 40, 4955–4963 (2022).
Only, H. et al. Developing health service delivery in a poor and marginalised community in North West Pakistan. Pak. J. Med. Sci. 34, 757–760 (2018).
Wootton, S. H. et al. Randomized quality improvement trial of opting-in versus opting-out to increase influenza vaccination rates during pregnancy. AJP Rep. 8, e161–e167 (2018).
Kahn, K. E. et al. Flu, Tdap, and COVID-19 Vaccination Coverage Among Pregnant Women—United States. https://www.cdc.gov/fluvaxview/coverage-by-season/pregnant-april-2024.html (2024).
Calhoun, K. et al. Association of Vaccine Hesitancy with Maternal Influenza and Tdap Vaccination Coverage—United States (2019–20 to 2022–23).
Higgins, J. P. T. & Thomas, J. 3.3 Determining which study designs to include. in Cochrane Handbook for Systematic Reviews of Interventions (Cochrane, 2024).
Liberati, A. et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 6, e1000100 (2009).
Higgins, J. P. & Green, S. 9.5.2 Identifying and measuring heterogeneity. In Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011] (The Cochrane Collaboration, 2011).
Sterne, J. A. C. et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 366, 4898 (2019).
Sterne, J. A. et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 355, 4919 (2016).
Sterne, J., Egger, M., Moher, D. & Boutron, I. Chapter 10: Addressing reporting biases. in Cochrane Handbook for Systematic Reviews of Interventions (Cochrane, 2017).
Sterne, J. A. C. et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ 343, d4002 (2011).
Neumann, I. & Schünemann, H. GRADE Book Version 1.0 (Updated September 2024) (The GRADE Working Group, 2024).
Acknowledgements
This study was supported by a Faculty Development Fund award (PI Regan) at the University of San Francisco and the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under Award Number R01AI169239. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors would like to thank Joanne Platt (National Health Services Foundation Trust) for developing the initial search strategy and Becky Skidmore (Independent Consultant, Ottawa Hospital Research Institute) for peer reviewing the search strategy. The authors are also grateful to Deshayne Fell (University of Ottawa) for reviewing and providing feedback on the study protocol.
Author information
Authors and Affiliations
Contributions
A.K.R. secured study funding and supervised all aspects of the study implementation. A.K.R., M.L.G., E.C., S.R., and A.G. contributed to the conception of the study and development of the study protocol. H.U. led the data collection in collaboration with E.J.O., B.A., and S.R., and A.K.R. performed data analysis and the preparation of the manuscript text, tables, and figures. All coauthors contributed to the writing of the study manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Regan, A.K., Uwimana, H., Rowe, S.L. et al. Systematic review and meta-analysis of interventions to increase the uptake of vaccines recommended during pregnancy. npj Vaccines 10, 76 (2025). https://doi.org/10.1038/s41541-025-01120-1
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41541-025-01120-1