Abstract
Vero cells, as approved by the World Health Organization, have been the most commonly used continuous cell line for viral vaccine production over the last 25 years, but their adherent phenotype continues to limit productivity. Adapting to a suspension culture would overcome this restriction and reduce production costs. First, a Vero suspension isolate was obtained and metabolically characterized. Second, RNA sequencing analysis was used to identify differentially expressed genes between adherent and suspension cells, which revealed complete downregulation of adhesion and matrix-associated genes. Additionally, signaling pathways involving Wnt and other tyrosine kinase receptors were identified as potential leads for growth optimization. In particular, supplementation with fibroblast growth factor 2 allowed for a 20% increase in cell density. Finally, a comparative viral productivity assay revealed a 30% increase in poliovirus production in suspension Vero cells compared to adherent cells depending on the serotype, as well as a 140% increase in respiratory syncytial virus production and a 150% increase in yellow fever virus production. This work establishes the potential of the suspension Vero cell line as a new cell platform for viral vaccine production.
Similar content being viewed by others
Introduction
Smallpox eradication, the 99% decline in cases of wild poliovirus since 19881, and, more recently, the severe acute respiratory syndrome coronavirus 2 epidemic all demonstrate the importance of vaccination in limiting the impact of pathogenic viruses on humans or animals. Among the historical choices for producing live, attenuated, or inactivated viruses, several production systems, particularly cell lines, have been developed to gradually replace other methods such as embryonated eggs. One of the main advantages of cell lines is better monitoring of all production phases, improved quality control of used biological materials, and higher reproducibility from one production batch to another. The scale-up is also greatly improved.
Among the continuous cell lines currently widely used in the vaccine industry, such as MDCK, CHO, PER.C6, and others, Vero cells have been both the first to be approved by the WHO and the most widely used cell line for viral vaccine production in the last 25 years2.
Vero cells are a continuous adherent cell line isolated from African green monkeys’ kidney epithelial cells in 19623. In 2014, genome sequencing revealed that the cells originated from a female Chlorocebus sabaeus4. Vero cells were characterized by their spontaneous establishment and aneuploidy, with an average of 58 chromosomes in more than 65% of the cells. They have been used as a substrate for vaccine production since the 1980s. Their popular use in viral vaccine production can be attributed to their significant advantages in this application. The Vero cell line is susceptible to infection by viruses from various families, including the poliovirus from Picornaviruses, the rabies virus from Rhabdoviruses, and the influenza virus from Orthomyxoviruses5. In addition, it facilitates viral replication due to a lack of type 1 interferon production6. Since its first US Food and Drug Administration approval in the 1980s7, the cell line has received greater attention and is currently deemed nontumorigenic and safe to use as long as it stays within the range of 150 passages8.
Traditionally, Vero cells have been cultivated in serum-containing media, and tremendous efforts have been made to adapt them to animal-component-free media. Indeed, removing serum reduces the risk of contamination with viruses, mycoplasma, or prions9, as well as batch variance and purification costs10. However, removing serum can reduce cellular growth and density, necessitating medium optimization and cell adaptation.
Currently, large-scale vaccine production with Vero cells is performed using microcarriers to expand the available adhesion surface. Despite achieving remarkable productivity, the process is still restricted by surface area. The use of nonadherent Vero cells would allow for overcoming this limitation. Furthermore, culture of suspension cells requires less time-consuming steps, such as adhesion or cell detachment by trypsinization for each passage. The scale-up of suspension processes compared to adherent ones is facilitated by the simplification of the process. It has also been assessed in the case of lentiviral production that suspension culture in single-use stirred tank bioreactor was the most cost-effective technology, preceding the fixed-bed technology for adherent cultures, when a suspension cell line was available11. Various actors have thus launched the development of suspension Vero cells in serum-free media9,12,13,14,15. However, the reported observations included an increased population doubling time (PDT) ranging from 40 to 500 h, depending on the reports, and a tendency toward aggregation. These characteristics have slowed down the scale up and eventually the industrialization of suspension Vero cells. We can note that for teams succeeding to obtain an increased PDT under 60 h, efforts towards industrialization have proven promising. For instance, competitive titers were obtained for different viral production in bioreactors such as VSV (1 L production16), Newcastle Disease Virus (1 L production17), and recently HSV-1 productions up to a pilot scale of 60 L18. These productions highlight the potential of the suspension Vero cells.
From a reference serum-free adherent Vero cell line16, we successfully obtained a novel isolate that actively proliferates in suspension. The PDT was around 40 h, and maximum densities ranging from 1.4 million cells/mL in Erlenmeyers to 2.5 million cells/mL in bioreactors could be obtained, depending on culturing conditions.
In this report, suspension and adherent Vero cells were compared in terms of growth features, transcriptomic profiles, and susceptibility to four different viruses: poliovirus serotype 1 (PV1) and serotype 3 (PV3), yellow fever virus (YFV), and respiratory syncytial virus (RSV), which belong to the Picornavirus, Flavivirus, and Paramyxovirus families, respectively. Altogether, these new findings will help us identify the levers of action needed to industrialize Vero cells in suspension.
Results
Growth rate of suspension Vero cells
Once derived from parental adherent cells, the growth rate of suspension Vero cells was assessed and compared to that of adherent Vero cells (Fig. 1A). Adherent cells went into a lag phase the day following the passage, then proliferation began to accelerate on Days 2–3, peaking on Days 3–4, before declining to a stop on Day 8. The maximum density was reached after 8 days of culture with 340,000 cells/cm², but corresponded to 230,000 cells/cm² on Day 4, when cells were passaged for amplification. The PDT was around 27 h between Days 1 and 4.
 ) versus adherent cells (
) versus adherent cells ( ) over 9 days.
) over 9 days.When centrifuged and diluted, suspension Vero cells grew slowly for the first 24 h, peaking between Days 2 and 3 before slowing down from Day 4 to Day 7. On Day 7, the maximal cell density (1.4 million cells/mL) was achieved. On Day 4, a passage density of 1.2 million cells/mL was observed. The PDT was around 39 h between Days 1 and 4.
Metabolite production
Since efficient energetic metabolism is a key component of cell growth, metabolites such as glucose, lactate, glutamine, and ammonium ions were analyzed in cell culture supernatant and compared between adherent and suspension Vero cells.
First, the media used for adherent and suspension Vero cells had different initial glucose concentrations (Fig. 1B). Higher rates of glucose consumption were observed during cell proliferation peaks. Accordingly, glucose consumption was higher during the first 3 days of culture for suspension cells and then tended to stagnate, whereas it increased between Days 3 and 4 for adherent cells. In all cases, the glucose concentration was not limiting for suspension cells even after 9 days of culture, and it only became limiting for adherent cells on Day 9. As a general observation, adherent cells consumed more glucose than suspension ones. In terms of lactate concentrations, suspension cells produced more lactate over the first 2 days, and then slowed, reaching a cumulative steady concentration (around 11 mM) on Day 9 (Fig. 1B). Adherent cells produced more lactate until Day 4 before slowing down. The highest concentration (26 mM) was reached after 9 days of culture.
Second, metabolites associated with glutaminolysis were observed. Adherent and suspension cells consumed glutamine at similar rates during the first 4 days of culture before a decrease was observed for adherent cells as the metabolite concentration became limiting, in contrast to suspension cells, which had a higher initial concentration (Fig. 1C). Finally, ammonium production was comparable between cell lines, peaking at around 3.5 mM on Day 9.
Transcriptomic analysis
Both adherent and suspension Vero cells were amplified for varying times, and RNA sequencing analysis (RNA-Seq) was performed on frozen pellets harvested at 5, 15, and 25 passages after thawing. Biological triplicates were generated for all points and cell types. Using a 3’ RNA-Seq analysis, FastQ files were processed and revealed differentially expressed genes (DEGs) between the two populations.
Differential expression through time
A principal component analysis was performed to assess the evolution of both cell lines over time as well as the variability between samples. Both principal component analysis axes covered more than 80% of the variance (Fig. 2).
In contrast to adherent cells, the transcriptomic profile of suspension Vero cells appeared to slightly evolve over time since the different passages of suspension cells were more separated, particularly on-axis PC1. However, this evolution represented only 74 genes (Supplementary Data 1). Gene ontology (GO) analysis revealed that DEGs that were upregulated over time within suspension cells were associated with cytokine activity (Table 1), with a significant increase in IL1A, IL1B, CXCL2, or CXCL8 expression (Fig. 3A). Increased passage was also associated with alterations in apoptotic signaling via intrinsic and extrinsic pathways (Table 1). The key upregulated genes in the intrinsic pathway were CHAC1, DDIT3, and TRIB3 (Fig. 3B), whereas those in the extrinsic pathway were NGF, IL1B, IL1A, and G0S2. The downregulated genes over 20 passages were associated with the extracellular matrix (Table 1), including CDH6, VCAN, MMP1, and ANOS1 (Fig. 3C).
Overview of differentially expressed genes
At Passage 25, for each isolate, 11,182 genes were expressed by adherent and/or suspension cell lines, as shown on the volcano plot (Fig. 4A). A total of 858 DEGs were identified between suspension and adherent cells with a log2 fold change of plus or minus 2 and a p-value of 0.05 (displayed in red on the volcano plot), accounting for 7.7% of global gene expression. According to the Venn diagram (Fig. 4B), compared to adherent cells, 657 genes among DEGs were downregulated in suspension cells, whereas 201 were upregulated. Interestingly, there was a greater decrease in gene expression in suspension cells than in adherent cells, with more than 75% of DEGs downregulated. The first finding is that the Vero cell adaptation to the suspension culture is characterized by a slight decrease in gene expression.
A A volcano plot of differentially expressed genes (DEGs) between adherent and suspension cells on Passage 25. The red dots represent significant DEGs (log2 fold change greater than 2). A positive log fold change indicates that the gene is upregulated in suspension cells compared to adherent cells, while a negative log fold change indicates that the gene is downregulated in suspension cells. B Venn diagram depicting the differences in expression between adherent and suspension Vero cell lines on Passage 25. Downregulated genes in adherent versus suspension Vero are indicated.
Analysis of differentially expressed genes
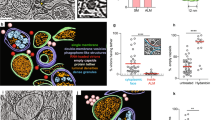
The GO analysis of downregulated genes in suspension cells revealed a substantial number of GO terms related to the extracellular matrix, adhesion junctions, or plasma membrane (Supplementary Table 1). The observation of metabolic pathway modifications revealed the downregulation of adhesion-related pathways such as cell-cell junction organization, tight junction interactions, or adherens junction interactions (Table 2). For example, the cell-cell junction organization metabolic pathway (Reactome) had 30% of its genes downregulated in suspension Vero cells (Fig. 5A), including key adhesion genes CDH6, CLDN10, CLDN4, CDLN3, CRB3, and CADM1 (Fig. 5B). “Transmembrane receptor protein tyrosine kinase activity” was one of the molecular functions GO terms that were also highlighted in downregulated genes with key genes such as CSF1R, FGFR2, NRP1, and RET (Fig. 5C).
A Representation of cell-cell junction organization metabolic pathway (Reactome), key downregulated genes in suspension cells associated with (B) cell-cell junction organization metabolic pathway and gene ontology (GO) bicellular tight junction, C GO transmembrane receptor protein tyrosine kinase activity, D Wnt signaling pathway and/or GO Wnt-protein binding.
The metabolic pathway analysis revealed another pathway associated with proliferation that was downregulated in suspension cells versus adherent cells: the Wnt signaling pathway (Table 2), with genes such as WIF1, IGFBP4, and FZD6 whose expressions were downregulated and DKK1 whose expression was strongly upregulated (Fig. 5D). These findings will help optimize cell growth in the future.
As previously indicated during the passage comparison, GO terms of genes whose expressions were upregulated in suspension culture were also associated with cytokine activity and cytokine-receptor binding (Supplementary Data 2), including inflammation-related signaling pathways (Table 2).
Viral receptors
As previously noted, Vero cells are well-known for their ability to replicate a wide range of viruses. Thus, the stability of viral receptor expression is critical. At passage 25, there were no significant differences in viral receptor expression between adherent and suspension cells (Table 3).
Optimization of cell growth
Following transcriptomic analysis, medium optimization was assessed to promote suspension cell growth. The Wnt signaling pathway was first activated by adding Wnt3A and DKK1 inhibitor WAY-262611. However, no change in cell proliferation was observed in contrast to a strong inhibitory effect of 30% loss of proliferative ability 3 days after the addition of the SKL2001 molecule, a potent Wnt agonist (data not shown). This would imply that the Wnt pathway is tightly controlled in those cells and plays a vital role. When fibroblast growth factor 2 (FGF2 or bFGF) was added to the medium, cell growth improved in a dose-dependent manner 3 days after passage, with an increase of about 8% at 2.5 ng/mL and 17% at 20 ng/mL (Fig. 6).
Viral productivity
Suspension and adherent Vero cells were infected with three viruses from different families in order to assess viral productivity in these cells. For poliovirus, AgD productivity was comparable for Serotype 1 for an identical culture volume of suspension and adherent cells, with 214 UI/mL and 199 UI/mL for suspension and adherent cells, respectively (Fig. 7A). However, AgD productivity was higher for Serotype 3, with 189.5 UI/mL for suspension cells compared to 146.5 UI/mL for adherent cells, showing a nearly 30% increase for a similar culture volume (Fig. 7B). For YFV, there was a significant 150% increase between cell types (p value = 0,003), with 8.08 log10 50% cell culture infectious dose/mL (CCID50/mL) for suspension cells and 7.68 log10 CCID50/mL for adherent cells for a similar culture volume (Fig. 7C). For RSV, the production peaked at 7.61 log10 CCID50/mL for suspension cells compared to 7.21 log10 CCID50/mL for adherent cells (Fig. 7D), and the titer was improved by 142% for a similar number of cells harvested.
The figure shows Antigen D maximal titers with (A) poliovirus Serotype 1 (PV1) and (B) poliovirus Serotype 3 (PV3), as well as the maximal infectious titer of suspension versus adherent Vero cells infected with (C) yellow fever virus or (D) respiratory syncytial virus (RSV). The error bars represent the standard deviation for N = 2 distinct cultures for each condition of PV1, PV3, and RSV infections and N = 3 distinct cultures for each condition of YFV infection. For YFV, mean titers were compared. The data were checked for normality using the Shapiro–Wilk test. The means were compared using the two-tailed and unpaired t-test (p value = 0.0028, df = 6).
Discussion
From a reference serum-free adherent Vero cell line, we successfully obtained a new isolate that actively proliferates in suspension. In order to characterize and compare these cells to the parental line, several analyses were performed. First, the PDT was estimated to be around 40 h before optimization for suspension cells against 30 h for the parental serum-free adherent cell line, indicating that PDT increases with adaptation. Previous reports revealed a comparable decrease in proliferation rate; nonetheless, our resultant isolates and culture conditions were deemed satisfactory when compared to other studies9,12,13,15. We also observed a shift in growth kinetics, with a maximum growth rate between Days 1 and 3 for suspension cells and between Days 3 and 4 for adherent cells. This difference is probably due to mild passage conditions for the suspension culture, which does not require enzymatic dissociation.
Second, in terms of central metabolism, we found that suspension cells consumed less glucose than adherent cells and produced less lactate, with Ylactate/glucose ratio of 1.47 for suspension cells versus 1.84 for adherent cells. The observed value for adherent cells is comparable to previously reported ones, ranging from 1.6516 to 1.917 or 2.018. Altogether, this suggests that these cells in suspension have a more efficient glucose metabolism because they are less prone to lactate production. Additionally, the maximum lactate concentration reached was 26 mM for adherent cells and only 11 mM for suspension cells. As some authors19 have shown that lactate concentrations up to 20 mM had no effect on Vero cell growth (adherent culture with serum conditions), a lower lactate concentration in suspension culture should not be responsible for slowing cell growth. For glutamine metabolism, both cell lines showed similar consumption rates, and the Yammonium ions/glutamine ratio was 0.74 for adherent cells and 0.80 for suspension cells. According to the literature, this ratio varies depending on the medium used, ranging from 0.218 to 0.617 for serum-free adherent Vero cells and from 0.43 17 to 1.020 for serum-cultivated adherent Vero cells. The maximum ammonium ion concentration reached by adherent and suspension cells was 3.5 mM. Hassell et al.19 found that ammonium ion concentrations up to 2.5 mM had no effect on adherent Vero cells cultured with serum, but higher concentrations were not tested. However, during the maximum time for passage (Day 4), the ammonium ion concentration in suspension culture was less than 2.5 mM and hence should not affect growth.
The comparative transcriptomic analysis revealed more differences in suspension cells compared to adherent cells across the passages, even if the number of DEGs between passages remained low. In suspension cells, the genes with upregulated expressions were mostly associated with the GO terms “cytokine activity,” “apoptotic signaling associated with intrinsic apoptotic signaling pathway in response to endoplasmic reticulum stress,” and “extrinsic apoptotic signaling pathway” Genes associated with the GO term “cytokine activity,” such as CXCL2, CXCL8, IL1A, and IL1B, wer associated with the promotion of the immune system as well as cell growth and survival. In addition, IL1A and IL1B can promote the extrinsic apoptotic signaling pathway. Furthermore, genes involved in the “extrinsic apoptotic signaling pathway,” such as CHAC1, DDIT3, and TRIB3, were upregulated. DDIT3, also known as CCAAT/enhancer-binding protein homologous protein (CHOP), is a transcription factor that is activated in response to cellular stress, such as metabolic perturbations, and promotes cell cycle arrest at the G1/S checkpoint21 as well as apoptosis22. CHAC1 overexpression promotes apoptosis via glutathione depletion after activation by CHOP23,24. TRIB3 is another CHOP target that negatively regulates CHOP in cases of transient stress and promotes apoptosis when endoplasmic reticulum stress is prolonged25. Therefore, activation of the apoptotic network could be regarded as a cell response and adaptation to the stress induced by the culture changes, explaining the slower growth of the adapted suspension cells.
The majority of the genes whose expression was downregulated in suspension cells over the passages were linked to the extracellular matrix, such as the chondroitin sulfate proteoglycan VCAN or the cadherin CDH6. Extracellular matrix modifications throughout the passages indicate that the suspension cell line is still adapting to its new culture conditions and further downregulating genes associated with cell adhesion. Analysis of DEGs between suspension and adherent cell lines revealed further downregulation of adhesion-associated pathways such as adherens and cell-cell junctions, as well as genes associated with the extracellular matrix and the plasma membrane. These findings explain why the suspension cell line can grow without contact with the surface or as a single-cell suspension. Other transcriptomic studies of Vero suspension cells are rare, but Sène et al.26 revealed a downregulation in adherens junction pathways. In contrast to our cell line, Sène et al.26 found an upregulation of pathways associated with extracellular matrix or adhesion regulation. A similar upregulation of cell adhesion pathways was reported in HEK293 cells transitioned to a suspension culture27, despite the absence of change observed in extracellular matrix-associated genes. The absence of similar upregulation of cell adhesion/extracellular matrix pathways would also indicate that the adaptation process to the suspension does not follow a unique course.
Suspension cell growth was slower than adherent cell growth, so culture conditions were optimized to achieve comparable growth. The identification of key genes associated with cell proliferation that were downregulated in suspension cells versus adherent cells can point to potential optimization strategies. Downregulation of the gene expression of receptors with tyrosine kinase activity, such as FGFR2 or RET, suggests a potential decrease in downstream proliferative stimulation through those receptors. Similarly, downregulations in Wnt pathway-related gene expression, such as WIF1, IGFBP4, and FZD6, and upregulation of DKK1 gene expression suggest a modification in this pathway, which we decided to investigate further in medium optimization.
Thus, the effect of the Wnt3A, the DKK1 inhibitor WAY-262611, and the Wnt agonist SKL2001 on cell growth was investigated. Wnt3A and WAY-262611 had no effect on cell proliferation, whereas SKL2001 inhibited cell growth by 30%. The effect of bFGF, an FGFR-activating growth factor, was then investigated. The supplementation of the medium with 20 ng/mL bFGF resulted in a 20% increase in cell density 3 days after the passage. The growth factor bFGF can activate all FGFRs with varying efficiencies, including FGFR1, FGFR2, FGFR3, and FGFR428. This particular cell stimulation is probably due to an increase in receptor activation and the Ras/MEK/MAPK/ERK signaling pathway, which promote proliferation29. Additional experimentation on a larger scale and in combination with other growth factors could further improve the PDT.
As a key element for an interest in suspension Vero cells, it is important to evaluate their potential for viral production relative to parental adherent cells. First and most interestingly, the transcriptomic findings revealed no significant differences in the expression of viral receptors for the three viruses studied, YFV, RSV, and polioviruses, implying the stability of viral receptor expression despite considerable changes in culture conditions. Upon testing, viral productivity for PV1 was shown to be comparable in both suspension and adherent Vero cells. The three YFV, RSV, and PV3 viral titers were higher in suspension cells than in adherent cells, with titers multiplying by 2.4, 2.5, and 1.3, respectively. Previous studies revealed that viral productivity was higher in suspension Vero cells than in adherent cells for rabies virus12, Type 5 adenovirus (titer multiplied by 1.514), HSV-1 (titer multiplied by 1.3 to 1230), and VSV (titer multiplied by 1.5–39,13). These novel findings confirm that suspension Vero cells, which lack adherent properties, are a very promising platform for diverse viral vaccine production, even if, currently, the choice of the duration of sampling after infection of adherent or suspended cells will definitively depend on the industrial process, which is still to be optimized.
In conclusion, Vero cells were successfully adapted to a suspension culture with a doubling time of about 40 h. The cell line transcriptomic analysis revealed that adaptation to suspension culture was driven by a general downregulation of genes associated with cellular junctions, adhesion, and extracellular matrix. Furthermore, the genes associated with the inflammatory response and apoptotic pathways were upregulated, while those associated with cell proliferation pathways, such as Wnt or FGF signaling pathways, were downregulated. These findings can explain the slower growth of suspension Vero cells compared to adherent ones. Subsequent medium optimization with bFGF increased cell density by 20%. Finally, no differences in the expression of viral receptors of interest were observed. Suspension cells were found to produce at least as much, if not more, polioviruses, YFV, and RSV as adherent cells, demonstrating the full potential of this cell line for viral vaccine production.
Materials and methods
Cell culture
Adherent Vero cells (ATCC CCL-81) were passaged every 3–4 days in a serum-free and chemically defined in-house medium (MAV) in 25 cm² to 175 cm² T-flasks. Cells were incubated in a humidified incubator with 5% CO2 at 37 °C (Reach-In CO2 incubator, ThermoScientific).
Suspension Vero cells were derived from adherent cells and cultivated in a serum-free and chemically defined in-house medium developed for suspension cells (MSV) on a shaker with agitation. Suspension Vero cells were passaged every 3–4 days by centrifugation and incubated in a humidified incubator with 5% CO2 at 37 °C (ISF1-X incubator shaker, Kuhner).
Cell growth and metabolite analysis
The cells were counted automatically using the NucleoCounter® NC-200™ (Chemometech). The key metabolites in the supernatant were titrated using the CEDEX Bio HT® analyzer (I&L Biosystem).
Transcriptomic and RNA sequencing analysis
RNAs were isolated from frozen samples using the TRIzol method. Briefly, 1 mL of TRIzol (ThermoFisher) was added to about 10 million cells and incubated for 5 min at room temperature. Chloroform (Carlo Erba) was added at a 1:5 ratio to TRIzol. Samples were vortexed, incubated for 2–3 min, and then centrifuged at 17,000 × g for 15 min at 4 °C. The aqueous phase was taken, and an equivalent volume of isopropanol (Carlo Erba) was added. After homogenization, samples were incubated at room temperature for 10 min and then centrifuged at 17,000 × g for 10 min at 4 °C. The pellet was washed at least twice in 1 mL of 70% ethanol (Carlo Erba), and the RNA pellet was left to dry for 5 to 15 min before being resuspended in RNA-free water (ThermoFisher). The quality was checked using the Nanodrop (Labtech), and the concentration was adjusted to 1000 ng/µL. The samples were stored at −80 °C.
Library preparation and sequencing
Libraries were prepared using the QuantSeq 3’ mRNA-Seq Library Prep Kit (Lexogen) for sequencing on Illumina platforms according to the supplier protocol using the unique molecular identifier (UMI) option (6 nucleotides). The library concentrations were determined using a fluorimeter Qubit® 2.0 (ThermoScientific), and the size and quality were determined using the Bioanalyzer (Agilent Technologies). The sequencing was outsourced (Helixio, Saint Beauzire, France) and performed with the NextSeq500 (Illumina), Single read (1 × 75 bp).
RNA-Seq analysis
FastQ files were collected and preprocessed with fastp 0.20.1, which enabled UMI preprocessing and polyA trimming. Data are available at the Gene Expression Omnibus (GEO; accession number GEO: GSE250417). The trimmed fastQ files were aligned with STAR 2.7.6a using the Chlorocebus sabaeus genome assembly (ChlSab1.1) with annotation 101. PCR duplicates were then removed using UMI-Tools 1.0.1. Using quant3p, 3’ annotations were extended before being counted by HTSeq. DEGs were analyzed using RNA-seq DRaMA with EdgeR. Metabolic pathways were realized with RNA-seq DRaMA using pathways from Reactome31, Kyoto Encyclopedia of Genes and Genomes32,33,34, WP35, and PID36. GO analysis37 was performed on significant DEGs with PANTHER (https://www.pantherdb.org/) using the human gene ID for the identified genes38,39.
Medium optimization
The supplement was screened using an Alamar Blue® (ThermoFisher) assay according to the supplier protocol in 6-, 24-, and 96-well ultra-low attachment plates (Corning). In the culture medium, FGF2 (Peprotech), Wnt-3A (produced by the ATCC CRL 2647 L Wnt-3A cell line), WAY-262611 (Sigma-Aldrich), and SKL2011 (Calbiochem) were added at the specified concentrations. The cells were inoculated in supplemented media in two distinct wells of a 6-well plate or four distinct wells of a 24-well plate for each biological replicate of Wnt signaling pathway-related supplementation and FGF2 supplementation, respectively. After 3 days of culture at 37 °C, 5% CO2, and under agitation, the wells were homogenized, and seven or three samples from each well were transferred to a 96-well plate and treated with Alamar Blue®. The absorbance was read on the Glomax Multi-Detection System (Promega).
Viral infections
Viral infections were realized without addition of supplements, tested in the medium optimization section. Two distinct cultures were performed for RSV and each poliovirus infection, and three distinct cultures were performed for YFV infections.
Respiratory syncytial virus
Adherent or suspension Vero cells were grown in their respective media in either T75 cm² or 125 mL Erlenmeyers for a maximum of 5 days before infection with RSV (Sanofi stock) and medium replacement. The media of adherent and suspension cells were changed 3 days after infection. Infected cells were harvested 5 days after infection for adherent cells (harvest by trypsinization) and 7 days after infection for suspension cells and stored at −80 °C for titration using the 50% cell culture infectious dose/mL (CCID50/mL) technique. The CCID50 value was calculated using the Spearman-Karber method and expressed as CCID50/mL. Titers are given for an equal number of infected adherent and suspension cells at time of infection.
Yellow fever virus and inactivated polio vaccine
For polioviruses and YFV assays, adherent Vero cells were grown in bioreactors (Ambr Modular, Sartorius) on Cytodex-1 microcarriers (Cytiva), while suspension Vero cells were grown in 1 L Erlenmeyers. For YFV and polioviruses infections, adherent and suspension cells were inoculated at ~0.3 million cells/mL and cultivated for a maximum of 5 days. Right before infection, the adherent medium was changed, and suspension cells were inoculated in bioreactors with a medium change at 1 million cells/mL for YFV and 2 million cells/mL for polioviruses. Infection was performed with Mahoney and Saukett stains for PV1 and PV3 (Sanofi stock), respectively, and with Strain 17D for YFV (Sanofi stock) in similar culture volumes for both cell types. The media was changed only for adherent cells 2 days after infection in the YFV process. Supernatants from infected cultures were harvested 2 to 3 days after infection for polioviruses and 3 to 4 days after infection for YFV and stored at −80 °C for further analysis.
The YFV infectious titer was determined using a CCID50/mL approach (endpoint dilution assay). The CCID50 value was calculated using the Spearman-Karber method and expressed as CCID50/mL. The Antigen D of PV1 and PV3 was titrated using BiacoreTM (T200, Cytiva) with Antigen D-specific antibodies for each Serotype 1 and 3 as described previously40,41,42. The error bars represent the standard deviation for N = 2 distinct cultures for each condition of PV1, PV3, and RSV infections and N = 3 distinct cultures for each condition of YFV infection.
Statistical analysis
All statistical analyses were performed using GraphPad Prism (GraphPad Software Inc.). The data were checked for normality using the Shapiro–Wilk test. The means were compared using the two-tailed and unpaired t-test. *P-values less than 0.05, **p-values less than 0.01, ***p-values less than 0.001, and ****p-values less than 0.0001 were considered statistically significant.
Data availability
Data are available at the GEO under accession number GSE250417.
References
Hussain, S. F., Boyle, P., Patel, P. & Sullivan, R. Eradicating polio in Pakistan: an analysis of the challenges and solutions to this security and health issue. Glob. Health 12, 63 (2016).
Meeting, W.E.C.o.B.S. & Standardization, W.H.O.E.C.o.B. WHO Expert Committee on Biological Standardization: Sixty-first Report, Vol. 978. (World Health Organization, 2013).
Yasumura, Y. & Kawakita, Y. Studies on SV40 in tissue culture-preliminary step for cancer research in vitro. Nihon Rinsho 21, 1201–1215 (1963).
Osada, N. et al. The genome landscape of the african green monkey kidney-derived Vero cell line. DNA Res. 21, 673–683 (2014).
Vlecken, D. H., Pelgrim, R. P., Ruminski, S., Bakker, W. A. & van der Pol, L. A. Comparison of initial feasibility of host cell lines for viral vaccine production. J. Virol. Methods 193, 28–41 (2013).
Hegde, N. R. Cell culture-based influenza vaccines: a necessary and indispensable investment for the future. Hum. Vaccin Immunother. 11, 1223–1234 (2015).
Duigou, A. Maîtrise des procédés de culture cellulaire pour la production de vaccins. Doctoral dissertation, UHP-Université Henri Poincaré (2010).
Grachev, V., Magrath, D., Griffiths, E. & Petricciani, J. WHO requirements for the use of animal cells as in vitro substrates for the production of biologicals (requirements for biological substances No. 50). Biologicals 26, 175–193 (1998).
Paillet, C., Forno, G., Kratje, R. & Etcheverrigaray, M. Suspension-Vero cell cultures as a platform for viral vaccine production. Vaccine 27, 6464–6467 (2009).
Caron, A. L., Biaggio, R. T. & Swiech, K. Strategies to Suspension Serum-Free Adaptation of Mammalian Cell Lines for Recombinant Glycoprotein Production. Methods Mol. Biol. 1674, 75–85 (2018).
Comisel, R.-M., Kara, B., Fiesser, F. H. & Farid, S. S. Lentiviral vector bioprocess economics for cell and gene therapy commercialization. Biochem. Eng. J. 167, 107868 (2021).
Rourou, S., Ben Zakkour, M. & Kallel, H. Adaptation of Vero cells to suspension growth for rabies virus production in different serum free media. Vaccine 37, 6987–6995 (2019).
Shen, C. F. et al. Development of suspension adapted Vero cell culture process technology for production of viral vaccines. Vaccine 37, 6996–7002 (2019).
Lee, D. K., Park, J. & Seo, D. W. Suspension culture of Vero cells for the production of adenovirus type 5. Clin. Exp. Vaccin. Res. 9, 48–55 (2020).
Logan, M., Rinas, K., McConkey, B. & Aucoin, M. G. Vero cells gain renal tubule markers in low-calcium and magnesium chemically defined media. Sci. Rep. 12, 6180 (2022).
Kiesslich, S., Kim, G. N., Shen, C. F., Kang, C. Y. & Kamen, A. A. Bioreactor production of rVSV-based vectors in Vero cell suspension cultures. Biotechnol. Bioeng. 118, 2649–2659 (2021).
Fulber, J. P. C. et al. Process development for Newcastle disease virus-vectored vaccines in serum-free Vero cell suspension cultures. Vaccines 9, 1335 (2021).
Shen, C. F. et al. Development, optimization, and scale-up of suspension Vero cell culture process for high-titer production of oncolytic herpes simplex virus-1. Biotechnol. J. 19, e2300244 (2023).
Petiot, E. Procédés de cultures de cellules VERO en milieu sans sérum : contributions au développement d'une stratégie PAT. Institut Polytechnique de Lorraine (2009).
Quesney, S. et al. Kinetics and metabolic specificities of Vero cells in bioreactor cultures with serum-free medium. Cytotechnology 42, 1–11 (2003).
Rourou, S., van der Ark, A., van der Velden, T. & Kallel, H. A microcarrier cell culture process for propagating rabies virus in Vero cells grown in a stirred bioreactor under fully animal component free conditions. Vaccine 25, 3879–3889 (2007).
Hassell, T., Gleave, S. & Butler, M. Growth inhibition in animal cell culture: The effect of lactate and ammonia. Appl. Biochem. Biotechnol. 30, 29–41 (1991).
Mendonça, R. Z. & Pereira, C. Cell metabolism and medium perfusion in VERO cell cultures on microcarriers in a bioreactor. Bioprocess Eng. 18, 213–218 (1998).
Barone, M. V., Crozat, A., Tabaee, A., Philipson, L. & Ron, D. CHOP (GADD153) and its oncogenic variant, TLS-CHOP, have opposing effects on the induction of G1/S arrest. Genes Dev. 8, 453–464 (1994).
Zinszner, H. et al. CHOP is implicated in programmed cell death in response to impaired function of the endoplasmic reticulum. Genes Dev. 12, 982–995 (1998).
Crawford, R. R. et al. Human CHAC1 protein degrades glutathione, and mRNA induction is regulated by the transcription factors ATF4 and ATF3 and a bipartite ATF/CRE regulatory element. J. Biol. Chem. 290, 15878–15891 (2015).
Mungrue, I. N., Pagnon, J., Kohannim, O., Gargalovic, P. S. & Lusis, A. J. CHAC1/MGC4504 is a novel proapoptotic component of the unfolded protein response, downstream of the ATF4-ATF3-CHOP cascade. J. Immunol. 182, 466–476 (2009).
Ohoka, N., Yoshii, S., Hattori, T., Onozaki, K. & Hayashi, H. TRB3, a novel ER stress-inducible gene, is induced via ATF4-CHOP pathway and is involved in cell death. Embo J. 24, 1243–1255 (2005).
Sène, M. A., Xia, Y. & Kamen, A. A. Comparative transcriptomic analyses of a vero cell line in suspension versus adherent culture conditions. Int J. Cell Biol. 2023, 9364689 (2023).
Malm, M. et al. Evolution from adherent to suspension: systems biology of HEK293 cell line development. Sci. Rep. 10, 18996 (2020).
Ornitz, D. M. et al. Receptor specificity of the fibroblast growth factor family. J. Biol. Chem. 271, 15292–15297 (1996).
Diez del Corral, R. & Morales, A. V. The multiple roles of FGF signaling in the developing spinal cord. Front. Cell Dev. Biol. 5, 58 (2017).
Fabregat, A. et al. The reactome pathway knowledgebase. Nucleic Acids Res. 46, D649–d655 (2018).
Kanehisa, M. & Goto, S. KEGG: kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 28, 27–30 (2000).
Kanehisa, M. Toward understanding the origin and evolution of cellular organisms. Protein Sci. 28, 1947–1951 (2019).
Kanehisa, M., Furumichi, M., Sato, Y., Kawashima, M. & Ishiguro-Watanabe, M. KEGG for taxonomy-based analysis of pathways and genomes. Nucleic Acids Res. 51, D587–d592 (2023).
Pico, A. R. et al. WikiPathways: pathway editing for the people. PLoS Biol. 6, e184 (2008).
Schaefer, C. F. et al. PID: the pathway interaction database. Nucleic Acids Res. 37, D674–D679 (2009).
Ashburner, M. et al. Gene ontology: tool for the unification of biology. Nat. Genet. 25, 25–29 (2000).
Mi, H., Muruganujan, A., Casagrande, J. T. & Thomas, P. D. Large-scale gene function analysis with the PANTHER classification system. Nat. Protoc. 8, 1551–1566 (2013).
Thomas, P. D. et al. PANTHER: making genome-scale phylogenetics accessible to all. Protein Sci. 31, 8–22 (2022).
Manin, C. et al. Method for the simultaneous assay of the different poliovirus types using surface plasmon resonance technology. Vaccine 31, 1034–1039 (2013).
Author information
Authors and Affiliations
Contributions
C. Bresson, L. Bourigault, B. Pain, and N. Sève designed and initiated the research. L. Bourigault performed the experiments with the help of C. Jean for the transcriptomic approach and C. Chevalard and M. Kloutz for cell culture and viral production. D. Soulet, F. Pelissier, S. Richard, and I. Bassard determined viral titers. L. Bourigault drafted the manuscript, which was edited and revised by C. Bresson, C. Charretier, and B. Pain.
Corresponding author
Ethics declarations
Competing interests
The authors declare the following financial interests and personal relationships as potential competing interests: This work was funded by Sanofi. Léa Bourigault, Corinne Bresson, Christophe Chevalard, Damien Soulet, Fleurine Pelissier, Stéphanie Richard, Isabelle Bassard, Nicolas Sève, and Cédric Charretier are Sanofi employees and may hold shares or stocks in the company.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Bourigault, L., Bresson, C., Jean, C. et al. Characterization of a suspension Vero cell line for viral vaccine production. npj Vaccines 10, 114 (2025). https://doi.org/10.1038/s41541-025-01157-2
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41541-025-01157-2




 ) and lactate measured in the supernatant (
) and lactate measured in the supernatant ( ), C concentrations of glutamine (
), C concentrations of glutamine ( ) and ammonium ions (
) and ammonium ions ( ) measured in the supernatant. The error bars represent the standard deviation for N = 3 distinct cultures for each condition.
) measured in the supernatant. The error bars represent the standard deviation for N = 3 distinct cultures for each condition.




