Abstract
RTS,S/AS01E is the first malaria vaccine implemented for young African children. However, it provides partial protection against Plasmodium falciparum (Pf) malaria, and a better understanding of the mechanisms and determinants of vaccine immunity will help develop second-generation improved vaccines. We measured IgG to vaccine target and Pf blood-stage off-target proteins before and after vaccination in 874 children aged 1–4 years in a phase 2b trial of RTS,S/AS02A in Mozambique. We found that naturally acquired PfCSP IgG levels pre-vaccination were positively associated with RTS,S immunogenicity. Increased levels of IgG to the C-terminus and NANP-repeat regions of PfCSP, and to PfMSP5 and PfMSP1 block 2, following vaccination, were significantly associated with a lower hazard of clinical malaria over 6 months. Thus, immune priming, anti-PfCSP C-terminus and off-target antibody responses contributed to malaria protection after adjusting for prior Pf exposure, and this could guide strategies for optimizing the immunogen and vaccine deployment.
Similar content being viewed by others
Introduction
After its approval in 2021, and following successful pilot implementation studies, the World Health Organization officially recommended the RTS,S/AS01E malaria vaccine in 2023 for African children in endemic regions with medium to high malaria transmission. RTS,S is a pre-erythrocytic vaccine from GSK designed to target the Plasmodium falciparum (Pf) circumsporozoite protein (CSP) and prevent liver stage infection. It combines the last 18 NANP repeats and the carboxy-terminal (C-term) segment of the PfCSP with the hepatitis B surface antigen (HBsAg) to form virus-like particles resembling the outer shell of the hepatitis B virus1,2. In a large multicenter phase 3 trial, RTS,S demonstrated approximately 55% efficacy against clinical malaria in children aged 5–17 months during the first 12 months following vaccination. However, this vaccine efficacy (VE) waned over time, decreasing to around 30% over a 48-month follow-up period, including a booster vaccine dose administered 18 months after primary vaccination3. Despite this decline, the implementation of RTS,S and R21/Matrix-M, another PfCSP-based vaccine, has already demonstrated effectiveness in preventing numerous deaths and cases of malaria in Africa4.
The mode of action of RTS,S still needs to be better understood. The main proposed mechanism of protection against Pf is antibody-mediated, particularly via IgGs targeting the central immunodominant NANP repeat region of PfCSP5. RTS,S-induced IgGs promote complement fixation and activation6,7, opsonic phagocytosis by neutrophils and monocytes, NK cell activation8,9, and inhibition of sporozoite motility10. The C-term fragment of PfCSP contains three known CD4+ and CD8+ T-cell epitopes11, but it is thought to be less immunogenic in terms of neutralizing antibodies, therefore its contribution to VE has received less attention. Some evidence indicates that anti-C-term IgGs contribute to malaria protection12,13,14,15. However, this has not been seen in other studies with pre-erythrocytic vaccines in adults, and the role of these antibodies remains unclear16. In previous studies with younger children from the phase 3 trial, we found three novel IgG-based correlates of protection independent of anti-PfCSP NANP repeat antibodies. First, IgGs to PfCSP C-term were associated with lower odds of clinical malaria; this association was stronger for antibody avidity than levels, in contrast to what was observed for NANP-specific IgGs13. Second, IgGs to HBsAg were unexpectedly associated with protection against malaria12. Third, IgGs to a set of Pf blood-stage (PfBSag) and pre-erythrocytic stage antigens not included in RTS,S but that increased 1 month after vaccination (‘off-target’ responses) were associated with malaria protection17,18,19. Whether those correlates are also relevant in older children, and with different adjuvanted CSP-based vaccines, still needs to be confirmed.
Other key knowledge gaps are how age and malaria transmission intensity (MTI) impact VE, particularly how to disentangle the contribution of those two factors that usually go in parallel, and to what extent vaccination affects naturally acquired immunity (NAI) to malaria20,21. In general, what is known is that very young children, particularly infants aged 6–12 weeks, exhibit lower immunogenicity and VE compared to older children aged 5–17 months22,23. However, as children age, this trend reverses, with older children and adults mounting lower immunogenicity and VE than younger children24. Additionally, it is generally hypothesized that increased exposure to the malaria parasite, including current infection, may negatively impact immunogenicity and VE, though definitive evidence supporting this assumption remains limited.
We had previously investigated some of those questions in the context of the RTS,S/AS02A pivotal phase 2b trial conducted in Mozambique, enrolling 2022 children aged 1–4 years in two areas of high (Ilha Josina) and moderate-low (Manhiça) MTI25. The VE against clinical malaria in this trial during the first 6 months of follow-up after vaccination was moderate (29.9%), although it persisted for 4 years in Manhiça25,26. We found that anti-PfCSP NANP repeat IgG levels and antibody functions7 following RTS,S vaccination were lower in children aged >24 months compared to those <24 months, but significantly inferior in low- compared to high-MTI settings20. In this trial, no association was initially found between peak anti-PfCSP NANP repeat ELISA IgG levels and protection against clinical malaria in a subset of 209 children evaluated by GSK during the first 6 months post-vaccination27, or in a subset of 737 children studied by Burnet over 1.5 years after vaccination15. Those analyses did not take into account the amount of malaria exposure prior to vaccination, despite the previous finding that higher baseline indirect fluorescent antibody test (IFAT) titers to PfBSag at the individual level correlated with higher vaccine immunogenicity20. Thus, it remained to be demonstrated in this trial whether antibodies to the PfCSP NANP repeat and C-term regions were correlates of protection, and whether antibodies to other vaccine epitopes had a role in protection. In addition, we had found evidence in this trial that RTS,S vaccination could affect blood-stage immunity, as IgG levels to asexual PfBSag measured by bead-based or by protein arrays were significantly lower in vaccinated than comparator recipients 6 months after vaccination28,29. This decrease was also observed in other phase 2b trials in Kenya30, likely reflecting fewer Pf infections due to the partial pre-erythrocytic stage protection provided by RTS,S vaccination.
In the present study, we aimed to further characterize RTS,S-induced IgG responses in the Mozambican phase 2b trial, investigating in more depth how malaria exposure prior to vaccination affects immunogenicity and VE. Specifically, we evaluated the role of IgG to PfCSP NANP, C-term, HBsAg and off-target responses in protection against clinical malaria following primary vaccination, in a different age group and using a different adjuvant than the phase 3 trial, including additional antigens and post-vaccination timepoints not tested in our prior studies, and taking into account the individual levels of baseline malaria exposure using PfBSag antibody markers. Through this analysis, we seek to gain further insight into RTS,S-induced immune correlates of protection and baseline determinants, and the roles of both on-target and off-target responses on efficacy, which are relevant for further optimizing the vaccine immunogen.
Results
Study participants
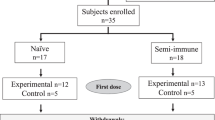
A total of 874 children aged 12 to 59 months old at first vaccination were included in the study (Fig. 1, Supplementary Fig. 1). Of these, 739 received RTS,S/AS02A (675 from Manhiça, 64 from Ilha Josina) and 135 received a comparator vaccine (68 from Manhiça, 67 from Ilha Josina) (Table 1).
Participants received either RTS,S/AS02A or a comparator vaccine administered at month (M) 0, M1 and M2. Blood samples were collected at baseline (M0), 2 weeks after the third dose (M3), and 6.5 months after the third dose (M8). 743 children were enrolled in cohort 1 (Manhiça, low malaria transmission intensity [MTI]) and followed up by passive surveillance to detect clinical malaria episodes as endpoint, and 131 were enrolled in cohort 2 (Ilha Josina, high MTI), where participants received a combination of amodiaquine and sulfadoxine-pyrimethamine 14 days before the third vaccine dose to clear asymptomatic parasitemia, and were followed up using active detection of infection during 4.5 months starting 2 weeks post-vaccination to measure vaccine efficacy against infection.
Immunogenicity to RTS,S vaccine target antigens
A robust PfCSP antibody response was observed among children receiving RTS,S in both trial sites, Manhiça and Ilha Josina (Fig. 2), with significant increases in IgG levels to all three constructs (full length [FL], NANP repeat, C-term). One month (M) post-third vaccine dose (M3), the fold difference in anti-PfCSP FL IgG geometric mean levels in the RTS,S relative to the comparator group was 3935.5 (95% confidence interval [CI] 2065.4–7498.9, p < 0.001) in Manhiça. For NANP repeat and C-term constructs, the fold differences were 602.6 (95% CI 342.8–1056.8, p < 0.001) and 285.8 (95% CI 166.3–490.9, p < 0.001), respectively. In Ilha Josina, the fold-changes were 1285.3 (95% CI 463.5–3564.5, p < 0.001) for PfCSP FL, 462.4 (95% CI 170.6–1253.1, p < 0.001) for NANP and 324.3 (95% CI 120.2–873.0, p < 0.001) for C-term.
a anti-PfCSP IgG levels in Manhiça, b anti-PfCSP IgG levels in Ilha Josina. Boxplots display median IgG levels against PfCSP full length (fl), PfCSP NANP and PfCSP C-terminus (C-term) and the interquartile range (IQR). p-values correspond to the FDR-adjusted from a t-test comparing the IgG levels to each PfCSP construct between treatment groups at month (M) 3. c IgG levels to Hepatitis B surface antigen (HBsAg) in Ilha Josina. Boxplots display median and IQR of IgG levels to HBsAg at each timepoint (month) for each of the age groups (from 12 to 24 [12 < 24] and from 24 to 59 [24 < 59] months). Children in the older group received the Engerix vaccine as a comparator. p-values were obtained from paired t-tests between IgG levels at M0 and M3 and from two-sample t-tests between IgG levels at M3 in each age group in the RTS,S treatment group.
Antibody levels to HBsAg in Ilha Josina had a significant increase from M0 to M3 after RTS,S vaccination in both children younger than 24 months (who had been previously vaccinated with the Engerix vaccine) and children older than 24 months (who had not been previously immunized against hepatitis B virus, because it had only been recently introduced as part of the local EPI programme) (p < 0.001), although M3 IgG levels were significantly higher in the younger children (p = 0.02)(Fig. 2). Among children aged 24 to 59 months, a greater increase (31.7 ± 95% CI 11.38–88.51, p < 0.001) in anti-HBsAg antibody levels was observed after RTS,S vaccination than after Engerix vaccination in the comparator group, consistent with previous analyses20. Thus, RTS,S elicited a stronger antibody response against HBsAg compared to the Engerix-B vaccine, particularly in children with pre-existing hepatitis B immunity.
IgG levels to PfCSP and HBsAg decreased over the subsequent 6 months post-vaccination in both age groups (Fig. 2).
Immunogenicity to vaccine off-target Pf antigens
RTS,S immunization elicited antibody responses against a set of PfBSag not included in the vaccine immunogen. In Manhiça, RTS,S-vaccinated children exhibited a significant increase from M0 to M3 in levels of IgG to PfRh4.2, PfMSP5, PfMSP1 Block (Bl) 2 and PfRh5, with geometric mean fold-changes ranging from 5.06 (95% CI 4.51–5.69, p < 0.001) for PfRh4.2 to 1.22 (95% CI 1.12–1.33, p < 0.001) for PfRh5 (Fig. 3). Conversely, IgG levels to PfEXP1 decreased, with a mean fold-change of 0.79 (95% CI 0.65–0.95, p = 0.002).
Boxplots show median and interquartile range (IQR) of IgG levels against each of the Pf-blood-stage antigens at each time point (Month [M]). p-values are adjusted by false discovery rate (FDR) from t-tests comparing IgG levels at M3 between RTS,S and comparator groups, and from paired t-tests comparing IgG levels at M0 and M3 in RTS,S vaccinees.
Comparing IgG levels at M3 between vaccination groups, children in Manhiça had significantly higher antibody levels against PfRh4.2 (5.31, 95% CI 3.45–8.15, p < 0.001) and PfMSP5 (6.08, 95% CI 3.07–12.05, p < 0.001) in the RTS,S group. A trend of higher IgG levels to PfRh5 was also observed, although this difference did not reach statistical significance (1.57, 95% CI 1.1–2.25, p = 0.089) (Fig. 3). In Ilha Josina, similar observations were noted for PfRh4.2 (3.06, 95% CI 1.5–6.27, p = 0.02) (Supplementary Fig. 2).
Post-vaccination levels of IgG to off-target PfBSag had a low correlation with levels of IgG to PfCSP constructs (Supplementary Fig. 3).
Effect of age and site on RTS,S immunogenicity
At M3, RTS,S-vaccinated children from Manhiça (N = 675) compared to those from Ilha Josina (N = 64) had lower IgG levels to PfCSP constructs (Supplementary Fig. 4), though these differences were not statistically significant (0.58 95% CI [0.26–1.27], p = 0.17 for NANP; 0.63 95% CI [0.34–1.16], p = 0.32 for C-term).
In Manhiça, older children (N = 569) had lower geometric mean antibody levels compared to younger children (N = 174), with fold differences of 0.46 (95% CI 0.27–0.78, p = 0.016) for NANP repeat and 0.54 (95% CI 0.32–0.90, p = 0.038) for C-term (Supplementary Fig. 5). In Ilha Josinha older children also exhibited lower antibody responses, with fold differences of 0.29 (95% CI 0.05–1.65) for NANP and 0.53 (95% CI 0.09–3.06) for C-term, though this difference did not reach significance (p = 0.21 and p = 0.47, respectively).
Effect of baseline Pf exposure on RTS,S immunogenicity
Analyzing correlations between PfCSP or PfBSag IgG levels prior to vaccination (M0), and PfCSP IgG levels post-vaccination (M3), we observed positive moderate associations between IgG to PfEXP1, PfMSP2 or PfCSP constructs at baseline and PfCSP IgG response at M3 (Fig. 4). In contrast, baseline IgG levels to other PfBSag showed very low associations, if any, with post-vaccination PfCSP responses (Supplementary Fig. 6). For baseline PfBSag antibodies that are considered good markers of naturally acquired Pf exposure, correlation coefficients ranged from 0.22 (PfEXP1 at M0 vs. PfCSP C-term at M3, p < 0.001) to 0.31 (PfMSP2 at M0 vs. PfCSP NANP at M3, p < 0.001) (Fig. 4).
Correlations between IgG levels against PfCSP NANP repeat and PfCSP C-terminus (C-term) at month (M) 3 and IgG to PfEXP1, PfMSP2, PfCSP NANP and C-term at M0, assessed by the Pearson’s correlation coefficients. Displayed p-values are adjusted by FDR. a Outcome variable is M3 anti-PfCSP NANP IgG levels. b Outcome variable is M3 anti-PfCSP C-term IgG levels.
Pre-vaccination anti-PfCSP IgG levels correlated positively with post-vaccine antibodies to the same construct (0.44 for PfCSP NANP, p < 0.001; 0.45 for PfCSP C-term, p < 0.001) (Fig. 4). These results did not change when adjusting for age at baseline. The positive effect of previous malaria exposure on RTS,S antibody responses could potentially be mediated by PfCSP-specific acquired immunity, through boosting by vaccination. To determine it, we regressed post-vaccination PfCSP-antibody responses on baseline anti-PfCSP, adjusting for baseline exposure, measured as the sum of IgG levels to PfEXP1 and PfMSP2, and for age. The previously observed correlations between pre-vaccination anti-PfEXP1 or PfMSP2 levels and post-vaccination anti-PfCSP IgG levels disappeared. In contrast, those involving pre-vaccination anti-PfCSP levels were maintained (all adjusted p-values for PfCSP FL, NANP and C-term were <0.001 when the outcome was PfCSP NANP and when it was PfCSP C-term) (Fig. 5).
Three multiple linear regression models are presented, each using one baseline anti-PfCSP IgG measurement (full-length [FL], NANP, or C-terminus [C-term]) as the predictor, with outcomes defined as the post-vaccination IgG levels at month (M) 3 against NANP (blue triangles) and C-term (red circles). These models were adjusted for baseline malaria exposure, measured as the sum of anti-PfEXP1 and anti-PfMSP2 IgG levels at M0, and age at first vaccination. Each forest plot displays the estimated effect sizes (multiplicative effect in the dependent variable per a 10-fold increase in the predictor) and their 95% confidence intervals.
We also analyzed the relationship between pre-existing (M0) anti-HBsAg IgG levels and post-vaccination PfCSP IgG levels, and age at first vaccination, but found no significant correlation (Supplementary Fig. 7).
Association of post-vaccination IgG levels and clinical malaria protection
To evaluate the relevance of post-vaccination PfCSP antibodies on protection against clinical malaria over a 6-month follow-up period, we took into account the heterogeneity of Pf exposure at baseline, as well as age. For the primary analysis including cohort 1 children (Manhiça), higher levels of IgG to all PfCSP constructs –including the C-term fragment– induced by RTS,S immunization, were significantly associated (p < 0.05) with a lower hazard of a first or only malaria episode, adjusted by baseline PfEXP1 and PfMSP-2 IgG levels, and by age (Table 2). These results were replicated using multiple malaria episodes as the endpoint. In the multivariable models, Pf exposure at baseline independently correlated with higher malaria risk subsequently (Table 2). In secondary analysis including both cohort 1 and 2 vaccinated children, higher levels of anti-PfCSP IgG also significantly correlated with a lower risk of a first or only clinical malaria event for FL (p = 0.042), NANP repeat (p = 0.022) and C-term (p = 0.027); Table 2 shows the results adjusting by age, site and Pf exposure at baseline. The associations between M3 IgG levels to the NANP repeat or C-term regions and protection were not significant if adjusted by M3 IgG levels to the other respective PfCSP construct, which means that we could not detect an independent association (data not shown).
Finally, we assessed the effect of PfBSag antibodies at M3 on malaria protection in both vaccine groups together, adjusting by their M0 IgG levels and by age at baseline (Table 3). Including Manhiça children only, higher levels of IgG to PfBSag showing an off-target profile were associated with a reduced hazard of having a first or only clinical malaria episode, with statistical significance for PfMSP5 (HR 0.81, 95% CI 0.69–0.96, p = 0.013) and PfMSP1 Bl2 (HR 0.75, 95% CI 0.58–0.96, p = 0.024), and borderline significance for PfRh4.2 (HR 0.80, 95% CI 0.63–1.01, p = 0.064) and PfRh5 (HR 0.77, 95% CI 0.58–1.01, p = 0.060). Adjusting by M3 PfCSP IgG levels, PfMSP1 Bl2 maintained its significant association with protection, and a trend continued to be observed for PfMSP5 and PfRh5 (Table 3). As expected, M0 levels of IgG to all PfBSag, used for adjustment as markers of exposure, positively correlated with malaria risk in the models. Including all study children, significant associations with a lower risk of having a first or only malaria episode were observed for levels of IgG to PfMSP5 (HR 0.78, 95% CI 0.68–0.90, p = 0.0005), PfMSP1 Bl2 (HR 0.72, 95% CI 0.59–0.87, p = 0.0006), PfRh5 (HR 0.74, 95% CI 0.59–0.94, p = 0.011), PfRh4.2 (HR 0.71, 95% CI 0.60–0.84, p = 8.77e-5), and PfEBA140 (HR 0.78, 95% CI 0.64–0.94, p = 0.010). Adjusting by site and M3 CSP IgG levels (Table 3), significant associations were maintained for IgG levels to PfMSP5 and PfMSP1 Bl2 and borderline significant for IgG levels to PfRh5, PfRh4.2 and PfEBA140. No protective associations were found for M3 IgG levels to antigen markers of Pf exposure or PfEMP1.
Discussion
Our study showed, first, that IgG antibodies induced by RTS,S/AS02A vaccination towards the understudied C-term fragment of PfCSP are associated with protection against clinical malaria also in older (1–4 years) children, as previously identified in younger children (around 1 year old) in the RTS,S/AS01E phase 3 clinical trial, despite targeting less immunogenic epitopes12,13,14,15. Furthermore, we confirm the more frequently reported association between IgG to the immunodominant NANP repeat domain of PfCSP and less malaria risk, which nevertheless was not identified in prior phase 2b trial analyses15,25. This different outcome was probably due to the fact that we adjusted the analyses by serological markers of Pf exposure at baseline, which are associated with higher vaccine-induced anti-PfCSP responses and an increased risk of malaria12,13,14,15. However, we could not detect an independent association of either anti-NANP or anti-C-term antibodies with VE, as this association was lost in survival models that adjusted for one another.
Second, we also showed that a rise in antibody levels to Pf antigens not included in the RTS,S immunogen, so-called off-target responses, which were initially identified in younger children participating in the phase 3 trial, also occurred in older children after vaccination with a different formulation, and that these antibodies were associated with malaria protection. Third, we shed more light on the relationship between baseline MTI and vaccine immunogenicity, showing that pre-vaccination antibody levels to PfCSP or to PfBSag that are markers of exposure, correlated positively with IgG to PfCSP responses post-vaccination, suggesting that prior natural Pf infections may prime rather than impair the B lymphocytes and that RTS,S vaccination boosts this response31.
Our findings, together with our previous results, build upon the importance of anti-PfCSP C-term antibodies for vaccine immune protection in the target population of African children and support taking this region into account for future refinement of second-generation CSP-based vaccine design. In the phase 3 trial, higher avidity of anti-C-term IgGs predicted protection, independently of NANP-specific IgG responses13. Moreover, we have shown that Fc-dependent functional antibodies targeting this epitope are associated with malaria protection in this phase 2b trial, particularly in younger male children15. This evidence contrasts with the strong prioritization of current next-generation vaccine candidates and monoclonal antibodies targeting other regions of the PfCSP, primarily the N-terminal junctional region and the central NANP repeat, largely based on vaccine studies in malaria-naive adults32.
Our data also confirm an off-target response to PfBSag. Specifically, we observed a significant increase in IgG levels upon RTS,S immunization, and/or differences between vaccine groups, for PfRh4.2, PfMSP5, PfRh5 and PfMSP1 Bl2. Fold-changes were, however, lower in comparison to those of PfCSP IgG, partly because the baseline levels of PfBSag were higher. This aligns with findings from the phase 3 trial, where infants aged 6–12 weeks and children aged 5–17 months exhibited higher M3 IgG levels against these antigens in RTS,S vaccinees compared to the comparator group19. Post-vaccination IgG levels to PfMSP1 Bl2 and PfMSP5 were the most consistently associated with protection against clinical malaria when controlling for baseline IgG levels to the same PfBSag, also identified in the previous phase 3 trial study19. Naturally acquired antibodies against these PfBSag have previously been shown to be protective33,34,35,36,37. This association could indicate that the increase in anti-PfBSag IgG levels reflects an overall enhanced immune state. However, we hypothesize that the off-target responses to RTS,S are more likely related to a higher quality anti-PfCSP antibody response, with cross-reactivity to these PfBSag17 (similar to what has been observed between antibodies targeting the NANP repeat and the N-junction region of PfCSP38), rather than being a surrogate marker of non-specific vaccine effect. Cross-reacting anti-PfCSP antibodies may confer cross-stage protection against Pf, if they can bind PfBSag in the parasites with sufficient affinity and directly or indirectly disrupt its lifecycle. PfRh5 is in advanced stages of development as a blood-stage vaccine39,40,41, since specific antibodies can neutralize Pf parasites by blocking the interaction with its receptor basigin, impeding erythrocyte invasion, although other mechanisms may also be involved39. Anti-PfMSP5 IgG may contribute to protection by opsonizing merozoites and inducing a neutrophil respiratory burst34. However, it is also possible that off-target antibodies simply reflect the quality of anti-PfCSP antibodies and do not play a mechanistic role in malaria protection.
It has been hypothesized that malaria exposure negatively affects VE and immunogenicity, in part due to the lower performance of many vaccines in sub-Saharan Africa42,43. Older age, which is associated with increased cumulative exposure, negatively correlated with immune responses to both PfCSP NANP and C-term constructs, with younger children exhibiting stronger post-vaccination responses. This has been seen in other studies of R21/Matrix-M and of PfSPZ vaccines44,45,46, and interpreted as evidence that cumulative exposure would be detrimental to vaccine responses. However, contrary to this general assumption, in our study, pre-vaccination antibody levels correlated positively with M3 IgG levels to PfCSP constructs at the individual level. Importantly, after adjusting for potential confounders, we identified that M0 IgG levels to all three PfCSP constructs were the strongest predictors of post-vaccination responses, rather than IgG levels to PfEXP1 or PfMSP2. This finding aligns with those from the phase 3 trial23, where we also did not observe a negative impact of malaria exposure at baseline on post-RTS,S immune responses in children older than 5 months, as well as with the trial conducted by Juraska et al in 202447, in which they observed a positive association of infection at baseline and VE. Higher baseline antibody levels were, therefore, associated with a stronger vaccine-induced response here, likely due to NAI to PfCSP priming the immune system.
It is important to note that the lower absolute M3 IgG levels observed in older children could result from differences in the maturation of the immune system and the tolerizing effects of prior malaria exposure. In many vaccine studies, younger children have been shown to mount more robust immune responses than older children or adults—a phenomenon attributed largely to differences in immune system maturation, rather than differences in previous exposure. For instance, vaccines such as MMR (administered after 12 months, when maternal antibodies no longer interfere) and varicella often elicit stronger responses in infants compared to older age groups48,49. At the same time, cumulative exposure to Pf (and to other common infections) with age could lead to the induction of tolerogenic and immunoregulatory mechanisms, potentially affecting vaccine immunogenicity. Nevertheless, we did not observe a negative impact of malaria exposure in our study.
The study has some limitations. The imbalance in sample sizes between cohort and treatment groups precludes the use of the comparator children in the PfCSP antibody correlates of protection analyses, which could have been then approached as vaccine effect modification analyses, making our results less sensitive to potential confounding effects. Instead, we chose to maximize the number of RTS,S group children in Manhiça as the primary analysis of the relationship between vaccine-induced PfCSP antibodies against the clinical malaria protection endpoint. Additionally, the smaller sample size in Ilha Josina compared to Manhiça likely reduced the statistical power to detect significant differences in post-vaccination IgG levels, which may explain the non-significant comparisons observed, as NANP repeat levels were previously found to be significantly different by site20. Moreover, as only primary vaccination was administered during the Mozambique phase 2b trial, we could not evaluate the antibody response to a booster dose. This limitation restricts our ability to assess the long-term dynamics of the immune response and to understand how the now-recommended booster dose may modify this response. The study design did not include HBsAg IgG measures in Manhiça, potentially limiting the understanding of HBsAg-related findings. These factors should be considered when interpreting our results and conclusions, and in the design of future studies.
In summary, this study confirms the strong immunogenicity elicited by RTS,S, particularly to PfCSP, including the C-term portion, and that the vaccine unintentionally elicits a response to PfBSag not contained in the immunogen, albeit at much weaker intensity. Our findings highlight the complexity of immune responses to RTS,S in malaria-endemic settings, whereby higher baseline malaria exposure at the individual level is positively associated with enhanced immunogenicity post-vaccination, but older children exhibit lower M3 IgG levels than the younger. Additionally, a protective role of C-term-specific IgGs besides NANP was found, although we could not fully disentangle whether their respective protective effects may reflect overall vaccine-induced immunity or an independent mechanism. Furthermore, the observed off-target responses, such as increased antibody levels against PfMSP1 Bl2 and PfMSP5, and their association with less future clinical malaria, suggest these may contribute to the RTS,S overall efficacy. These findings underscore the interest of C-term and off-target immune responses, which could lead to new strategies for optimizing malaria vaccines, and call for further investigation into the determinants, mechanisms and implications of cross-reactive antibody responses upon antimicrobial vaccination.
Methods
Study design
A subset of 874 out of 2022 participants in the RTS,S/AS02A phase 2b trial (NCT00197041), conducted in Mozambique from 2003 to 200725, was included in this study according to the following selection criteria: having received all three vaccine doses, having serum samples available from the first three timepoint visits (study M0, M3, M8), and having been previously randomly selected and analyzed for antibody functionality analyses in collaboration with the Burnet Institute7.
Out of the 874 selected children, 743 were enrolled from cohort 1 (Manhiça, low MTI, with a mean Pf incidence rate of 294.75 newly diagnosed cases per 1000 population50) and followed up by passive surveillance to detect clinical malaria episodes as primary endpoint. In addition, 131 were enrolled in cohort 2 (Ilha Josina, high MTI, with a mean Pf incidence rate of 403.30 newly diagnosed cases per 1000 population50), where participants received a combination of amodiaquine and sulfadoxine-pyrimethamine 14 days before the third vaccine dose to clear asymptomatic parasitaemia, and were followed up by active detection of infection during 4.5 months starting 2 weeks post-vaccination, to measure VE against infection as endpoint. Given that long-term vaccine protection had been previously documented in Manhiça51, sample inclusion from this site was prioritized to maximize statistical power for assessing immune responses and correlates of protection. Among the selected participants, 739 received RTS,S/AS02A (675 from Manhiça and 64 from Ilha Josina) while 135 received comparator vaccines (68 from Manhiça and 67 from Ilha Josina) (Supplementary Fig. 1), administered at M0, M1 and M2. Venous blood samples were collected at baseline (M0), 2 weeks after the third dose (M3), and 6.5 months after the third dose (M8).
Ethics
The clinical trial research protocols were approved by the National Mozambican Ethics Review Committee, the Hospital Clínic of Barcelona Ethics Review Committee in Spain, and the PATH-Malaria Vaccine Initiative Research Ethics Committee, and all parents or legal guardians of the participants provided written informed consent.
Antibody assays
An in-house multiplex bead-based antibody assay applying the xMAP technology (Luminex Corporation) was performed to quantify total IgG levels against three PfCSP constructs (FL, NANP repeat, and C-term recombinant proteins)52 and a panel of eight specific PfBSag selected based on our objectives: exported protein 1 (PfEXP1, from Sanaria, USA) and merozoite surface protein 2 (PfMSP2, from University of Edinburgh, UK), as primary markers of exposure to Pf infection; reticulocyte binding protein homologs 4.2 and 5 (PfRh4.2, from Burnet Institute, Australia; PfRh5, from ICGEB, India), merozoite surface proteins 1 and 5 (PfMSP1 Bl2, from University of Edinburgh; PfMSP5, from Monash University, Australia), and erythrocyte binding antigen 140 (PfEBA140, from Burnet), as off-target antigens, based on our previous phase 3 trial studies12,17,19, and erythrocyte membrane protein 1 (PfEMP1, from INSERM, France) as a major target of variant immunity and severe malaria pathogenesis (Supplementary Table 1). Antigens coupled to magnetic beads were multiplexed, added to a 96-well plate and incubated with 50 µL of the test samples, positive or negative controls52. Test samples were tested at different dilutions depending on the time point (1/500 and 1/80,000 for M0 and M8.5, and 1/500 and 1/150,000 for M3). The negative controls consisted of serum from naive individuals (1/500 dilution), and the positive control consisted of a standard curve with 12 serial dilutions (4-fold) made of a mixture of a WHO reference reagent (NIBSC code: 10/198) and an RTS,S vaccines pool. After the incubation, beads were washed and incubated with anti-human IgG biotinylated secondary antibody (Sigma), washed again, and incubated with streptavidin-R-phycoerythrin (Sigma). Finally, beads were washed, resuspended and acquired using a Luminex xMAP 100/200 analyzer. At least 50 microspheres per analyte were acquired per sample.
The IgG levels were measured as median fluorescence intensity (MFI) and transformed to levels in arbitrary units (AU) using the modeled calibration curves from serial dilutions of the positive reference sample. Because the magnitude of antibody responses varied across the different Pf antigens, different dilutions were selected as optimal for the estimation of IgG levels to the different antigens. For IgG to the three PfCSP constructs and against the most immunogenic PfBS antigens, PfEXP1 and PfMSP2, the highest dilutions were used (1/80,000 at M0 and M8 and 1/150,000 at M3). For the antibody measures against the rest of the antigens included in the study (PfRh4.2, PfRh5, PfMSP1 Bl 2, PfMSP5, PfEMP1 and PfEBA140), the dilution selected was 1/500 for the three timepoints (M0, M3 and M8).
Antibody levels to HBsAg were determined only in samples from Ilha Josina at GSK laboratories by ELISA with a commercial kit (AUSAB EIA, Abbott Laboratories, Abbott Park, IL)20.
Statistical analysis
For each cohort, RTS,S immunogenicity was evaluated by comparing post-vaccine anti-PfCSP and anti-HBsAg IgG geometric means between treatment groups, and by comparing log-transformed IgG levels to PfCSP constructs pre- vs. post-vaccination in the RTS,S group. 95% CI and p-values were obtained using Welch’s two-tailed, two-sample t-tests and paired t-tests on log-transformed IgG levels, respectively. Linear regression models were used to assess how site and age were associated with log10-transformed M0 and M3 anti-Pf IgG levels. Geometric mean changes/differences and their 95% CI were estimated by exponentiation of regression coefficient estimates, and p-values were obtained by t-tests on the regression coefficients. Multiple regression was used to assess the relationship between pre- and post-vaccination anti-PfCSP-constructs log-transformed IgG levels when controlling for age and exposure, estimated as the sum of pre-vaccination anti-PfEXP1 and PfMSP2 log-transformed IgG levels, by including them as covariates. p-values were adjusted for the False Discovery Rate (FDR) within each block of results of repeated simple regressions and multiple regressions.
To evaluate the relationship between post-vaccination antibody levels to the PfCSP constructs and clinical malaria during the first 6 months post-vaccination, Cox proportional hazard models analyzed the time to both first or only episode and multiple episodes, in RTS,S vaccinees. The models included post-vaccination antibody levels to the PfCSP constructs expressed in log10-transformed calibrated antibody levels, adjusting by baseline age (as a continuous variable), and malaria exposure (sum of IgG levels to PfEXP1 and PfMSP2). Hazard ratios (HR) represent the relative risk of malaria per a 10-fold increase in antibody levels. Primary analysis involved children from Manhiça only, where clinical malaria was the primary endpoint (cohort 1), and secondary analysis also included Ilha Josina children (cohorts 1 and 2), where clinical malaria episodes were detected both by active and passive surveillance, adjusted by site. We applied clustered survival analysis, employing the Andersen-Gill approximation, to account for the non-independence of multiple clinical malaria episodes within the same individual in Manhiça. This approach allows for the estimation of HR while adjusting for within-individual correlation53. Similar Cox proportional hazard models were used to analyze the relationship between M3 IgG to PfBSag that are markers of NAI and time to first clinical malaria episode in vaccinated and comparator groups. The analysis was first conducted in Manhiça, adjusting for baseline PfBSag levels, age at baseline and M3 PfCSP antibodies induced by vaccination, and then extended to both cohorts combined, with additional adjustment for site. The follow-up period started at the first visit 1 month after the third vaccine and lasted for 6 months (183 days). We used the R survival package for all time-to-event (TTE) analysis53. For each TTE analysis sample size (N), number of events, HR (estimated as ten-fold unit increases) with their 95% CI and p-values were reported. Statistical significance for all tests was determined at an alpha level of 0.05 or below.
Data availability
The datasets generated and/or analyzed in the current investigation are available from the corresponding author upon reasonable request.
Code availability
The underlying code for this study [and training/validation datasets] is not publicly available but may be made available to qualified researchers on reasonable request from the corresponding author.
References
Nielsen, C. M. et al. RTS,S malaria vaccine efficacy and immunogenicity during Plasmodium falciparum challenge is associated with HLA genotype. Vaccine 36, 1637–1642 (2018).
Rutgers, T. et al. Hepatitis B surface antigen as carrier matrix for the repetitive epitope of the circumsporozoite protein of Plasmodium falciparum. Bio/Technology6, 1065–1070 (1988).
Penny, M. A., Galactionova, K., Tarantino, M., Tanner, M. & Smith, T. A. The public health impact of malaria vaccine RTS,S in malaria endemic Africa: country-specific predictions using 18 month follow-up Phase III data and simulation models. BMC Med. 13, 170 (2015).
Asante, K. P. et al. Feasibility, safety, and impact of the RTS,S/AS01E malaria vaccine when implemented through national immunisation programmes: evaluation of cluster-randomised introduction of the vaccine in Ghana, Kenya, and Malawi. Lancet403, 1660–1670 (2024).
Chaudhury, S. et al. The biological function of antibodies induced by the RTS,S/AS01 malaria vaccine candidate is determined by their fine specificity. Malaria J. https://malariajournal.biomedcentral.com/articles/10.1186/s12936-016-1348-9 (2016).
Kurtovic, L. et al. Human antibodies activate complement against Plasmodium falciparum sporozoites, and are associated with protection against malaria in children. BMC Med. 16, 61 (2018).
Kurtovic, L. et al. Induction and decay of functional complement-fixing antibodies by the RTS,S malaria vaccine in children, and a negative impact of malaria exposure. BMC Med. 17, 45 (2019).
Feng, G. et al. Induction, decay, and determinants of functional antibodies following vaccination with the RTS,S malaria vaccine in young children. BMC Med. 20, 289 (2022).
Suscovich, T. J. et al. Mapping functional humoral correlates of protection against malaria challenge following RTS,S/AS01 vaccination. Sci. Transl. Med. 12, eabb4757 (2020).
Behet, M. C. et al. The complement system contributes to functional antibody-mediated responses induced by immunization with Plasmodium falciparum malaria sporozoites. Infect. Immun. 86, e00920–17 (2018).
Kaslow, D. C. & Biernaux, S. RTS,S: toward a first landmark on the Malaria Vaccine Technology Roadmap. Vaccine 33, 7425–7432 (2015).
Ubillos, I. et al. Baseline exposure, antibody subclass, and hepatitis B response differentially affect malaria protective immunity following RTS,S/AS01E vaccination in African children. BMC Med. 16, 197 (2018).
Dobaño, C. et al. Concentration and avidity of antibodies to different circumsporozoite epitopes correlate with RTS,S/AS01E malaria vaccine efficacy. Nat. Commun. 10, 2174 (2019).
Kurtovic, L. et al. Multifunctional antibodies are induced by the RTS,S malaria vaccine and associated with protection in a phase 1/2a trial. J. Infect. Dis. 224, 1128–1138 (2021).
Kurtovic, L. et al. Antibody mechanisms of protection against malaria in RTS,S-vaccinated children: a post-hoc serological analysis of phase 2 trial. Lancet Microbe 5, 100898 (2024).
Scally, S. W. et al. Rare PfCSP C-terminal antibodies induced by live sporozoite vaccination are ineffective against malaria infection. J. Exp. Med. 215, 63–75 (2017).
Macià, D. et al. Strong off-target antibody reactivity to malarial antigens induced by RTS,S/AS01E vaccination is associated with protection. JCI Insight 7, e158030 (2022).
Dobaño, C. et al. Differential patterns of IgG subclass responses to Plasmodium falciparum antigens in relation to malaria protection and RTS,S vaccination. Front. Immunol. https://www.frontiersin.org/journals/immunology/articles/10.3389/fimmu.2019.00439/full (2019).
Dobaño, C. et al. RTS,S/AS01E immunization increases antibody responses to vaccine-unrelated Plasmodium falciparum antigens associated with protection against clinical malaria in African children: a case-control study. BMC Med. 17, 157 (2019).
Aide, P. et al. Four year immunogenicity of the RTS,S/AS02A malaria vaccine in Mozambican children during a phase IIb trial. Vaccine 29, 6059–6067 (2011).
Olotu, A. et al. Four-year efficacy of RTS,S/AS01E and its interaction with malaria exposure. N. Engl. J. Med. 368, 1111–1120 (2013).
RTS,S Clinical Trials Partnership et al. A phase 3 trial of RTS,S/AS01 malaria vaccine in African infants. N. Engl. J. Med. 367, 2284–2295 (2012).
Macià, D. et al. The effect of Plasmodium falciparum exposure and maternal anti-circumsporozoite protein antibodies on responses to RTS,S/AS01E vaccination in infants and children: an ancillary observational immunological study to a phase 3, randomised clinical trial. Lancet Infect. Dis. https://doi.org/10.1016/S1473-3099(24)00527-9 (2024).
White, M. T. et al. A combined analysis of immunogenicity, antibody kinetics and vaccine efficacy from phase 2 trials of the RTS,S malaria vaccine. BMC Med. 12, 117 (2014).
Alonso, P. L. et al. Efficacy of the RTS,S/AS02A vaccine against Plasmodium falciparum infection and disease in young African children: randomised controlled trial. Lancet 364, 1411–1420 (2004).
Campo, J. J. et al. Duration of vaccine efficacy against malaria: 5th year of follow-up in children vaccinated with RTS,S/AS02 in Mozambique. Vaccine 32, 2209–2216 (2014).
Guinovart, C. et al. Insights into long-lasting protection induced by RTS,S/AS02A malaria vaccine: further results from a phase IIb trial in Mozambican children. PLoS ONE 4, e5165 (2009).
Campo, J. J. et al. RTS,S vaccination is associated with serologic evidence of decreased exposure to Plasmodium falciparum liver- and blood-stage parasites. Mol. Cell. Proteom. 14, 519–531 (2015).
Campo, J. J. et al. Impact of the RTS,S malaria vaccine candidate on naturally acquired antibody responses to multiple asexual blood stage antigens. PLoS ONE 6, e25779 (2011).
Ndungu, F. M. et al. A seven-year study on the effect of the pre-erythrocytic malaria vaccine candidate RTS,S/AS01 E on blood stage immunity in young Kenyan children. Wellcome Open Res. 4, 42 (2019).
Schrum, J. E. et al. Cutting Edge: Plasmodium falciparum induces trained innate immunity. J. Immunol. 200, 1243–1248 (2018).
Gaudinski, M. R. et al. A monoclonal antibody for malaria prevention. N. Engl. J. Med. 385, 803–814 (2021).
Medeiros, M. M. et al. Natural antibody response to Plasmodium falciparum merozoite antigens MSP5, MSP9 and EBA175 is associated to clinical protection in the Brazilian Amazon. BMC Infect. Dis. 13, 608 (2013).
Perraut, R. et al. Association of antibody responses to the conserved Plasmodium falciparum merozoite surface protein 5 with protection against clinical malaria. PLoS ONE 9, e101737 (2014).
Conway, D. J. et al. A principal target of human immunity to malaria identified by molecular population genetic and immunological analyses. Nat. Med. 6, 689–692 (2000).
Tham, W.-H. et al. Antibodies to reticulocyte binding protein-like homologue 4 inhibit invasion of Plasmodium falciparum into human erythrocytes. Infect. Immun. https://journals.asm.org/doi/10.1128/iai.00048-09 (2009).
Reiling, L. et al. The Plasmodium falciparum erythrocyte invasion ligand Pfrh4 as a target of functional and protective human antibodies against malaria. PLoS ONE. https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0045253 (2012).
Oludada, O. E. et al. Molecular and functional properties of human Plasmodium falciparum CSP C-terminus antibodies. EMBO Mol. Med. 15, e17454 (2023).
Douglas, A. D. et al. The blood-stage malaria antigen PfRH5 is susceptible to vaccine-inducible cross-strain neutralizing antibody. Nat. Commun. 2, 601 (2011).
Douglas, A. D. et al. Neutralization of Plasmodium falciparum merozoites by antibodies against PfRH5. J. Immunol. 192, 245–258 (2014).
Reddy, K. S. et al. Bacterially expressed full-length recombinant Plasmodium falciparum RH5 protein binds erythrocytes and elicits potent strain-transcending parasite-neutralizing antibodies. Infect. Immun. 82, 152–164 (2014).
Jongo, S. A. et al. Safety, immunogenicity, and protective efficacy against controlled human malaria infection of Plasmodium falciparum sporozoite vaccine in Tanzanian adults. Am. J. Trop. Med. Hyg. 99, 338–349 (2018).
Muyanja, E. et al. Immune activation alters cellular and humoral responses to yellow fever 17D vaccine. J. Clin. Invest. 124, 3147–3158 (2014).
Datoo, M. S. et al. Safety and efficacy of malaria vaccine candidate R21/Matrix-M in African children: a multicentre, double-blind, randomised, phase 3 trial. Lancet 403, 533–544 (2024).
Jongo, S. A. et al. Safety and immunogenicity of radiation-attenuated PfSPZ vaccine in Equatoguinean infants, children, and adults. Am. J. Trop. Med. Hyg. 109, 138–146 (2023).
Steinhardt, L. C. et al. Safety, tolerability, and immunogenicity of Plasmodium falciparum sporozoite vaccine administered by direct venous inoculation to infants and young children: findings from an age de-escalation, dose-escalation, double-blind, randomized controlled study in western Kenya. Clin. Infect. Dis. 71, 1063–1071 (2020).
Juraska, M. et al. Genotypic analysis of RTS,S/AS01E malaria vaccine efficacy against parasite infection as a function of dosage regimen and baseline malaria infection status in children aged 5–17 months in Ghana and Kenya: a longitudinal phase 2b randomised controlled trial. Lancet Infect. Dis. 24, 1025–1036 (2024).
Ceyhan, M., Kanra, G., Erdem, G. & Kanra, B. Immunogenicity and efficacy of one dose measles–mumps–rubella (MMR) vaccine at twelve months of age as compared to monovalent measles vaccination at nine months followed by MMR revaccination at fifteen months of age. Vaccine 19, 4473–4478 (2001).
Silber, J. L., Chan, I. S. F., Wang, W. W., Matthews, H. & Kuter, B. J. Immunogenicity of Oka/Merck varicella vaccine in children vaccinated at 12–14 months of age versus 15–23 months of age. Pediatr. Infect. Dis. J. 26, 572 (2007).
Malaria Atlas Project. Global Plasmodium falciparum Incidence Rate map. Available from: https://data.malariaatlas.org/maps?layers=Malaria:202406_Global_Pf_Parasite_Rate&extent (2024).
Alonso, P. L. et al. Duration of protection with RTS,S/AS02A malaria vaccine in prevention of Plasmodium falciparum disease in Mozambican children: single-blind extended follow-up of a randomised controlled trial. Lancet 366, 2012–2018 (2005).
Ubillos, I. et al. Analysis of factors affecting the variability of a quantitative suspension bead array assay measuring IgG to multiple Plasmodium antigens. PLoS ONE 13, e0199278 (2018).
Therneau, T. A package for survival analysis in R. Available from: https://CRAN.R-project.org/package=survival (2024).
Acknowledgements
We thank the phase 2b clinical trial volunteers and their families for their participation. We are grateful to the clinical, field, and laboratory teams performing the phase 2b clinical trial at the research institutions; Deepak Gaur for providing the PfRh5 antigen; Joe Campo and Itziar Ubillos for database support. The phase 2b clinical trial (NCT00197041) was previously funded by PATH Malaria Vaccine Initiative and GSK (clinical trial sponsor), which provided the opportunity to review a draft of this manuscript for factual accuracy, but the authors are solely responsible for the final content and interpretation. The immunology study was supported by funds from the USA National Institute of Allergy and Infectious Diseases, National Institutes of Health (NIH-NIAID, R01AI095789, U01AI165745), and the Spanish Ministerio de Economía y Competitividad (Instituto de Salud Carlos III, PI11/00423 and PI17/02044), co-funded by FEDER funds/European Regional Development Fund (ERDF). C.J. was supported by an AGAUR-FI scholarship (2019 FI_B 00986) granted by the Secretaria d’Universitats i Recerca del Departament d’Empresa i Coneixement de la Generalitat de Catalunya and co-funded with the Social European Fund. L.M. was supported by Erasmus+Mundus Joint Master Degree Leading International Vaccinology Education, co-funded by the Education, Audiovisual and Culture Executive Agency the European Commission, and by a Fondation Mérieux scholarship. J.G.B. was supported by an Investigator Grant from the National Health and Medical Research Council of Australia. G.M. was supported by RYC 2020-029886-I/AEI/10.13039/501100011033, co-funded by European Social Fund (ESF). This research was supported in part by the Intramural Research Program of the NIAID-NIH. The Manhiça Health Research Centre receives core funding from the Spanish Agency for International Cooperation and Development (AECID). This research is part of ISGlobal’s Program on the Molecular Mechanisms of Malaria, which is partially supported by the Fundación Ramón Areces. We acknowledge support from the grant CEX2023-0001290-S funded by MCIN/AEI/10.13039/501100011033, and support from the Generalitat de Catalunya through the CERCA Program. The funders had no role in study design, data collection and analysis, the decision to publish, or the preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
C.D. and G.M. contributed conceptualization. C.J., J.P.T.-Y., L.M. and M.V. performed laboratory experiments. R.A., C.D. and G.M. supervised laboratory work. D.M., R.S. and E.F. performed data curation and analysis. J.S. coordinated the clinical trial. E.F., D.M., R.A., G.M. and C.D. wrote the original draft. D.L.N., R.L.C., D.C., B.G., J.G.B. and S.D. supplied antigens. All authors critically read, revised, and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Fuentes, E., Jairoce, C., Macià, D. et al. Role of malaria exposure and off-target responses on RTS,S/AS02A vaccine immunogenicity and protection in Mozambican children. npj Vaccines 10, 116 (2025). https://doi.org/10.1038/s41541-025-01167-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41541-025-01167-0