Abstract
Adults aged 65 years and older are at increased risk for infectious diseases, including invasive meningococcal disease (IMD), yet data on meningococcal vaccine immunogenicity in this population remain limited. In this randomized clinical trial (CTIS: 2024-513640-29-00, 13-05-2024), 222 older adults (65–85 years) received a quadrivalent meningococcal conjugate vaccine (MenACWY-TT), with 104 adults receiving a booster dose one year later. Serum bactericidal activity (rSBA) and polysaccharide-specific IgG and IgM concentrations were assessed. One month post-primary vaccination, 91–98% of participants had protective rSBA titers (≥8). Booster vaccination transiently increased bactericidal responses, but titers returned to pre-booster values within a year for MenC, -W, and -Y. rSBA titers correlated stronger with IgM than IgG, particularly for MenW and -Y. Interestingly, IgM depletion markedly reduced rSBA titers, while IgG depletion had minimal impact. These findings highlight that MenACWY-TT vaccination elicits functional antibody responses in older adults, largely driven by IgM.
Similar content being viewed by others
Introduction
Neisseria meningitidis is a Gram-negative diplococcus that asymptomatically colonizes the human nasopharynx in up to 25% of the population1. However, on rare occasions, the encapsulated bacteria become pathogenic and causes invasive meningococcal disease (IMD), which may lead to meningitis and/or septicemia1,2. The incidence of IMD, particularly caused by meningococcal serogroups W and Y, is increasing among older adults with case-fatality rates of up to 26%, which may be an underestimation given to sometimes atypical presentation of IMD in elderly3,4,5,6,7,8. Older adults surviving IMD often experience substantial sequelae, which significantly impact quality of life and long-term health outcomes3,9. With the global proportion of individuals aged over 60 years rapidly increasing10, the number of IMD cases among older adults is expected to rise, presenting a growing public health concern3,5.
Given the rapid onset and severity of IMD, meningococcal vaccination is the key strategy in IMD prevention2,11. Among the available meningococcal vaccines, the quadrivalent MenACWY-TT polysaccharide-tetanus toxoid-conjugate vaccine (MenACWY-TT), which targets meningococcal serogroups A, C, W, and Y, is licensed for use in all age groups12. Although aging is associated with a gradual decline in immune function which often results in low vaccine responsiveness13, primary MenACWY-TT vaccination induces robust bactericidal responses - the established correlate of protection against IMD14,15—in older adults12,16,17,18. However, data on bactericidal responses following a second (so-called booster) dose of MenACWY-TT in this age group are scarce19,20. Moreover, to our knowledge, no studies have investigated meningococcal polysaccharide (ps)-specific immunoglobulin G (IgG) and M (IgM) antibody responses, which drive bactericidal activity by facilitating complement-mediated bacterial lysis21, following MenACWY-TT primary and booster vaccination in adults aged 65 years and older.
This study aims to determine the immunogenicity of a primary MenACWY-TT vaccination as well as a booster dose, administered one year later, in adults aged 65 to 85 years, by measuring meningococcal-specific serum bactericidal antibody (SBA) titers. Additionally, we investigated the contribution of MenACWY ps-specific IgM and IgG antibodies to the bactericidal activity. Altogether, these observations provided new insights that may guide future vaccination strategies.
Results
Study population
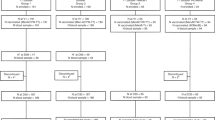
Out of the 5570 invitations sent to eligible older adults, 512 individuals (9.5%) expressed interest in participating in the study and 223 participants were included to receive a primary MenACWY-TT dose (Fig. 1). Following the loss of one participant before the first post-vaccination sampling, analyses were performed on a cohort of 222 participants (median age 73 years; IQR: 69–76; 42.8% female; see Supplementary Table 2 for detailed baseline characteristics). One year after primary vaccination, a subset of 104 participants (baseline median age 73 years; IQR: 69–77; 44.2% female; Fig. 1, and Supplementary Table 2) received a booster MenACWY-TT dose.
MenACWY-specific rSBA responses following primary MenACWY-TT vaccination
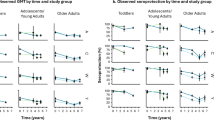
At baseline, proportions of participants with rSBA titers ≥8 varied per serogroup, ranging from 12.2% for MenY to 23.9% for MenA (Fig. 2B and Table 1). One month following vaccination, rSBA titers significantly increased for all four serogroups compared to baseline (all p < 0.0001; Fig. 2A and Table 1). Additionally, 96% (MenA), 99% (MenC), 90% (MenW), and 92% (MenY) of older adults achieved protective titers (rSBA ≥8), and 90% (MenA), 86% (MenC), 88% (MenW), and 90% (MenY) of older adults reached rSBA titers of ≥128 (Fig. 2B and Table 1). One year post-vaccination, rSBA titers and the proportions of participants with rSBA titers ≥8 and ≥128 remained significantly higher than baseline (all p < 0.0001), although a significant decline was observed compared to one month post-vaccination (all p < 0.0001; Fig. 2 and Table 1). At one year post-vaccination, 75% (MenA), 82% (MenC), 78% (MenW), and 75% (MenY) of participants had rSBA titers ≥8, while 54% (MenA), 56% (MenC), 73% (MenW), and 63% (MenY) maintained titers ≥128 (Fig. 2B, Table 1). At two-year post-vaccination, rSBA titers were still significantly higher than baseline (p < 0.0001) in primary-only vaccinees (n = 110; Fig. 2A and Table 1). Of these participants, 73% (MenA), 74% (MenC), 62% (MenW), and 58% (MenY) still had rSBA titers ≥8. Although no effect of age on rSBA responses was observed, MenA- and MenC-specific rSBA titers were significantly higher in females than in males (p = 0.014 and p < 0.001, respectively, one-month post vaccination; Supplementary Table 2).
MenACWY-specific rSBA responses A and corresponding percentages of participants with rSBA titers ≥8 and ≥128 (B), ps-specific IgG (C) and IgM (D) concentrations were determined pre-vaccination (pre-), at one month (1 m) and one year (1 y) post-primary MenACWY-TT vaccination for all participants (n = 222). At two years (2 y) post-primary vaccination, responses were assessed only in participants who did not receive a booster vaccination at 1 y (n = 110). Geometric mean titers (GMTs) and geometric mean concentrations (GMCs) are indicated in black, with error bars representing the 95% confidence intervals. Following log10-transformation, rSBA and antibody responses were compared between timepoints using paired generalized linear mixed models (GLMM), followed by post-hoc Emmeans tests and subsequent Bonferroni correction for multiple testing. Proportions of participants with rSBA ≥8 or ≥128 (with 95% CI) were calculated with the Wilson score interval with continuity correction.
MenACWY ps-specific IgG and IgM responses following primary MenACWY-TT vaccination
At one month post-vaccination, significant increases in ps-specific IgG and IgM concentrations were observed compared to baseline for all serogroups (all p < 0.0001), which remained elevated for two years (all p < 0.0001; Fig. 2C, D and Table 1).
Interestingly, while MenA- and MenC-specific IgG and MenACWY-specific IgM concentrations peaked at one month, followed by a decline at one year post-vaccination (all p < 0.0001), MenW-specific IgG concentrations increased significantly from one month to one year post-vaccination (p < 0.0001; Fig. 2C, D, and Table 1). Moreover, no significant change in MenY-specific IgG concentrations was observed between these timepoints. Upon further analysis, a substantial number of participants demonstrated a ‘slow’ IgG response, with higher IgG concentrations at one year compared to one month post-vaccination for MenW (n = 143) and MenY (n = 98; Supplementary Fig. 1). Interestingly, while these individuals had significantly lower IgG concentrations at one month post-vaccination compared to those exhibiting the expected antibody peak at one month (p < 0.0001 for both serogroups), IgG concentrations were similar at one year post-vaccination (Supplementary Fig. 1). A similar slow increase in rSBA or IgM responses was observed in only small numbers of participants.
MenACWY-specific rSBA responses following booster MenACWY-TT vaccination
In the booster cohort (n = 104), significant increases in rSBA titers were observed at one month post-booster vaccination compared to pre-booster titers for all serogroups (all p < 0.0001; Fig. 3A and Table 2). Additionally, proportions of older adults with rSBA titers ≥8 increased for MenA (98%), MenC (95%), MenW (98%), and MenY (94%), though not significant for MenC (p = 0.068; Fig. 3B and Table 2), whereas proportions with titers ≥128 increased for all serogroups (p < 0.01; Fig. 3B and Table 2). One year post-booster vaccination, MenACWY-specific rSBA titers declined significantly compared to one month post-booster (p < 0.0001; Fig. 3A and Table 2). Interestingly, no significant differences were found between pre-booster and one year post-booster vaccination rSBA titers, except for an increase for MenA (p < 0.0001; Fig. 3A and Table 2). One month post-booster vaccination rSBA titers for MenA (p = 0.0033) and MenC (p < 0.0001) were lower, and those for MenW and MenY were comparable compared to one month post-primary vaccination. No effect of age or sex was observed on post-booster vaccination rSBA responses (Supplementary Table 2). Two years post-primary vaccination, rSBA titers in the booster cohort were significantly higher than those observed in the primary-only cohort for MenA (p < 0.0001), MenW (p = 0.0002), and MenY (p = 0.0030), but not for MenC (Supplementary Fig. 2A).
Data shown are for the booster cohort (n = 104), which includes only participants who received a MenACWY-TT booster vaccination at one year (1 y) post-primary vaccination. MenACWY-specific rSBA responses (A), corresponding percentages of participants with rSBA titers ≥8 and ≥128 (B), ps-specific IgG (C), and IgM (D) concentrations were assessed pre-booster (1 y), at one month (1 y + 1 m), and one year (1 y + 1 y) following booster vaccination. For reference, responses measured before and after primary vaccination in booster cohort participants are also shown. Geometric mean titers (GMTs) and geometric mean concentrations (GMCs) are shown in black, with error bars representing 95% confidence intervals. Following log10-transformation, rSBA and antibody responses were compared between timepoints using paired generalized linear mixed models (GLMM), followed by post-hoc Emmeans tests and subsequent Bonferroni correction for multiple testing. While statistical comparisons were performed across all timepoints, the significant p-values are only shown for the following comparisons: 1 y vs. 1 y + 1 m, 1 y vs. 1 y + 1 y, 1 m vs. 1 y + 1 m, 1 y + 1 m vs. 1 y + 1 y. Proportions of participants with rSBA ≥8 or ≥128 (with 95% CI) were calculated with the Wilson score interval with continuity correction.
MenACWY ps-specific IgG and IgM responses following booster MenACWY-TT vaccination
One month post-booster vaccination, ps-specific IgG concentrations significantly increased for all serogroups and remained significantly higher at one year (for MenA, MenW, and MenY) compared to pre-booster concentrations (p < 0.0001; Fig. 3C and Table 2). Nevertheless, a significant decline in IgG was noted between one month and one year post-booster vaccination for all serogroups (p < 0.0001). Notably, IgG concentrations were significantly higher one month post-booster vaccination compared to one month post-primary vaccination for MenA, MenW, and MenY (p < 0.01), but not for MenC (p = 0.76).
Ps-specific IgM concentrations increased significantly one month post-booster compared to pre-booster vaccination (p < 0.0001; Fig. 3D and Table 2). However, for MenC, MenW, and MenY, IgM returned to pre-booster concentrations by one year. Additionally, IgM concentrations at one month post-booster vaccination did not exceed those observed at one month post-primary vaccination.
Two years after primary vaccination, MenACWY ps-specific IgG and MenA ps-specific IgM concentrations were significantly higher in the booster cohort compared to the primary-only cohort (all p < 0.05; Supplementary Fig. 2B, C).
Correlations between MenACWY-specific rSBA, IgG, and IgM responses following primary and booster MenACWY-TT vaccination
Correlations between ps-specific IgG and IgM concentrations were either absent or weak across all serogroups and timepoints following primary as well as booster MenACWY-TT vaccination (Fig. 4A, B).
The Spearman correlation coefficient and corresponding levels of significance are shown for each pairwise combination of MenACWY-specific rSBA titers, IgG levels, and IgM levels for the whole cohort (n = 222) following primary vaccination (A). At two years (2 y) post-primary vaccination, responses were only assessed in participants who did not receive a booster vaccination at 1 y (n = 110). Additionally, correlations following booster vaccination are shown for the booster cohort (n = 104) (B), which includes only participants who received a MenACWY-TT booster vaccination at one year (1 y) post-primary vaccination. For reference, correlations observed before and after primary vaccination are also shown for the booster cohort, to allow for direct comparison between timepoints. Correction for multiple testing was performed using the Bonferroni method. The strength of the correlation is depicted by color intensity, while the significance level is indicated by the size of the dot. Spearman rho values are displayed for each pairwise correlation.
Following primary vaccination, correlations between rSBA titers and IgG concentrations were moderate for MenA and MenC, and weak for MenW and MenY (p < 0.001; Fig. 4A). Interestingly, strong correlations were found between post-primary vaccination MenW- and MenY-specific rSBA titers and IgM concentrations (p < 0.001; Fig. 4A). Besides, these correlations were moderate for MenA and MenC (p < 0.001). Overall, one month post-primary vaccination rSBA titers showed stronger correlations with ps-specific IgM than with IgG for all serogroups.
One month post-booster vaccination, rSBA titers correlated stronger with ps-specific IgM than with IgG for MenC and MenY (Fig. 4B). For MenA and MenC, these correlations were moderate-to-strong for both IgG and IgM. Notably, MenW and MenY-specific rSBA titers demonstrated stronger correlations with IgG at one month post-booster vaccination, than correlations observed at one month post-primary vaccination (Fig. 4B).
The role of MenACWY ps-specific IgM in conferring bactericidal activity
To assess the relative importance of ps-specific IgG and IgM responses in conferring bactericidal activity, individuals were ranked high for IgG and/or IgM at one-month post-primary vaccination (Fig. 5A). As such, four groups were allocated: both high, both low, high IgG-low IgM and vice versa. MenW and MenY-specific rSBA titers were significantly higher in participants with low ps-specific IgG but high IgM concentrations, as compared to participants with high IgG but low IgM concentrations (p < 0.0001 for both; Fig. 5A). Nevertheless, this was not observed for MenA and MenC.
Participants were ranked high or low for IgG and/or IgM concentrations at one-month post-primary vaccination. As such, four groups were assigned: both high, both low, high IgG-low IgM and vice versa. Based on these groups, rSBA titers at one month post-primary vaccination were compared between the different Ig-profile groups (A) using the Kruskal-Wallis test, followed by Dunn’s post-hoc test and Bonferroni correction for multiple testing. The effect of IgG or IgM depletion on post-vaccination rSBA titers were determined in a subset of one-month post-vaccination serum samples (n = 28). MenC- (B) and MenW- (C) specific IgG concentrations (left) and rSBA titers (right) following IgG depletion. MenC- (D) and MenW- (E) specific IgM concentrations (left) and rSBA titers (right) following IgM depletion. Original and depleted samples were compared using the Wilcoxon matched-pairs signed rank test.
Lastly, we depleted either IgG or IgM from a subset of post-vaccination serum samples (n = 28) and determined MenC and MenW-specific rSBA titers (Fig. 5B–E). Upon IgM depletion, significant decreases in rSBA titers were found in depleted versus original sera (MenC: p = 0.0008; MenW: p < 0.0001, Fig. 5D, E), but not following IgG depletion (MenC: p = 0.49; MenW: p = 0.37, Fig. 5B, C).
Discussion
In this study, we demonstrated that both primary and booster MenACWY-TT vaccination elicited robust bactericidal activity and polysaccharide-specific IgG and IgM responses in adults aged 65-85 years. However, booster-induced bactericidal responses were found to be short-lived. Moreover, we showed that rSBA titers were generally better explained by IgM than by IgG, underscoring the key role of IgM in functional immunity against meningococci.
Remarkably, despite the initial increase in bactericidal activity upon booster vaccination, rSBA titers following the booster dose were lower than (MenA and MenC) or comparable to (MenW and MenY) titers observed after primary immunization. Moreover, the booster-induced bactericidal responses appeared to be short-lived, as MenC-, MenW-, and MenY-specific rSBA titers declined to pre-booster levels within one year. This limited persistence may be partly attributed to the short interval of one year between the two vaccine doses in this study. Although Robertson et al.19,20, observed similar increases in rSBA titers one month after a MenACWY-TT booster dose administered 3 or 6–7 years post-primary vaccination, their studies did not assess bactericidal responses beyond one month. Therefore, the potential benefit of a delayed booster for sustaining long-term protection in older adults remains unclear. Further evaluation of persistence of bactericidal responses following booster doses administered at different time intervals (e.g., 5–10 years) and persistence of functional antibody responses beyond one year post MenACWY-TT booster vaccination in older adults will be useful to support preventive vaccination strategies.
Interestingly, our data show that bactericidal activity is largely driven by ps-specific IgM responses. Considering that IgM is more efficient in complement fixation and bacterial opsonization than IgG22,23, low booster rSBA responses are likely explained by low IgM concentrations, even despite the presence of high IgG concentrations. More so, depletion of IgM from serum resulted in a strong reduction in bactericidal activity, whereas rSBA titers were mostly sustained upon IgG depletion, emphasizing the cruciality of ps-specific IgM responses in antibody-mediated complement-dependent killing of meningococci and consequent protection against disease. The latter is consistent with an earlier study by van der Heiden et al.24, demonstrating that rSBA responses after MenACWY-TT vaccination in middle-aged adults (50–65 y) were strongly associated with IgM responses, particularly in case of MenW and MenY. Moreover, Park et al.22, reported functional antibody responses upon vaccination with a pneumococcal polysaccharide vaccine were more dependent on ps-specific IgM than IgG concentrations. Lastly, a recent study by Hendriks et al.25, has shown that IgM outperforms IgG in antibody-mediated complement-mediated opsonophagocytic killing of Streptococcus aureus. Altogether, these findings underscore the essential role of ps-specific IgM in bactericidal activity.
The relatively slow and relatively weaker IgG responses against MenW and MenY, alongside strong correlations between rSBA titers and IgM (but not IgG), suggest a more naïve-like immune response. In contrast, the strong IgG responses against MenA and MenC, which correlated well with rSBA titers, may reflect the boosting the pre-existing memory immunity from prior natural exposure (carriage)26. However, historical meningococcal circulation patterns provide only partial support for this, as both MenC and MenW have circulated and caused outbreaks in the Netherlands in 200127 and 201828, respectively. Notably, MenA disease is virtually absent in The Netherlands29. Therefore, pre-existing immunity against MenA might be the result of polysaccharide cross-reactivity with Bacillus pumilus30 or Streptococcus faecalis31, bacterial species commonly found in soil and in the gastro-intestinal tract, respectively. Besides prior meningococcal exposure, structural differences in the capsular polysaccharides and their conjugation to the carrier protein may also play a role in immune activation32,33: MenW and MenY share a more comparable capsule structure, whereas the polysaccharides of MenA and MenC are more chemically distinct. In addition, MenW- and MenY-Ps are directly conjugated to the tetanus toxoid carrier protein, whereas MenA- and MenC-Ps are conjugated indirectly by an adipic dihydrazide, which might result in differences in antigen presentation and subsequent stronger IgG responses33.
A key strength of this study is the evaluation of antibody responses following both primary and booster MenACWY-TT vaccination, including direct comparison between booster and non-boosted individuals within the same cohort. Moreover, the evaluation of (functional) antibody responses beyond one month post-vaccination allows for assessment of antibody persistence and waning over time. However, the generalizability of our findings may have some limitations, as the study population consisted of community-dwelling older adults in relatively good health. Additionally, although we focused in this study only on antibody responses as it has been shown to correlate strongly with protection14,15,34, it cannot be excluded that also cellular immunity and complement activity are important aspects of the immune response, warranting further investigation.
Lastly, even though IMD cases among older adults contribute to a significant proportion of the overall IMD burden in developed countries, IMD prevalence is low. To illustrate, in 2017, the MenW IMD incidence in adults aged 65 years and older in the Netherlands was approximately 0.6 per 100,00028. Hence, it is unlikely that meningococcal vaccination will be implemented in national immunization programs as policymakers must weigh the cost-effectiveness of broad vaccination strategies for older adults against the potential benefits. Nevertheless, insights in how (well) older adults respond to MenACWY-TT vaccination may become relevant in outbreak settings3. Moreover, data on conjugate vaccine immunogenicity in older adults are valuable in itself to increase our understanding of immune responses in elderly, including antibody functionality and the contribution of different antibody isotypes as shown for IgG and IgM in our study. In this regard, responses following meningococcal conjugate vaccination can be compared to antibody kinetics following pneumococcal conjugate vaccination; a vaccine which is offered to seniors on a regular basis.
In conclusion, primary MenACWY-TT vaccination elicits robust bactericidal antibody responses which are sustained for at least two years in the majority of older adults. The administration of a booster dose one year post-primary vaccination only transiently enhances rSBA titers. Importantly, our findings underscore the important role of ps-specific IgM responses in conferring bactericidal activity, which may be especially relevant in the context of reduced IgM responses upon booster vaccination. Further studies are warranted to explore bactericidal activity (along with the underlying dynamics of ps-specific IgM and IgG responses), over longer follow-up periods, as well as the clinical implications of these findings in protection against invasive meningococcal disease in aging populations.
Methods
Study design and participants
A total of 5570 adults aged 65 to 85 years, living in the Dutch municipality of De Bilt, were invited to participate in this phase IV, single-center, open-label randomized clinical trial. Exclusion criteria can be found in Supplementary table 1. The study population size was determined based on a prior study conducted by our group investigating the immunogenicity of the MenACWY-TT vaccine in middle-aged adults24. According to the study protocol, the primary objective was to compare functional antibody responses between two age groups: adults aged 65–74 years and those aged 75–85 years. However, the recruitment targets for the distinct age groups were not fully met. Therefore, the two age strata were combined into a single cohort of older adults aged 65–85 years for the current analysis.
Ethical approval was obtained through the Medical Research Ethics Committees United (MEC-U), Nieuwegein, the Netherlands (reference number: R20.003, clinical trial number: NL72728.100.20) on 24 April 2020. The clinical trial was registered in the Clinical Trials Information System (CTIS) under registration number 2024-513640-29-00 on 13 May 2024. All participants provided written informed consent, and all procedures were performed in compliance with Good Clinical Practice and the Declaration of Helsinki.
Vaccination and sample collection
During the summer of 2022, all participants received a single intramuscular dose of the quadrivalent meningococcal vaccine conjugated to tetanus toxoid (MenACWY-TT, Nimenrix, Pfizer). Venous blood was sampled prior to vaccination, one month, and one year after primary immunization. At enrollment, participants were randomly assigned to either receive or not receive a MenACWY-TT booster dose 1 year after the primary vaccination using a random-number table generated in Microsoft Excel (Microsoft), with blocked randomization ensuring an equal distribution of sex and 5-year age intervals. Blood was sampled one month post-booster vaccination and two years post-primary vaccination, which was 1-year post-booster for the booster cohort. Participants who were originally allocated to receive a booster MenACWY-TT dose but developed a coagulation disorder prior to booster vaccination were reassigned to the primary-only cohort for an as-treated analysis. Serious adverse events (SAEs) were considered exclusionary from further participation in the study if they occurred within two weeks following vaccination or within one week following blood collection.
Blood samples were collected using blood collection tubes containing serum separator clot activator (Vacuette tubes, Greiner Bio-one), centrifuged, and serum was aliquoted and stored at −80 °C until further analysis. All samples were processed within 8 h after collection. Health characteristics were collected from all participants during the first study visit using a questionnaire. Frailty scores were determined for each participant using the Tilburg Frailty Indicator self-report questionnaire35.
Serological analysis
Functional MenACWY-specific antibody titers were determined using the serum bactericidal assay with baby rabbit complement (rSBA) (Pelfreez, lot: 21044-C) as described previously15,36. Target strains included MenA (strain 3125), MenC (strain C11), MenW (strain MP01240070), and MenY (strain S-1975). rSBA titers were expressed as the reciprocal of the highest serum dilution resulting in ≥50% killing after 60 min of incubation, whereby an rSBA titer of ≥8 was considered as correlate of protection14,15,34. Moreover, an rSBA titer ≥128 was considered as correlate of prolonged duration of protection14,15. rSBA titers below the lower limit of quantification (<4) were assigned a value of 2.
MenACWY ps-specific IgG and IgM serum concentrations were measured using a fluorescent bead-based multiplex immunoassay (MIA) as described previously37. Antibody concentrations below the detection limit were assigned an arbitrary value of 0.01 μg/ml.
To assess the contribution of IgG and IgM to bactericidal activity to MenC and MenW, a subset of post-vaccination sera (n = 28) was depleted of either antibody isotype. IgG depletion was achieved using EUROSORB (Euroimmun)38, while IgM was removed using goat anti-human IgM (µ-chain-specific) agarose beads (Sigma-Aldrich, A9935)24. Antibody depletion was confirmed using the MIA prior to rSBA measurements.
Statistical analysis
Participants sampled at baseline and at least once post-vaccination were included in the analysis. Geometric mean rSBA titers (GMTs) and geometric mean antibody (IgG and IgM) concentrations (GMCs) with corresponding 95% confidence intervals (95% CI) were determined for each meningococcal serogroup at each time point. All serology data were log10-transformed prior to further statistical analyses.
rSBA and antibody responses were compared between timepoints using paired generalized linear mixed models (GLMM), followed by post-hoc Emmeans tests. Proportions of participants with rSBA ≥8 or ≥128, along with 95% CI, were calculated with the Wilson score interval with continuity correction for each serogroup, and proportions were compared between timepoints using Fisher’s exact test. Correlations between rSBA titers and IgG or IgM responses were determined using Spearman’s rho. Differences in rSBA titers between high (above median) and low (below median) Ig groups were evaluated using the Kruskal-Wallis test, followed by Dunn’s post-hoc test. The Wilcoxon matched-pairs signed rank test was used to compare pre- and post-IgG or IgM depletion antibody concentrations or rSBA titers. Lastly, the effects of age and sex on rSBA responses were analyzed using generalized linear models (GLMs), whilst adjusting for pre-vaccination rSBA titers. All multiple comparisons were corrected for multiple testing using the Bonferroni method if needed. A p-value of <0.05 was considered statistically significant. Statistical analyses were performed using RStudio (version 4.4.0).
Data availability
The datasets containing participant-specific data used in the current study are available under restricted access since (1) the study is still ongoing and (2) to comply with EU legislation on the General Data Protection Regulation (GDPR) and participant privacy and ethical rights. Access to the redacted data can be obtained via the corresponding author in the form of pseudonymized data as long as data transfer is in agreement with the clinical protocol and GDPR and takes into account participants’ privacy and ethical rights. Data sharing will be regulated in a data sharing agreement in the timeframe of 2 months after receipt of the request.
Code availability
The underlying code for this study is not publicly available but may be made available on reasonable request from the corresponding author.
References
Stephens, D. S., Greenwood, B. & Brandtzaeg, P. Epidemic meningitis, meningococcaemia, and Neisseria meningitidis. Lancet 369, 2196–2210 (2007).
Pace, D. & Pollard, A. J. Meningococcal disease: clinical presentation and sequelae. Vaccine 30, B3–B9 (2012).
Weil-Olivier, C. et al. Invasive meningococcal disease in older adults: current perspectives and call for action. Eur. Geriatr. Med. 15, 729–741 (2024).
Wang, B., Santoreneos, R., Giles, L., Haji Ali Afzali, H. & Marshall, H. Case fatality rates of invasive meningococcal disease by serogroup and age: a systematic review and meta-analysis. Vaccine 37, 2768–2782 (2019).
Guedes, S., Bertrand-Gerentes, I., Evans, K., Coste, F. & Oster, P. Invasive meningococcal disease in older adults in North America and Europe: is this the time for action? A review of the literature. BMC Public Health 22, 380 (2022).
Campbell, H. et al. Variable clinical presentation by the main capsular groups causing invasive meningococcal disease in England. J. Infect. 80, 182–189 (2020).
Deghmane, A. E., Taha, S. & Taha, M. K. Global epidemiology and changing clinical presentations of invasive meningococcal disease: a narrative review. Infect. Dis.54, 1–7 (2022).
Taha, S., Deghmane, A. E. & Taha, M. K. Recent increase in atypical presentations of invasive meningococcal disease in France. BMC Infect. Dis. 24, 640 (2024).
Marshall, G. S. et al. Correction to: understanding the sequelae of invasive meningococcal disease in the United States. Infect. Dis. Ther. 13, 2221–2222 (2024).
Kanasi, E., Ayilavarapu, S. & Jones, J. The aging population: demographics and the biology of aging. Periodontology 72, 13–18 (2016).
Parikh, S. R. et al. The everchanging epidemiology of meningococcal disease worldwide and the potential for prevention through vaccination. J. Infect. 81, 483–498 (2020).
Assaf-Casals, A. & Dbaibo, G. Meningococcal quadrivalent tetanus toxoid conjugate vaccine (MenACWY-TT, Nimenrix): a review of its immunogenicity, safety, co-administration, and antibody persistence. Hum. Vaccin Immunother. 12, 1825–1837 (2016).
Allen, J. C., Toapanta, F. R., Chen, W. & Tennant, S. M. Understanding immunosenescence and its impact on vaccination of older adults. Vaccine 38, 8264–8272 (2020).
Borrow, R., Andrews, N., Goldblatt, D. & Miller, E. Serological basis for use of meningococcal serogroup C conjugate vaccines in the United Kingdom: reevaluation of correlates of protection. Infect. Immun. 69, 1568–1573 (2001).
Borrow, R., Balmer, P. & Miller, E. Meningococcal surrogates of protection-serum bactericidal antibody activity. Vaccine 23, 2222–2227 (2005).
Dbaibo, G. et al. Immunogenicity and safety of a quadrivalent meningococcal serogroups A, C, W-135 and Y tetanus toxoid conjugate vaccine (MenACWY-TT) administered to adults aged 56 Years and older: results of an open-label, randomized, controlled trial. Drugs Aging 30, 309–319 (2013).
Esteves-Jaramillo, A. et al. Immunogenicity and safety of a quadrivalent meningococcal tetanus toxoid-conjugate vaccine (MenACYW-TT) in >/=56-year-olds: a Phase III randomized study. Vaccine 38, 4405–4411 (2020).
Kirstein, J., Pina, M., Pan, J., Jordanov, E. & Dhingra, M. S. Immunogenicity and safety of a quadrivalent meningococcal tetanus toxoid-conjugate vaccine (MenACYW-TT) in adults 56 years of age and older: a Phase II randomized study. Hum. Vaccin Immunother. 16, 1299–1305 (2020).
Robertson, C. A., Jacqmein, J., Selmani, A., Galarza, K. & Oster, P. Immune persistence and booster response of a quadrivalent meningococcal conjugate vaccine (MenACYW-TT) 5 years after primary vaccination of adults at >/=56 years of age. Hum. Vaccin Immunother. 20, 2426868 (2024).
Robertson, C. A., Jacqmein, J., Selmani, A., Galarza, K. & Oster, P. Immunogenicity and safety of a quadrivalent meningococcal conjugate vaccine (MenACYW-TT) administered as a booster to adults aged >/=59 years: a phase III randomized study. Hum. Vaccin Immunother. 19, 2160600 (2023).
Lewis, L. A. & Ram, S. Meningococcal disease and the complement system. Virulence 5, 98–126 (2014).
Park, S. & Nahm, M. H. Older adults have a low capacity to opsonize pneumococci due to low IgM antibody response to pneumococcal vaccinations. Infect. Immun. 79, 314–320 (2011).
Shyur, S. D., Raff, H. V., Bohnsack, J. F., Kelsey, D. K. & Hill, H. R. Comparison of the opsonic and complement triggering activity of human monoclonal IgG1 and IgM antibody against group B streptococci. J. Immunol. 148, 1879–1884 (1992).
van der Heiden, M. et al. Novel intervention in the aging population: a primary meningococcal vaccine inducing protective igm responses in middle-aged adults. Front. Immunol. 8, 817 (2017).
Hendriks, A. et al. Glycan-specific IgM is critical for human immunity to Staphylococcus aureus. Cell Rep. Med. 5, 101734 (2024).
Pollard, A. J. & Frasch, C. Development of natural immunity to Neisseria meningitidis. Vaccine 19, 1327–1346 (2001).
de Voer, R. M. et al. Immunity against Neisseria meningitidis serogroup C in the Dutch population before and after introduction of the meningococcal c conjugate vaccine. PLoS ONE 5, e12144 (2010).
Knol, M. J., Ruijs, W. L., Antonise-Kamp, L., de Melker, H. E. & van der Ende, A. Implementation of MenACWY vaccination because of ongoing increase in serogroup W invasive meningococcal disease, the Netherlands, 2018. Eur. Surveill 23, 18-00158 (2018).
Knol, M. et al. Meningococcal disease in the Netherlands. Background information for the Health Council. Bilthoven, The Netherlands: National Institute for Public Health and the Environment; (2017).
Vann, W. F., Liu, T. Y. & Robbins, J. B. Bacillus pumilus polysaccharide cross-reactive with meningococcal group A polysaccharide. Infect. Immun. 13, 1654–1662 (1976).
Filice, G. A., Hayes, P. S., Counts, G. W., Griffiss, J. M. & Fraser, D. W. Risk of group A meningococcal disease: bacterial interference and cross-reactive bacteria among mucosal flora. J. Clin. Microbiol. 22, 152–156 (1985).
Bhattacharjee, A. K., Jennings, H. J., Kenny, C. P., Martin, A. & Smith, I. C. Structural determination of the polysaccharide antigens of Neisseria meningitidis serogroups Y, W-135, and BO1. Can. J. Biochem. 54, 1–8 (1976).
Broker, M., Berti, F. & Costantino, P. Factors contributing to the immunogenicity of meningococcal conjugate vaccines. Hum. Vaccin Immunother. 12, 1808–1824 (2016).
Andrews, N., Borrow, R. & Miller, E. Validation of serological correlate of protection for meningococcal C conjugate vaccine by using efficacy estimates from postlicensure surveillance in England. Clin. Diagn. Lab. Immunol. 10, 780–786 (2003).
Gobbens, R. J., van Assen, M. A., Luijkx, K. G., Wijnen-Sponselee, M. T. & Schols, J. M. The Tilburg frailty indicator: psychometric properties. J. Am. Med. Dir. Assoc. 11, 344–355 (2010).
Maslanka, S. E. et al. Standardization and a multilaboratory comparison of Neisseria meningitidis serogroup A and C serum bactericidal assays. The Multilaboratory Study Group. Clin. Diagn. Lab. Immunol. 4, 156–167 (1997).
Ohm, M. et al. Different long-term duration of seroprotection against Neisseria meningitidis in adolescents and middle-aged adults after a single meningococcal acy conjugate vaccination in the Netherlands. Vaccines 8, 624 (2020).
Lederer, S. et al. Indirect immunofluorescence assay for the simultaneous detection of antibodies against clinically important old and new world hantaviruses. PLoS Negl. Trop. Dis. 7, e2157 (2013).
Acknowledgements
This work was supported by the Dutch Ministry of Health, Welfare, and Sport. We thank all the participants of the study, the nurses who performed the vaccinations and venipunctures, the site assistants who supported the study visits, and the laboratory technicians who helped process the blood samples.
Author information
Authors and Affiliations
Contributions
M.V., L.B., M.O., A.M.B., and G.d.H. were involved in conceptualization and design the study. M.V., M.O., A.M.B., and G.d.H. wrote the medical ethical application. M.V. and L.B. performed the clinical trial. M.V., D.v.R., and J.W. performed sample processing and laboratory data acquisition. M.V. was responsible for data management and analyzed the data. M.V., A.M.B., M.I.d.J., and G.d.H. discussed the data and wrote the manuscript. All authors critically revised the manuscript before publication.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Visser, M., van Rooijen, D.M., Wolf, J. et al. Immunogenicity of primary and booster MenACWY-TT vaccination in older adults and the importance of IgM. npj Vaccines 10, 115 (2025). https://doi.org/10.1038/s41541-025-01180-3
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41541-025-01180-3