Abstract
Outbreaks of H5 highly pathogenic avian influenza A viruses (HPAIVs) in animals pose a threat to humans immunologically naïve to avian influenza viruses. However, annual vaccination, such as for seasonal influenza is not planned because the number of human patients infected with H5 HPAIVs is small, and the possibility of human-to-human transmission of H5 HPAIVs is low at present. However, various clades of H5 HPAIVs have emerged continuously. Therefore, a vaccine that confers long-term and cross-clade immunity is required. To examine the long-term effectiveness and cross-clade reactivity of an H5 influenza virus vaccine, cynomolgus macaques were infected with an H5N1 HPAIV 5 years after two subcutaneous vaccinations with inactivated H5N1 whole-virus particles (H5 clade classical/outlier), which showed higher immunogenicity than did split vaccines in our previous studies. Neutralization titers against the vaccine strain were maintained for 5 years, and a recall immune response was observed on challenge infection against the challenge strain (clade 1) and other H5N1 HPAIV strains (clades 2.2, 2.3.2.1, and 2.3.4.4b). Compared with unvaccinated macaques, viral titers were low, and the cytokine signaling pathways related to the pathogenesis of an influenza virus infection were not activated in the vaccinated macaques. Thus, a whole-virus particle vaccine induced long-term memory sufficient to prevent severe pneumonia caused by an H5N1 HPAIV in cynomolgus macaques.
Similar content being viewed by others
Introduction
Wild aquatic birds serve as a natural reservoir of influenza viruses. Several avian influenza viruses have crossed the species barrier, with some causing a substantial higher mortality than do seasonal influenza viruses in humans1,2. Globally, more than 900 human cases of H5N1 highly pathogenic avian influenza virus (HPAIV) infections have been reported to the World Health Organization since 2003, with a cumulative case fatality rate of greater than 40%3. The pathological findings from human autopsies demonstrate the most severe form of lung injury, diffuse alveolar damage, and viral pneumonia4.
The large outbreaks of H5 viruses in animal populations pose a threat to humans because more than 90% of humans do not possess antibodies against H5N1 avian influenza viruses (seronegative)5,6,7,8. Direct contact with infected birds and cattle has been the cause of infection in humans, but there is a low possibility of human-to-human transmission at present. However, if reassortment of internal genes of influenza A viruses were to occur in mammals, especially in swine9, an H5N1 virus that easily transmits among humans might emerge. In addition, adaptation of the current H5N1 viruses for mammals accompanied by amino acid substitutions is a concern with respect to human transmissibility10. Thus, we need to prepare countermeasures to reduce mortality in case of pandemics.
Because vaccination is the most effective measure to induce antigen-specific immunity prior to infection and reduce mortality, candidate H5 vaccines have been developed. In our previous studies, split vaccines, a current formulation of the seasonal influenza vaccine, showed very low immunogenicity11,12. As a result, vaccination with an H5N1 split vaccine did not protect mice from lethal infection, whereas an inactivated whole-virus particle vaccine (WPV) conferred protective immunity11. In a nonhuman primate model that closely recapitulates human influenza13,14, two doses of an H5N1 WPV (H5 clade classical/outlier) were effective against challenge infection with H5N1 HPAIVs (H5 clades 1 and 2.3.2.1) 3 weeks after the second vaccination (5 weeks after the first vaccination)15,16,17. All vaccinated macaques survived with improved clinical signs of disease, reduction of viral titers, and ameliorated viral pneumonia. However, the long-term immune responses have not been assessed in nonhuman primates. Therefore, to assess the long-term immune responses after vaccination with an H5N1 WPV (H5 clade classical/outlier), cynomolgus macaques were infected with H5N1 HPAIV (H5 clade 1) 5 years after vaccination.
Results
A whole-virus particle H5N1 vaccine prevented viral replication in macaques vaccinated 5 years prior to challenge infection
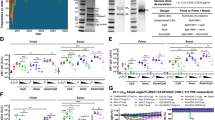
An inactivated WPV of a low pathogenic avian influenza virus A/duck/Hokkaido/Vac-3/2007 (H5N1) (Vac-3) (H5 clade classical or outlier)18,19 was subcutaneously injected into six cynomolgus macaques twice with a two-week interval between injections as described in a previous study (Fig. 1a)17. Five years after the first and second vaccinations (the prime-boost vaccination) (5Y vaccination), macaques were challenged with A/Vietnam/UT3040/2004 (H5N1) (clade 1), an HPAIV isolated from a patient who died from infection1. After challenge infection, swab samples were taken from the nostrils, trachea, and bronchus for 7 days, and infectious viral titers were measured. For comparison, viral titers of control macaques challenged without vaccination and macaques challenged 5 weeks after the first vaccination and 3 weeks after the second vaccination (5 W vaccination) were taken from the previous study (Supplementary Table 1)17. In nasal swabs, virus was detected at high titers from day 1 to day 7 after challenge in macaques without vaccination, whereas in those challenged after 5Y vaccination, the viral titer was less than ten times lower on day 1 after challenge infection, which dropped to below the detection limit on day 6 after challenge. Nasal viral titers detected each day and the viral titer area under the curve (AUC), which was relatively unaffected by daily variation in individual macaques, were at a level comparable to those in macaques challenged after 5 W vaccination (Fig. 1b, Supplementary Table 2). In tracheal and bronchial swabs, viral titers and the viral titer AUC were lower in macaques challenged after 5Y vaccination than in macaques without vaccination, although the degree of reduction was lower than that in the nasal swabs and macaques challenged after 5 W vaccination (Fig. 1c, d, Supplementary Table 2). No virus was detected in lung, brain, heart, jejunum, and ileum tissues of vaccinated macaques 7 days after challenge infection (Supplementary Table 3). Thus, the WPV conferred a protective effect on viral replication in macaques challenged after 5Y vaccination, although prevention of viral replication in the trachea and bronchus was less effective after 5Y vaccination than after 5 W vaccination.
a Cynomolgus macaques were subcutaneously vaccinated twice with inactivated whole particles of a low pathogenic avian influenza virus A/duck/Hokkaido/Vac-3/2007 (H5N1) (Vac-3) (H5 clade classical/outlier) with a two-week interval. Plasma was collected on week 0 (before vaccination), 2, 4, 8, and 32 weeks, and 1, 2, 3, 4, and 5 years after the prime-boost vaccination to measure Vac-3-specific IgG and neutralization titers. Five years later, macaques were challenged with an H5N1 highly pathogenic avian influenza virus A/Vietnam/UT3040/2004 (H5N1) (H5 clade 1). Autopsy was performed 7 days after challenge. b–d Virus titers after challenge infection. Nasal swab (b), tracheal swab (c), and bronchial brush (d) samples were collected on the indicated days (Supplementary Table 2). Left: each symbol shows the average titer calculated from titers in vaccinated macaques (red: n = 6 for those challenged 5 years after the prime-boost vaccination and blue: n = 3 for those challenged 5 weeks after the first and 3 weeks after the second vaccinations) and unvaccinated macaques (black: n = 3). Right: area under the virus titer and time curve (AUC). e Vac-3 specific IgG levels before and after challenge indicated as 50% max dilution. f Neutralization titers against Vac-3 before and after challenge infection. Each symbol indicates each macaque, with identification numbers in the graph. Neutralization titers higher than 9 and lower than 2 were calculated as 9 and 1, respectively. Statistical analysis was performed with the Mann-Whitney U test in b–d and the Wilcoxon test in e and f: *p < 0.05, **p < 0.01.
Neutralization activity and antigen-specific IgG increased rapidly upon challenge infection 5 years after WPV vaccination
Antibody responses for 5 years after the prime-boost vaccination were examined because viral propagation was low in macaques vaccinated 5 years prior to challenge infection. Vac-3-specific IgG was increased 6 weeks after the second vaccination (8 weeks after the first vaccination) and was maintained at low levels for 5 years without a significant difference between year 1 and year 5 (Fig. 1e). Levels of IgG specific for NAs of A/Thailand/1(KAN-1)/2004 and A/Texas/37/2024 were increased in two macaques (1744 and 1746) and two macaques (1691 and 1744) 6 weeks after the second vaccination, respectively, and in six macaques and three macaques (1686, 1691, and 1744) 7 days after the challenge, respectively (Supplementary Fig. 1). The neutralization titer against Vac-3 (H5 clade classical/outlier) peaked 6 weeks after the second vaccination, decreased at 32 weeks, and was maintained at higher levels than pre-vaccination for 5 years, because there was no significant difference in neutralization titers between year 1 and year 5 (Fig. 1f). Neutralization activity against the challenge strain A/Vietnam/UT3040/2004 (clade 1), A/whooper swan/Mongolia/3/2005 (clade 2.2), A/whooper swan/Hokkaido/1/2008 (clade 2.3.2.1), and A/Ezo red fox/Hokkaido/1/2022 (H5N1) (clade 2.3.4.4b) was also detected 7 days after challenge, all of which had similarities in the amino acid sequence of hemagglutinin (HA) over 89% to Vac-3 (Table 1, Supplementary Table 4). In contrast, neutralization activity against A/peregrine falcon/Hong Kong/810/2009 (clade 2.3.4.5) was not detected. The rapid increase in antibody responses 7 days after challenge infection, which was not seen in unvaccinated macaques, indicates a memory recall response with cross-reactivities against H5N1 strains different from the vaccine strain, demonstrating that the WPV induced persistent and durable immunological memory for as long as 5 years.
WPV partially prevented viral pneumonia in the lungs of macaques vaccinated 5 years prior to challenge
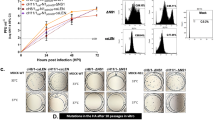
To evaluate the severity of pneumonia, lungs were collected at autopsy 7 days after challenge, and tissue slices including bronchi were examined. Exudates and inflammatory cells filled the air spaces in macaques without vaccination, whereas the area of inflammation was partial in macaques after 5Y vaccination, and the inflammatory lesion was very limited in those after 5 W vaccination (Fig. 2a). Histological pneumonia was less severe in macaques after 5 W vaccination than in those without vaccination based on criteria established for influenza virus infection20 (Fig. 2a, right graph). To assess the infiltration of immune cells in the vaccinated macaques, immunohistochemistry was performed against marker molecules for myeloid cells, including monocytes, granulocytes, inflammatory macrophages (stained with antibody clone MAC387), and CD163-positive inhibitory macrophages. The numbers of MAC387-positive and CD163-positive macrophages were significantly lower in the lungs of macaques after 5 W vaccination than in unvaccinated macaques by Student’s t-test, although the differences were not significant by the Mann-Whitney U-test (Fig. 2b, c). The number of MAC387-positive and CD163-positive macrophages was slightly lower in the lungs of macaques after 5Y vaccination than in unvaccinated macaques, although the differences were not significant. The numbers of cells positive for granzyme B (cytotoxic T-lymphocytes (CTL) and natural killer (NK) cells), a family of serine proteases, and CD3-positive T cells (CTL and helper T cells) were also lower in the vaccinated macaques despite large heterogeneity among macaques after 5Y vaccination, indicating that viral pneumonia with lymphocyte infiltration was ameliorated by the vaccination 5 years prior to challenge due to low viral propagation (Fig. 2d, e). The number of NKG2A-positive NK cells was slightly lower in macaques after 5Y vaccination (Fig. 2f). Fibrin clots, assessed by a Carstairs method that stains fibrin (orange-red), red blood cells (red or yellow), and platelets (light gray) showed the presence of fibrin clots in small vessels of macaques, especially in a macaque without vaccination (Fig. 2g). The number of bronchus-associated lymphoid tissues (BALTs) was similar between vaccinated macaques and those without vaccination (Supplementary Fig. 2). In summary, histological analysis showed that the WPV partially prevented the infiltration of inflammatory cells in the lungs of the macaques 5 years after vaccination, although the differences were not significant.
Cynomolgus macaques were subcutaneously vaccinated and infected as described in Fig. 1a. Lung tissues were collected 7 days after challenge infection. For each macaque, at least one section was made from the proximal part of the lobe vertical to the bronchus: left upper, left middle, left lower, right upper, right middle, and right lower lobes. a H & E staining and lung histological score (right graph). Histological scores were evaluated in each section. Each dot represents the average score of 6–8 sections for each macaque. b–f Immunohistochemical analysis of infiltrating cells. A section that showed the closest histological score to the average was selected. Left panels: b myeloid cells (monocytes and granulocytes), c CD163-positive inhibitory macrophages, d granzyme B-positive killer cells, e CD3-positive T cells, and f NKG2A-positive natural killer cells. Brown: positive cells; blue: nucleus. Right graphs: quantification of positive cells detected by immunohistochemistry. Each dot represents the average number of more than ten fields for each macaque. g Fibrin clots were visualized by Carstairs staining, and the number of clots was counted manually under the light microscope for each section. Left: Carstairs staining. Fibrin, red blood cells, collagen and platelets are stained in orange-red, yellow, blue, and gray, respectively. The asterisk indicates a fibrin clot in blood vessels. Right graph: the number of fibrin clots per pixel (area). Each dot represents the average number of 6–8 sections for each macaque. The area of each slide was measured by Image J, and the number of clots per area was calculated. a–g Left panel, representative photos are shown with macaque identification numbers in photos. Right panel, black: unvaccinated macaques; red: macaques challenged 5 years after vaccination; blue: macaques challenged 5 weeks after vaccination. **: p < 0.01, statistical analysis was performed with the two-tailed Student’s t-test. There is no significant difference by the analysis with the two-tailed Mann-Whitney U-test.
Vaccination prevented the activation of the interferon signaling pathway in the lungs of macaques infected with HPAIV
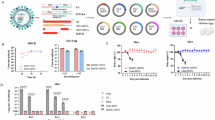
Because the prevention of pneumonia was partial at the histological level in the challenged macaques 5 years after vaccination, bulk RNA-seq was performed to identify differences in the lung tissues between macaques challenged without vaccination and those vaccinated 5 years before challenge to validate the efficacy of the long-term effect of WPV at the transcript level. Principle component analysis showed that the genes of the vaccinated macaques tended to cluster together, whereas those of the unvaccinated macaques were scattered in different directions (Fig. 3A). A total of 15,443 differentially expressed genes (DEGs) were found, with 266 DEGs higher and 311 DEGs lower in the vaccinated macaques than in the unvaccinated macaques (q value < 0.05), as shown in the volcano plot (Fig. 3B). Ingenuity Pathway Analysis showed that vaccination significantly prevented the activation of interferon signaling and hypercytokinemia/hyperchemokinemia pathways related to the pathogenesis of influenza virus in the vaccinated macaques (z score < 2) (Fig. 3C). A graphical summary showed that the gene expressions of interferon signaling, recruitment of phagocytes, hematopoiesis of mononuclear leukocytes, immune responses of leukocytes, necrosis of epithelial tissue, and cell death/apoptosis of epithelial cells were low in the lungs of vaccinated macaques (Fig. 3D). Gene expressions lower in the vaccinated macaques included those that belonged to the interferon signaling pathway, innate immunity pathway, cell death pathway, and coagulation pathway (Supplementary Fig. 3).
Cynomolgus macaques were subcutaneously vaccinated and infected as described in Fig. 1a. Three unvaccinated cynomolgus macaques infected with a same H5N1 strain were used for comparison. Seven days after challenge, lung tissues were collected from inflamed lesions of each lobe determined by gross pathology (redness, exudate, and hardness of the lung). RNA was extracted from each lobe and pooled for each macaque for RNA-seq. Ingenuity Pathway Analysis was performed with differentially expressed genes (q value < 0.05). A Principal component analysis. Black dots: unvaccinated macaques; Red dots: macaques challenged 5 years after vaccination. B Volcano plot of the differentially expressed genes. Red dots represent major interferon-regulated genes. C Altered canonical signaling pathways in vaccinated macaques with z score < -2. PKR: protein kinase receptor, TREM1: triggering receptor on myeloid cells-1. D Graphical summary of differential pathways in the lung of macaques challenged 5 years after vaccination vs without vaccination. Blue: predicted low activation of canonical pathways, cytokines, transcription regulators, and kinases.
Prevention of hypercytokinemia/hyperchemokinemia in macaques vaccinated 5 years prior to challenge
Finally, to assess inflammatory signatures at the protein level, levels of acute inflammatory cytokines in lung homogenates were measured. IFN-γ, interleukin (IL)-6, IL-1β, IL-8, monocyte chemotactic protein 1 (MCP-1), macrophage inflammatory protein-1α (MIP-1α), and MIP-1β were significantly lower in vaccinated macaques (Fig. 4A). Tumor necrosis factor-α was detected at a high level in a macaque without vaccination, but none was detected in those with vaccination. Consistent with lower levels of acute inflammatory cytokines, body temperature greater than 39 °C was seen in macaques without vaccination, but it started to decrease on days 4 and 3 after challenge in macaques challenged after 5Y vaccination and 5 W vaccination, respectively (Fig. 4B and Supplementary Fig. 4). Thus, vaccination with WPV prevented the upregulation of pyrogenic cytokines/chemokines in the lungs 5 years after vaccination, contributing to the early reduction of high body temperature caused by challenge with A/Vietnam/UT3040/2004 (H5N1).
Cynomolgus macaques were subcutaneously vaccinated and infected as described in Fig. 1a. A Lung cytokine levels. Seven days after challenge infection, inflamed red lung tissues were collected. Cytokine concentrations in lung homogenates were measured by a multiplex bead array assay. Lungs from macaques challenged without vaccination were collected in a previous study17, and were measured for this study for comparison. B before challenge infection (Supplementary Fig. 4). Body temperature data for macaques challenged without vaccination and those after 5 W vaccination were obtained from a previous study17. Because body temperatures between 10 A.M. and 6 P.M. were affected by anesthesia, average temperatures from 6 P.M. to 10 A.M. of individual macaques were calculated every day (64 and 96 points in 5 W/Without vaccination and 5Y, respectively). Means and standard deviations of six, three, and three macaques are shown as the ‘5Y post vaccination’, ‘5 W post vaccination’, and ‘Without vaccination’ groups, respectively. Black dots: unvaccinated macaques; red dots: macaques challenged after 5Y vaccination; blue dots: macaques challenged after 5 W vaccination. *: p < 0.05, statistical analysis was performed with the Mann-Whitney U test.
Discussion
In the present study, the long-term efficacy of H5N1 WPV (H5 clade classical/outlier) lasted for 5 years in cynomolgus macaques. Challenge infection with HPAIV (H5 clade 1) induced rapid memory recall immune responses with cross-reactive antibodies, which resulted in low viral titers in the upper and lower respiratory tracts. Pathway analysis based on bulk RNA sequencing in the lungs showed that vaccination prevented the upregulation of IFN signaling and hypercytokinemia/hyperchemokinemia pathways related to the pathogenesis of influenza virus, which was verified by less infiltration of inflammatory cells, lower levels of lung cytokine production and a lower increase in body temperature. Collectively, vaccination with the H5N1 WPV ameliorated pneumonia caused by H5N1 HPAIV challenge in macaques 5 years after vaccination. This study sheds light on the long-term memory response induced by WPV, which may be transferable to humans in the context of the efficacy of WPV after challenge.
Neutralization titers against the vaccine strain were maintained for 5 years, and a rapid memory recall immune response was observed on challenge. Of note, neutralization titers against the challenge strain (clade 1) and other H5N1 HPAIV strains that belonged to clades 2.2, 2.3.2.1, and 2.3.4.4b were also induced on infection, demonstrating the presence of cross-reactive antibodies induced by WPV (H5 clade classical/outlier). Although neutralization activity against H5 clade 2.3.4.5 was not detected, antibodies against neuraminidase, which bound to two N1 proteins of 2004 and 2024 strains in the present study, may also play a role in preventing replication of different strains, as demonstrated in our previous study17.
The present study demonstrated that WPV was sufficient to induce long-term memory immunity without addition of adjuvants. Our previous studies and studies from other groups support the efficacy of WPV over split vaccines, which might be attributed to the fundamental structure of the vaccine. WPVs contain single-stranded viral RNAs that stimulate innate immune receptors such as toll-like receptor 7, whereas viral RNAs are separated from protein antigens in split vaccines12,21,22. The subsequent effects on the immune system are: 1) activation of plasmacytoid dendritic cells by WPVs23; 2) antibody responses induced by WPV not only against the HA-head but also against the HA-stem as a relatively conserved epitope, associated with higher HA-specific-B cell and IFN-γ-producing CD4+ T cell responses24,25 and epitope spreading induced by the IFN-γ response, as reported in the cancer vaccine and autoimmune models26,27; 3) induction of vaccine antigen-specific CD8+ T cells after WPV immunization as shown in our previous study using Mafa-haplotype-matched macaques immunized with WPV25; and 4) induction of a high frequency of somatic hypermutation and high-affinity IgG responses accelerated upon prime and booster vaccination with WPV28,29. Thus, the nature of WPV activates innate and adaptive immune cells more efficiently, leading to longer lasting protective immunity than does the split vaccine.
Cross-reactive neutralizing antibodies were detected after the challenge infection, but not after the prime-boost vaccination in the present study. No neutralization activity was detected in the plasma of unvaccinated macaques on day 7 after the challenge infection, indicating that the neutralization activity detected on day 7 in the vaccinated macaques is a recall memory response activated by the challenge infection with the clade 1 H5N1 HPAIV. Therefore, it is thought that memory B cells induced by the prime-boost vaccination 5 years earlier were reactivated by the challenge infection. Although the virus titers were low in vaccinated macaques, the challenge virus might be a stimulator of memory B cells and/or dendritic cells that activated memory T cells for B cell help, especially B cells and T cells specific for common epitopes conserved in the vaccine and challenge strains. This result also suggests that the prime-boost vaccination and infection with different strains enhance the responses against conserved epitopes among strains24,30,31. In our previous study using WPV for the pandemic influenza 2009 influenza virus25, neutralization titers in vaccinated macaques were increased 2 days after the challenge infection. This result is consistent with the result in the present study, that the virus titers decreased by days 2–3 after the challenge infection.
In the present study, the efficacy of the vaccine to prevent viral replication was more evident in nasal swabs than in tracheal and bronchial swabs. This may differ in the degree to it elicits recall memory responses after subcutaneous vaccination and mucosal challenge. Nasal-associated lymphoid tissues (NALTs) were found in the paranasal sinuses of SARS-CoV-2-infected macaques32, and the involvement of NALTs in subcutaneous vaccination and intranasal infection needs to be elucidated. To increase the protective immunity in the lower airway, a booster vaccination or immunization intervals greater than 4 months may maximize neutralizing responses and provide higher protective immunity33.
Bulk RNA sequencing and pathway analyses of the lungs separated the macaques into those 5 years after vaccination and those without vaccination, demonstrating that WPV significantly prevented IFN signaling and hypercytokinemia/hyperchemokinemia related to the pathogenesis of influenza virus. It has been reported that high levels of IFN and inflammatory cytokines cause cytokine storm with increased mortality, as was the case with the 2009 pandemic influenza virus and HPAIV34,35,36,37,38,39,40. Thus, because prevention of cytokine production is especially important in regulating the influenza virus-induced pathology, WPV is a promising vaccine to provide long-lasting efficacy against pneumonia and hypercytokinemia caused by HPAIV.
A few experimental limitations need to be considered in the present study. The first limitation was the lack of comparison of gene expression in lung samples of non-vaccinated and uninfected macaques or vaccinated and uninfected macaques to infected macaques for a comparison with expression levels under homeostatic (baseline) conditions due to the absence of samples of uninfected macaques. The second limitation was that two challenge experiments were performed over five years apart. Therefore, batch effects were involved. However, the virus titers were examined every time and adjusted for challenge infections and neutralization assays. Therefore, the challenge virus titers were compatible in two challenge experiments and neutralization assays. The third limitation is the difficulty in predicting protective efficacy based on the levels of neutralizing titers before challenge infection, because the levels of neutralizing antibody against the challenge strain were very low before challenge infection in this study, but 5Y vaccination showed partial protection against challenge infection. In this case, the evaluation of non-neutralizing antibody and T-lymphocyte responses may help to predict protective immunity.
The present study provides the first evidence that a WPV induces memory immune responses for as long as 5 years without a significant decrease of antibody levels from 1 to 5 years after vaccination in nonhuman primates. It is known that antibody titers after seasonal influenza vaccination wane in several months, and the efficacy may not last until the next season41. The COVID-19 pandemic has shown that an mRNA vaccine can be manufactured promptly to reduce the morbidity and mortality of infection. However, several lines of evidence show that immune responses wane within several months and annual vaccination may be required to obtain immunity sufficient for protection, especially against variant strains42,43. It is difficult to evaluate the immunological memory induced by authentic vaccination because humans are repeatedly vaccinated and infected by influenza viruses, resulting in a mixture of heterogeneous immune responses. A five-year follow-up study of healthcare workers after vaccination with an adjuvanted influenza A/H1N1pdm09 split vaccine showed seroconversion in 34% of the healthcare workers after a single vaccination44. In addition, the antibody responses up to 180 or 208 days in donors primed with live attenuated H5N1 influenza vaccines and boosted with an unadjuvanted inactivated H5N1 vaccine showed a lower HA inhibition antibody response at 6 months45. The present study is in line with these long-term human observations, although the impact on infection was not investigated in those studies. Therefore, we suggest that WPV is an alternative modality of vaccines to compensate for the relatively short-term infection control induced by split vaccines and mRNA vaccines. Direct comparison of efficacy among different modalities of vaccines, e.g., WPVs, split vaccines, and mRNA vaccines, in a nonhuman primate model will be required to propose practical guidelines for effective vaccinations, especially against emerging viruses to which humans have no pre-existing immunity.
Methods
Ethics statement
This study was carried out in strict accordance with the Guidelines for the Husbandry and Management of Laboratory Animals of the Research Center for Animal Life Science at Shiga University of Medical Science, Standards relating to the Care and Keeping and Reducing Pain of Laboratory Animals (Notice of the Ministry of the Environment No. 88 of 2006, Japan), and Fundamental Guidelines for Proper Conduct of Animal Experiment and Related Activities in Academic Research Institutions under the jurisdiction of the Ministry of Education, Culture, Sports, Science and Technology (Notice No. 71, 2006). The Research Center for Animal Life Science at the Shiga University of Medical Science has a permit for the importation of cynomolgus macaques. Regular veterinary care and monitoring, balanced nutrition, and environmental enrichment were provided by the Research Center for Animal Life Science at the Shiga University of Medical Science. The protocols were approved by the Shiga University of Medical Science Animal Experiment Committee (Permit numbers: 2006-11-2, 2012-10-8, and 2017-9-13). The macaques were euthanized at endpoint (7 days after virus inoculation) using ketamine/xylazine followed by intravenous injection of pentobarbital (200 mg/kg). Animals were monitored every day during the study to be clinically scored as shown in Supplementary Table 5 and to undergo veterinary examinations to help alleviate suffering. Animals would be euthanized if their clinical scores reached 15 (a humane endpoint), but no macaque reached this endpoint in the present study.
Animals
Nine-year-old female cynomolgus macaques from Vietnam were used (Supplementary Table 1). They were healthy adults regularly monitored by a veterinarian. A maximal observed age of female cynomolgus macaques was reported as 23.5 years46. All procedures were performed under ketamine (5 mg/kg) and xylazine (1 mg/kg) anesthesia, and all efforts were made to minimize suffering. Food pellets of CMK-2 (CLEA Japan, Inc., Tokyo, Japan) were provided once a day after recovery from anesthesia, and drinking water was available ad libitum. Animals were singly housed in the cages with bars to climb up and puzzle feeders for environmental enrichment under controlled conditions of humidity (26–64%), temperature (24 °C–26 °C), and light (12 h light/12-h dark cycle, lights on at 8:00 A.M.). Individual macaques were distinguished by identification numbers (Supplementary Table 1). The absence of influenza A virus nucleoprotein-specific antibodies in their plasma was confirmed before experiments using an antigen-specific enzyme-linked immunosorbent assay (ELISA), AniGen AIV Ab ELISA (Animal Genetics Inc., Kyonggido, Korea). Two weeks before virus inoculation, a telemetry probe (M00, Data Sciences International, St. Paul, MN) was implanted in the peritoneal cavity of each macaque under ketamine/xylazine anesthesia followed by isoflurane inhalation with oxygen saturation and heart rate monitored during the operation. Body temperatures were recorded every 10 or 15 min as shown in Supplementary Fig. 4. Average temperatures between 6 P.M. and 10 A.M. were calculated for a comparison among groups in Fig. 4b because body temperatures during the daytime were affected by anesthesia and body temperatures during the night showed up-and-down changes in the range of 1 °C to 2 °C every night. The macaques used in this study were free from herpes B virus, hepatitis E virus, Mycobacterium tuberculosis, Shigella spp., Salmonella spp., and Entamoeba histolytica. The results of macaques without vaccination and macaques challenged 5 weeks after vaccination were re-analyzed using previously acquired data and materials17. There were no exclusions of animals from the study.
Vaccine strain and inactivation method
Influenza virus A/duck/Hokkaido/Vac-3/2007 (H5N1) (Vac-3, National Center for Biotechnology Information (NCBI) taxonomy database ID: 463698, H5 clade classical/outlier) is a reassortant virus between A/duck/Hokkaido/101/2004 (H5N3) and A/duck/Hokkaido/262/2004 (H6N1)15. Vac-3 was propagated in allantoic cavities of 10-day old embryonated hen’s eggs at 35 °C for 48 h. To prepare an inactivated whole particle vaccine, the allantoic fluid infected with Vac-3 was concentrated and purified by high-speed centrifugation (112,500 × g for 90 min) through a 10–50% sucrose density gradient and then treated in 0.2% formalin at 4 °C for one week. Inactivation of viruses in the vaccine was confirmed by the absence of detectable hemagglutination activity following inoculation of the material into embryonated eggs after one passage. The amount of the whole particle vaccine is indicated as the total amount of proteins, including HA and other viral proteins.
Vaccination and infection
The inactivated WPV (1 mg/dose) was injected subcutaneously into the back of macaques twice with a two-week interval under ketamine/xylazine anesthesia. A HPAIV A/Vietnam/UT3040/2004 (H5N1) (H5 clade 1) (NCBI taxonomy ID: 755291, kindly provided by Dr. Yoshihiro Kawaoka) isolated from a human patient1,47 was used for challenge infection. The macaques were challenged with A/Vietnam/UT3040/2004 (H5N1) (3 × 106 PFU50/7 mL) inoculated into the nostrils (0.5 mL for each nostril), oral cavity (0.5 mL on the surface of each tonsil), and trachea (5 mL) with pipettes and catheters 5 years after the prime-boost vaccination under ketamine/xylazine anesthesia. Experiments using challenge virus were performed in the biosafety level 3 facility of the Research Center for Animal Life Science, Shiga University of Medical Science. Details on vaccination and challenge have been described previously17.
Sample collection, measurements, and analysis
Under ketamine/xylazine anesthesia, two cotton sticks (TE8200, Eiken Chemical, Ltd., Tokyo, Japan) were used to collect fluid samples in nasal cavities, oral cavities and the trachea, and the sticks were subsequently immersed in 1 mL of PBS containing 0.1% bovine serum albumin (BSA) and antibiotics, as described previously17. A bronchoscope (MEV-2560, Machida Endoscope Co. Ltd., Tokyo, Japan) and cytology brushes (BC-203D-2006, Olympus Corporation, Tokyo, Japan) were used to obtain bronchial swabs.
Plasma was used for the neutralization assay and detection of antigen-specific IgG. Viral titration was performed in nasal and tracheal swabs and bronchial brushes. On day 7 after challenge, autopsy was performed, and lungs were cut vertically to the bronchus into 5 mm slices. One to two slices were fixed in 10% neutral buffered formalin for 3 days for histopathological analysis. Then, 5 × 5 × 5 mm3 pieces were cut out from inflamed lesions based on gross pathology and divided into two tubes to be 1) immersed in 500 μL of RNAlaterTM Stabilization Solution (AM7021, Thermo Fisher Scientific Inc, Waltham, MA), which were left overnight at 4 °C and stored at −80 °C until total RNA extraction and 2) stored immediately at −80 °C until use for virus titration and cytokine measurement.
Virus titration
Serial dilutions of swab samples and 10% w/v tissue homogenates were inoculated onto confluent MDCK cells in quadruplicate in 96-well plates. Cytopathic effects were examined under a microscope 72 h later. The Reed-Muench method was used to calculate titers.
Virus neutralization assay
Plasma samples were pretreated with a receptor-destroying enzyme (RDE) (RDEII, Denka Seiken, Tokyo, Japan) at 37 °C overnight and then inactivated at 56 °C for 1 h. Then, 200 PFU of Vac-3, A/Vietnam/UT3040/2004 (H5N1), A/whooper swan/Mongolia/3/2005 (H5N1) (clade 2.2)48, A/whooper swan/Hokkaido/1/2008 (H5N1) (clade 2.3.2.1)18, A/peregrine falcon/Hong Kong/810/2009 (H5N1) (clade 2.3.4.5)16 and A/Ezo red fox/Hokkaido/1/2022 (H1N1) (clade 2.3.4.4b)49 were mixed with the diluted samples for 1 h. The mixtures were added onto MDCK monolayers in quadruplicate in 96-well plates or 6-well plates. After 1 h, MDCK cells were covered by MEM containing 0.1% BSA or MEM containing 0.1% BSA and 1% agar. After incubation at 35 °C for 2 days, the number of wells with cytopathic effects and the number of plaques were counted. Neutralization titers are expressed as the dilution in which the numbers of plaques or wells with cytopathic effect were reduced to 50% of those without plasma.
Detection of antibody specific for virus antigen by ELISA
The antibody titers of plasma and swab samples against Vac-3 antigens were determined using ELISA. Ninety-six-well plates were coated with 50 μL of purified Vac-3 (20 mg/mL) disrupted with 0.05 M Tris-HCl (pH 7.8) containing 0.5% Triton X-100 and 0.6 M KCl and purified NA proteins (A/Thailand/1(KAN-1)/2004 (H5N1) (Cat# 40064-V07H) and A/Texas/37/2024 (H5N1) (Cat# 41016-V08B)) (1 μg/50 μL) (Sino Biological Inc., Beijing, China). Serially diluted samples were incubated overnight in the coated plates. After washing five times, horseradish peroxidase (HRP)–conjugated anti-monkey IgG antibodies (MP Biomedicals, Inc./Cappel, Aurora, OH) (1:2000, 50 μL) were added and incubated for 1 h at room temperature. HRP activity was assessed using 3, 3’, 5, 5’-tetramethyl benzidine substrate (100 μL). The reaction was stopped by the addition of 1 M hydrogen chloride (100 μL). Optical density (OD) was measured at 450 and 620 nm. Results are shown after subtraction of OD at 620 nm from OD at 450 nm.
Histological scoring of the lungs
Lungs were cut vertically to the bronchus into 5 mm-thick slices, which were fixed in 10% neutral buffered formalin for 3 days. They were embedded in paraffin and were cut to a thickness of 3 μm on glass slides. After deparaffinization in xylene and ethanol, sections were stained with hematoxylin and eosin (H & E) and observed under a light microscope. Histological scoring was performed by two pathologists, both blinded to sample identification, based on criteria established for influenza virus infection20 as follows: 0, normal lung; 1, mild destruction of bronchial epithelium; 2, mild peribronchiolar inflammation; 3, inflammation in the alveolar walls resulting in alveolar thickening; 4, mild alveolar injury accompanied by vascular injury; 5, moderate alveolar injury and vascular injury; and 6 and 7, severe alveolar injury with hyaline membrane-associated alveolar hemorrhage (under or over 50% of the section area, respectively). The mean score for the six to eight sections was calculated for each macaque, and the mean score of the two pathologists was defined as the histological score.
Immunohistochemistry of the lungs
Formalin-fixed, paraffin-embedded lung sections were deparaffinized and immersed in 0.3% hydrogen peroxide for 13 min. Antigen retrieval was performed, and sections were incubated with primary antibodies overnight at 4 °C (Supplementary Table 6). An anti-mouse and anti-rabbit universal immune-peroxidase polymer was used as a secondary antibody (Histofine Simple Stain MAX-PO (MULTI), Nichirei Biosciences Inc., Tokyo, Japan) followed by incubation with 3,3’-diaminobenzidine (DAB, Nichirei Biosciences). Nuclei were counterstained with hematoxylin. The number of cells positive with DAB visualization was quantified with NIS Elements version 5.41.00 (Nikon Solutions Co., Ltd., Tokyo, Japan) in which each slide was scanned randomly for more than ten fields (× 20). The total number of BALTs on each section was counted manually under the light microscope by detection of follicle-like structures composed of densely packed CD20-positive cells, of which the longest diameter exceeded 0.1 mm, and flanked by the bronchus or vessels. The number of BALTs of which the longest diameter was under 0.1 mm was also counted separately.
Fibrin clot detection by Carstairs staining
Formalin-fixed, paraffin-embedded lung sections were deparaffinized and treated with 5% ferric ammonium sulfate for 5 min and hematoxylin for 5 min. They were then stained with 10-fold diluted orange G picric acid solution (Muto Pure Chemicals Co., Ltd., Tokyo, Japan) for 30–60 min. Next, the sections were treated in a solution containing ponceau xylidine, fuchsin acid, and azophloxine (FUJIFILM Wako Pure Chemical Corporation, Osaka, Japan) for 5 min, a phosphotungstic acid hydrate solution (Muto Pure Chemicals Co., Ltd.) for 5 min, followed by an aniline solution (Muto Pure Chemicals Co., Ltd.) for 5 min.
Bulk RNA sequencing of lung tissues
From each lobe (six lobes per macaque), 5 × 5 × 5 mm3 pieces of lung were cut out from lesions that had the strongest inflammation determined by careful examination based on gross pathology (redness, exudate, and hardness of the lung). They were immersed in RNAlaterTM Stabilization Solution, left overnight at 4 °C, and stored at −80 °C until use. Then, 5 mg of lung tissue was cut out from each lobe, which resulted in a total of 30 mg per macaque, and they were excised and placed in a tube for homogenization twice at 5000 rpm for 15 sec with a TissueRuptor (Bertin Technologies, Montigny-le-Bretonneux, France, Precellys 24). Then, 600 μL of RLT Plus supplemented with 1% volume of 2-mercaptoethanol (SIGMA, #46496KMV) and 0.5% volume of Reagent Dx (QIAGEN, 19088) was added to the tube, and they were again homogenized twice at 5000 rpm for 15 s. Lysate was centrifuged at 8000 rpm for 3 min, and the supernatant was transferred to a gDNA elimination column. Total RNA was extracted using the RNeasy Plus Mini Kit (QIAGEN, 74136) according to the manufacturer’s instructions. Stranded RNA-seq for mammalian samples was performed by a NovaSeq System (Takara Bio Inc., Shiga, Japan). Briefly, poly(A) + RNA was purified and fragmented, and single-stranded cDNA was synthesized by reverse transcription with random primers. Double-stranded cDNA was synthesized with the integration of dUTP. Adapters were added to both terminals of the double-stranded cDNA, PCR amplification was performed with a polymerase that does not selectively amplify strands with dUTP, and templates for a DNA library were made. The DNA library was mixed and sequenced with an Illumina sequencer. Lead sequences were obtained, and amino acid sequences were classified according to tag sequences, mapped to a reference genome of cynomolgus macaques. Fragments per kilobase of exon per million fragments mapped were obtained to quantify the expression levels of mapped genes.
Bioinformatics analysis
Genes with a false discovery rate (q value) <0.05 were considered differentially expressed, and they were analyzed by the IPA software (version 2018; Ingenuity Systems; QIAGEN). RNA sequencing outcomes were uploaded to IPA for core analysis and jointly analyzed with the global molecular network in the Ingenuity Knowledge Base (QIAGEN, Valencia, CA). Canonical signaling pathways enriched by the DEGs were identified and rated according to p values. The z-scores of significantly involved canonical signaling pathways were also determined. Z-scores indicated the activation of the canonical signaling pathways, but the significantly involved pathways in the vaccinated macaques were inhibitory, thus shown only in blue.
Cytokine measurement
Tissue pieces of lungs collected at autopsy were stored at −80 °C until use. After homogenization with medium (10% weight/volume), levels of cytokines were measured using the Milliplex MAP nonhuman primate cytokine panel and Luminex200 (Millipore Corp., Billerica, MA, USA) according to the manufacturer’s instructions.
Statistical analysis
Statistical analysis was performed with the Mann-Whitney U-test, the Wilcoxon test, and the two-tailed Student’s t-test, and p < 0.05 was considered to indicate a significant difference.
Data availability
Sequence data that support the findings of this study have been deposited in the DNA DataBank of Japan (DDBJ) with the bioproject ID PRJDB35569. Sequence data (SAMD01593399 - SAMD01593428) (DRR703651-DRR703665) are found at https://ddbj.nig.ac.jp/search.
References
Tran, T. H. et al. Avian influenza A (H5N1) in 10 patients in Vietnam. N. Engl. J. Med. 350, 1179–1188 (2004).
Webster, R. G., Peiris, M., Chen, H. & Guan, Y. H5N1 outbreaks and enzootic influenza. Emerg. Infect. Dis. 12, 3–8 (2006).
World Health Organization. Avian Influenza Weekly Update Number 999. https://cdn.who.int/media/docs/default-source/wpro---documents/emergency/surveillance/avian-influenza/ai_20250530.pdf?sfvrsn=30965cc4_1&download=true. (WHO, 2025).
Korteweg, C. & Gu, J. Pathology, molecular biology, and pathogenesis of avian influenza A (H5N1) infection in humans. Am. J. Pathol. 172, 1155–1170 (2008).
Shi, J., Zeng, X., Cui, P., Yan, C. & Chen, H. Alarming situation of emerging H5 and H7 avian influenza and effective control strategies. Emerg. Microbes Infect. 12, 2155072 (2023).
Burrough, E. R. et al. Highly pathogenic avian influenza A(H5N1) clade 2.3.4.4b virus infection in domestic dairy cattle and cats, United States, 2024. Emerg. Infect. Dis. 30, 1335–1343 (2024).
Wang, T. T., Parides, M. K. & Palese, P. Seroevidence for H5N1 influenza infections in humans: meta-analysis. Science 335, 1463 (2012).
Toner, E. S. et al. Assessment of serosurveys for H5N1. Clin. Infect. Dis. 56, 1206–1212 (2013).
Kida, H., Shortridge, K. F. & Webster, R. G. Origin of the hemagglutinin gene of H3N2 influenza viruses from pigs in China. Virology 162, 160–166 (1988).
Gu, C. et al. A human isolate of bovine H5N1 is transmissible and lethal in animal models. Nature 636, 711–718 (2024).
Sawai, T. et al. Induction of cytotoxic T lymphocyte and antibody responses against highly pathogenic avian influenza virus infection in mice by inoculation of apathogenic H5N1 influenza virus particles inactivated with formalin. Immunology 124, 155–165 (2008).
Shingai, M. et al. Potent priming by inactivated whole influenza virus particle vaccines is linked to viral RNA uptake into antigen presenting cells. Vaccine 39, 3940–3951 (2021).
Itoh, Y. et al. A vaccine prepared from a non-pathogenic H5N1 avian influenza virus strain confers protective immunity against highly pathogenic avian influenza virus infection in cynomolgus macaques. Vaccine 26, 562–572 (2008).
Muramoto, Y. et al. Disease severity is associated with differential gene expression at the early and late phases of infection in nonhuman primates infected with different H5N1 highly pathogenic avian influenza viruses. J. Virol. 88, 8981–8997 (2014).
Soda, K. et al. Development of vaccine strains of H5 and H7 influenza viruses. Jpn. J. Vet. Res. 55, 93–98 (2008).
Shichinohe, S. et al. Potency of an inactivated influenza vaccine prepared from a non-pathogenic H5N1 virus against a challenge with antigenically drifted highly pathogenic avian influenza viruses in chickens. Vet. Microbiol. 164, 39–45 (2013).
Nakayama, M. et al. Protection against H5N1 highly pathogenic avian and pandemic (H1N1) 2009 influenza virus infection in cynomolgus monkeys by an inactivated H5N1 whole particle vaccine. PLoS One 8, e82740 (2013).
Okamatsu, M. et al. Antigenic, genetic, and pathogenic characterization of H5N1 highly pathogenic avian influenza viruses isolated from dead whooper swans (Cygnus cygnus) found in northern Japan in 2008. Virus Genes 41, 351–357 (2010).
Sakurai, A. et al. Broad-spectrum detection of H5 subtype influenza A viruses with a new fluorescent immunochromatography system. PLoS One 8, e76753 (2013).
Ogiwara, H. et al. Histopathological evaluation of the diversity of cells susceptible to H5N1 virulent avian influenza virus. Am. J. Pathol. 184, 171–183 (2014).
Gross, P. A. et al. A controlled double-blind comparison of reactogenicity, immunogenicity, and protective efficacy of whole-virus and split-product influenza vaccines in children. J. Infect. Dis. 136, 623–632 (1997).
Beyer, W. E., Palache, A. M. & Osterhaus, A. D. Comparison of serology and reactogenicity between influenza subunit vaccines and whole virus or split vaccines: a review and meta-analysis of the literature. Clin. Drug. Investig. 15, 1–12 (1998).
Ohno, M. et al. Assessing the pyrogenicity of whole influenza virus particle vaccine in cynomolgus macaques. Vaccine 41, 787–794 (2023).
Chua, B. Y. et al. Immunization with inactivated whole virus particle influenza virus vaccines improves the humoral response landscape in cynomolgus macaques. PLoS Pathog. 18, e1010891 (2022).
Arikata, M. et al. Memory immune responses against pandemic (H1N1) 2009 influenza virus induced by a whole particle vaccine in cynomolgus monkeys carrying Mafa-A1*052:02. PLoS One 7, e37220 (2012).
Anderson, B. W. et al. Induction of determinant spreading and of Th1 responses by in vitro stimulation with HER-2 peptides. Cancer Immunol. Immunother. 49, 459–468 (2000).
Yu, M., Johnson, J. M. & Tuohy, V. K. Generation of autonomously pathogenic neo-autoreactive Th1 cells during the development of the determinant spreading cascade in murine autoimmune encephalomyelitis. J. Neurosci. Res. 45, 463–470 (1996).
Onodera, T. et al. Whole-virion influenza vaccine recalls an early burst of high-affinity memory B cell response through TLR signaling. J. Immunol. 196, 4172–4184 (2016).
Shiohara, M. et al. Inactivated whole influenza virus particle vaccines induce neutralizing antibodies with an increase in immunoglobulin gene subclones of B-lymphocytes in cynomolgus macaques. Vaccine 40, 4026–4037 (2022).
Van Reeth, K. et al. Heterologous prime-boost vaccination with H3N2 influenza viruses of swine favors cross-clade antibody responses and protection. NPJ Vaccines 2, 11 (2017).
Haveri, A., Ikonen, N., Savolainen-Kopra, C. & Julkunen, I. Long-lasting heterologous antibody responses after sequential vaccination with A/Indonesia/5/2005 and A/Vietnam/1203/2004 pre-pandemic influenza A(H5N1) virus vaccines. Vaccine 39, 402–411 (2021).
Shimizu, S. et al. SARS-CoV-2 induces inflammation and intracranial infection through the olfactory epithelium-olfactory bulb pathway in nonhuman primates. J. Neuroimmunol. 387, 578288 (2024).
Miyamoto, S. et al. Saturation time of exposure interval for cross-neutralization response to SARS-CoV-2: Implications for vaccine dose interval. iScience 26, 106694 (2023).
Gao, R. et al. Cytokine and chemokine profiles in lung tissues from fatal cases of 2009 pandemic influenza A (H1N1): role of the host immune response in pathogenesis. Am. J. Pathol. 183, 1258–1268 (2013).
To, K. K. et al. Delayed clearance of viral load and marked cytokine activation in severe cases of pandemic H1N1 2009 influenza virus infection. Clin. Infect. Dis. 50, 850–859 (2010).
Beigel, J. H. et al. Avian influenza A (H5N1) infection in humans. N. Engl. J. Med. 353, 1374–1385 (2005).
Peiris, J. S. et al. Re-emergence of fatal human influenza A subtype H5N1 disease. Lancet 363, 617–619 (2004).
Cheung, C. Y. et al. Induction of proinflammatory cytokines in human macrophages by influenza A (H5N1) viruses: a mechanism for the unusual severity of human disease? Lancet 360, 1831–1837 (2002).
de Jong, M. D. et al. Fatal outcome of human influenza A (H5N1) is associated with high viral load and hypercytokinemia. Nat. Med. 12, 1203–1207 (2006).
Yang, W. H. et al. Long-term immunogenicity of an AS03-adjuvanted influenza A(H1N1)pdm09 vaccine in young and elderly adults: an observer-blind, randomized trial. Vaccine 31, 4389–4397 (2013).
Doyon-Plourde, P., Przepiorkowski, J., Young, K., Zhao, L. & Sinilaite, A. Intraseasonal waning immunity of seasonal influenza vaccine - a systematic review and meta-analysis. Vaccine 41, 4462–4471 (2023).
Levin, E. G. et al. Waning immune humoral response to BNT162b2 Covid-19 vaccine over 6 months. N. Engl. J. Med. 385, e84 (2021).
Zhang, Z. et al. Humoral and cellular immune memory to four COVID-19 vaccines. Cell 185, 2434–2451 (2022).
Trieu, M. C. et al. Antibody responses to influenza A/H1N1pdm09 virus after pandemic and seasonal influenza vaccination in healthcare workers. A 5-year follow-up study. Clin. Infect. Dis. 68, 382–392 (2019).
Talaat, K. R. et al. A live attenuated influenza A(H5N1) vaccine induces long-term immunity in the absence of a primary antibody response. J. Infect. Dis. 209, 1860–1869 (2014).
Huber, H. F. et al. Comparative lifespan and healthspan of nonhuman primate species common to biomedical research. Geroscience 47, 135–151 (2025).
Le, Q. M. et al. Pathogenicity of highly pathogenic avian H5N1 influenza A viruses isolated from humans between 2003 and 2008 in northern Vietnam. J. Gen. Virol. 91, 2485–2490 (2010).
Kajihara, M. et al. The PB2, PA, HA, NP, and NS genes of a highly pathogenic avian influenza virus A/whooper swan/Mongolia/3/2005 (H5N1) are responsible for pathogenicity in ducks. Virol. J. 10, 45 (2013).
Hiono, T. et al. Virological, pathological, and glycovirological investigations of an Ezo red fox and a tanuki naturally infected with H5N1 high pathogenicity avian influenza viruses in Hokkaido, Japan. Virology 578, 35–44 (2023).
Acknowledgements
The authors would like to thank Yoshihiro Kawaoka and Takahiro Hiono for sharing viruses, Kyoka Matsumoto, Ai Tanaka and Naomi Chihaya for technical assistance, Hideaki Tsuchiya, Iori Itagaki, Takahiro Nakagawa and Ikuo Kawamoto for regular animal care and Takefumi Yamamoto and Yasuhiro Mori for their technical support. This study was funded by JSPS KAKENHI Grant Nos. 16K19105 and 15H04720 to Y.I. and 16K19105 to M.N. and supported by the Japan Agency for Medical Research and Development (AMED) under Grant Nos. JP19fm0108008 to K.O. and H.K., JP223fa627008 to Y.I. and JP233fa827012 to Y.I. and H.K. The funder played no role in study design, data collection, analysis and interpretation of data, or the writing this manuscript. M.N. is a recipient of a Naito Memorial Foundation fellowship for female researchers. H. Ishigaki is a recipient of Takeda Science Foundation Grant.
Author information
Authors and Affiliations
Contributions
M.N., K.O. and Y.I. designed the study. M.N., N.K., C.T.N., T.S., K.T., H.I., H.I., S.S. and Y.I. participated in data acquisition. Y.S., Q.M.L. and H.K. prepared materials. M.N., Y.I. and K.T. analyzed data. M.N. and Y.I. wrote the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declares no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Nakayama, M., Kitagawa, N., Nguyen, C.T. et al. Long-term efficacy of an inactivated H5N1 whole-particle influenza vaccine in nonhuman primates. npj Vaccines 10, 164 (2025). https://doi.org/10.1038/s41541-025-01221-x
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41541-025-01221-x