Abstract
Molecular weight (MW) of organics was one of the important factors influencing membrane fouling propensity. This study identified critical foulants of reverse osmosis (RO) membranes in reclaimed water by MW fractionation. MW > 10 kDa component was identified as the critical fouling contributor (CFC) in secondary effluent (SE), which accounted for only 13 ± 5% of dissolved organic carbon (DOC) but contributed to 86 ± 11% of flux decline. Throughout 12-month monitoring, SE and MW > 10 kDa component showed a similar fouling variation tendency: apparently higher fouling potential in winter and lower in summer, while MW < 10 kDa component presented minor fouling changes. Morphology of membrane fouled by CFC characterized a smooth and thick foulant layer on membrane surface. CFC was mainly composed of proteins and polysaccharides, and a protein-polysaccharide-protein “sandwich” fouling layer structure was preferentially formed on membrane surface. extended Derjaguin–Landau-Verwey–Overbeek (xDLVO) analysis demonstrated that strong attractive interactions between CFC and membrane surface dominated the fouling process. Furthermore, computational fluid dynamics (CFD) simulation revealed strong filtration resistance of CFC, confirming its significant fouling potential. Dual effects including attractive interactions and advantageous ridge-and-valley surface appearance accounted for the significant fouling propensity of MW > 10 kDa component and glean valuable insights into RO fouling mechanisms of reclaimed water in practical application.
Similar content being viewed by others
Introduction
With the rapid urbanization and industrialization, the conflict between high demands for water and severe water scarcity has become increasingly prominent1. Wastewater reclamation for reusable water has occupied a pivotal position to solve water scarcity. Membrane technologies have been widely employed in wastewater reclamation ascribed to the superior separation performances2,3, among which reverse osmosis (RO) served as the core unit of high-standard pure water production owing to its effective removal of salts, organics, fine particles, bacteria and colloids4,5,6. However, membrane fouling spoiled RO systems in product water quality, water recovery, operating costs, and membrane lifespan7,8, which was considered to be a crucial problem.
The high occurrence of organic fouling in RO application and syngenetic interaction with colloid or biological fouling made it critical in RO fouling investigation9,10. Furthermore, as for wastewater, high organics concentrations (2.5–10 times that of surface water and seawater) and high RO treatment concentration ratio (1.2–2.4 times that of seawater system) made it more likely for organics to accumulate on membrane surface, and thus contributing to severe organic fouling11,12. Therefore, organic fouling was quite troublesome in the RO system for wastewater reclamation, and the identification and isolation of critical fouling contributor (CFC) needed in-depth investigation for a better understanding of composite fouling and fouling control.
Relevant researches focused on organic fouling have investigated model organics including bovine serum albumin (BSA), sodium alginate (SA) and humic acid (HA) to uncover the fouling characteristics of model foulant, mixed foulants and co-existing systems with ions in RO process. Ang et al. reported that BSA fouling of RO membrane was aggravated as increased Ca2+ concentration and exhibited significant fouling at pH = 4.7 (isoelectric point of BSA)13. Li et al. concluded that great fouling rates could be observed in the co-existence of BSA and SA, owing to the synergistic effect of forming BSA-SA aggregates with large particle size14. Fouling behaviors of several polysaccharides with different molecular structures, revealing that presence of Ca2+ ions could increase the fouling potential of polysaccharides with branched chains15,16. Zhao et al. reported that the presence of Ca2+ and Mg2+ ions obviously enlarged the interaction of HA-membrane and HA-HA through the bridging effects of ions and HA17. Li et al. demonstrated the initial flux decline of RO system was due to adsorption of the organics on membrane surface, and the later stage flux decline was attributed to the synergetic effects of the hydraulic resistance and the hydrophobicity of the fouling layer18. However, the intrinsic fouling behaviors of real reclaimed water was much more complicated than that of model foulants due to its complex organic compositions and interactions, so the fouling mechanisms were certainly different19.
As for real reclaimed water, CFC has been commonly investigated from the perspective of organic composition, hydrophobicity/hydrophilicity, molecular weight (MW), etc. A few studies reported humic-like substances exhibited significant fouling propensities20,21, while protein and polysaccharide-like substances were majorly considered as CFC in RO system22,23,24,25. Concentrations of proteins and polysaccharides were usually determined by Lowry method and phenol-sulfuric acid method with BSA and glucose as for standard substances. On one hand, these standards might not be appropriate to represent the proteins and polysaccharides in reclaimed water26,27. On the other hand, conventional colorimetric methods resulted in significant estimation errors of concentrations when applied to wastewater samples28,29.
Based on the intrinsic properties of reclaimed water, resin fractionation was carried out to separate fractions with different hydrophobicity/hydrophilicity to evaluate their corresponding membrane fouling behavior30,31,32. Tang et al. revealed hydrophobic neutral and hydrophobic acid fractions were the dominant foulants sampled from fouled RO membrane surface in full-scale wastewater treatment plant12. While in Wang’s study, hydrophilic neutrals and hydrophobic neutrals from chemical coal wastewater exhibited a higher propensity to RO membrane fouling33. On one hand, physiochemical characteristics of the separated fractions might be influenced by the use of acids, alkalis and organic solvents during the fractionation procedures, thus impacting their membrane fouling propensities34. On the other hand, hydrophobicity/hydrophilicity was a relative concept which highly depending on the fractionating resins and procedures, leading to different conclusions of CFC based on membrane fouling effects.
MW was proved to be one of the most critical factors influencing membrane fouling35,36,37. Abundant literatures were carried out to investigate the MW effects in MBR, MF, UF and NF fouling, and among those studies high MW organics were primarily reported as CFC38,39,40. It was widely recognized that fouling mechanism was associated with size exclusion effects based on porous filtration, and pore blocking or cake layer formation mainly accounted for fouling behaviors. Although studies on MW effects of membrane fouling in the aforementioned membrane technologies were relatively plentiful, but that in RO system was quite insufficient.
Despite the controversy about pores on RO membrane, size exclusion effect in RO process has been reported22,41, which accordingly attracted attention to corresponding MW effects on RO membrane fouling. Yu et al. found that high MW components (specifically, >10 kDa) in bacterial extracellular polymeric substances (EPS) had higher biofouling potential, which could be revealed by MW distribution analysis42. Yin et al. isolated biopolymer (MW>1k Da) from dissolved organic matter (DOM) in seawater, which was proved to be the major fouling substance43, but they also pinpointed that low MW organic fractions (MW < 350 Da) were more dominant as for biofouling in longer operation time44. Moreover, the organic composition and concentration whether of EPS collected from bacterial cultivation or DOM in seawater were quite different with that of organics in real reclaimed water. Hence, the identification of CFC was varied by water systems, and it was necessary to carry out systematic MW investigations on organic fouling in reclaimed wastewater and provide direct evidences for fouling behaviors of different MW organics in RO system.
This study, therefore, aimed to identify the CFC in wastewater reclamation from the perspective of MW effects. Molecular weight cut-off (MWCO) membrane was used to fractionate reclaimed water into fractions with different MWs, and the isolated MW > 10 kDa component was identified as CFC in RO system throughout 12-month monitoring. The fouled membranes were characterized by field emission scanning electron microscope (FESEM), atomic force microscope (AFM), and contact angle goniometer. Component analysis of MW > 10 kDa component were carried out by three-dimensional excitation-emission matrix fluorescence spectra (EEM), attenuated total reflection fourier transformed infrared spectroscopy (ATR-FTIR) and confocal laser scanning microscopy (CLSM), revealing its major foulant composition of proteins and polysaccharides. Strong attractive interaction between MW > 10 kDa component and RO membrane was demonstrated by the extended Derjaguin–Landau-Verwey–Overbeek (xDLVO) theory, and computational fluid dynamics (CFD) simulation manifested the significant filtration resistance. This study identified and isolated the CFC in reclaimed water responsible for RO membrane fouling, revealed the fouling behaviors, uncovered the fouling mechanisms, and helped glean valuable insight into effective RO fouling control in practical applications.
Results and discussion
Molecular weight effect on membrane fouling: identification of critical fouling contributor
Secondary effluent (SE) samples were collected from three wastewater reclamation plants (WWRPs) in Beijing, namely GBD, XHM, and BS WWRPs. After MW fractionation with molecular weight cut-off (MWCO) of 10 kDa, water samples with MW > 10 kDa and MW <10 kDa could be prepared from SE of three WWRPs. And the corresponding water qualities of samples were listed in Table 1. A relatively minor discrepancy between water qualities of SE in GBD and XHM was found, while the conductivity, dissolved organic carbon (DOC) and UV254 of SE from BS were obviously lower than those of the other two WWRPs. Specifically, MW > 10 kDa and MW <10 kDa components separated from the same SE sample exhibited huge differences: DOC and UV254 of MW > 10 kDa were significantly lower than those of MW <10 kDa. And the DOC and specific ultraviolet absorbance at 254 nm (SUVA) of MW > 10 kDa from BS were obviously different from those of the other two WWRPs.
As shown in Fig. 1a, similar flux declines of SE and MW > 10 kDa, and apparently discrepant flux declines of MW <10 kDa component were observed in fouling experiments of water samples from all three WWRPs. Although the water qualities and total capacity of these WWRPs were different, the consistent observations in fouling experiments demonstrated that MW > 10 kDa component showed similar membrane fouling potential to SE, while MW < 10 kDa made a minor contribution to membrane fouling.
a Normalized flux decline of three cases: GBD, XHM, and BS WWRPs; (b) membrane fouling evaluation of different MW components (ΔJ: the flux decline of MW > 10 kDa and MW < 10 kDa at the operating time of 60 h, ΔJSE: the flux decline of SE at the operating time of 60 h). Error bars refer to the standard deviations.
The flux decline ratio of MW > 10 kDa and MW < 10 kDa to SE were calculated as ΔJ/ΔJSE to evaluate fouling contributions of component with different MW. The larger ΔJ/ΔJSE, the higher fouling contribution. As shown in Fig. 1b, the average ΔJ/ΔJSE of MW > 10 kDa was calculated as 86 ± 11%, which was approximately twice than that of MW < 10 kDa (41 ± 4%). Taking DOC into consideration, it was interestingly found that the DOC of MW > 10 kDa component only accounted for 13 ± 5% of SE, while the its fouling contribution was quite significant. Comparatively, MW < 10 kDa component with much higher DOC proportion exhibited much slighter fouling contribution. According to DOC and fouling contribution analysis, MW > 10 kDa component was accordingly inferred to be the CFC during RO treatment in WWRPs.
Seasonal water quality variations of critical fouling contributor
Subsequent studies focused on GBD WWRP due to its typical water qualities and largest treatment capacity (1,000,000 m3/d) in China. A successive monthly water quality monitoring of water samples collected from GBD WWRP was carried out from November in 2021 to February in 2023 for about 12 months. Seasonal variations of water quality indexes including DOC, UV254, and SUVA were displayed in Supplementary Fig. 1, and the variations of these indexes in different water samples (SE, MW > 10 kDa and MW < 10 kDa) showed a similar tendency as season changes. Generally, higher DOC, UV254 and lower SUVA were observed in autumn and winter with lower temperatures (from November in 2021 to February in 2022 and October in 2022 to February in 2023). The opposite observation occurred in spring and summer with higher temperatures (from March to September in 2022). It was speculated that temperature variations as season changes were responsible for the water quality and fouling performance of reclaimed waters in WWRPs.
Seasonal fouling potential variations of critical fouling contributor
Fouling experiments were conducted based on water samples collected monthly from GBD WWRP. As displayed in Fig. 2a, it was found that the fouling behaviors of MW > 10 kDa component (purple symbols) were approaching that of SE (blue symbols), while MW < 10 kDa component (green symbols) obviously maintained slighter fouling potential by contrast. The repeatability of these results was verified at varied seasons throughout 12-month monitoring, further demonstrating that the MW > 10 kDa component was the CFC in reclaimed water of RO fouling. Moreover, SE and MW > 10 kDa component exhibited a similar fouling variation tendency as season changes: higher fouling potential in autumn and winter and lower fouling potential in spring and summer, while minor similar variations were found as for MW < 10 kDa component. The flux decline after 60-h operation (ΔJ) was further calculated to demonstrate the fouling potential variations at varied seasons (Supplementary Fig. 2). The average ΔJ of SE, MW > 10 kDa and MW < 10 kDa throughout the 12 months were calculated as 0.53 ± 0.10, 0.45 ± 0.11 and 0.26 ± 0.06, respectively. It was mentioning that ΔJ/ΔJSE of MW > 10 kDa was around 85%, along with almost consistent variation tendency as season changes, verifying the remarkable fouling contribution of MW > 10 kDa component.
Filtration resistance analysis was carried out as displayed in Fig. 2b. The Rm (intrinsic membrane filtration resistance) and Rp (polarization filtration resistance) of water samples varied relatively slightly in each fouling experiment. Rf (fouling filtration resistance) variation mainly contributed to that of RT (total filtration resistance). It was obvious to find that Rf ranked in the sequence of SE, MW > 10 kDa and MW < 10 kDa, with average Rf value of (14.5 ± 5.6) × 1013, (9.2 ± 4.0) × 1013 and (4.4 ± 1.4) × 1013 m−1, respectively. Specifically, Rf of MW > 10 kDa accounted for nearly 63% of SE, while MW < 10 kDa accounted for only 30%. The Rf variation (Fig. 2c) explicitly demonstrated that varied seasons significantly presented similar effects on fouling behaviors of SE and MW > 10 kDa, while much slighter effect on MW < 10 kDa, which agreed well with flux results. In conclusion, according to the fouling behaviors of water samples in different WWRPs and the repeatable verification fouling results at varied seasons, MW > 10 kDa component could be sufficiently identified as CFC in RO treatment process. As shown in the results above, both DOC concentration and fouling potential indexes (flux decline, ΔJ and Rf) of water samples exhibited similar variation tendency as season changes: higher in autumn and winter and lower in spring and summer. In our opinion, fouling variations as seasonal changes were essentially related to the influences of temperature. On one hand, higher foulant loading (DOC concentration) led to more severe membrane fouling, which might be attributed to more microbial secretion (EPS and SMP) in lower temperatures to resist adverse environment44. On the other hand, decreased water flux and permeability were caused by higher water viscosity and increased concentration polarization as temperatures decreased45. Moreover, literatures have reported that low temperature would lead to the shrinkage of the membrane pores and reduction of porosity of porous membrane like MF and UF46,47, reducing membrane flux and higher TMP. As for RO membrane, it could be accordingly referred that the distinct “ridge-and-valley” structure might be impacted by low temperature, thus affecting the water permeability.
Correlation analysis of water qualities and fouling potential
As discussed in the section above, seasonal effects on fouling potential of reclaimed water samples were revealed. To uncover the influence of water qualities on fouling behaviors and identify the key water quality index in practical application, correlations of water quality indexes (DOC, UV254, SUVA and EEM) and fouling potential indexes (ΔJ and Rf) of each sample were plotted in Supplementary Fig. 3a–c, respectively. To be specific, the majority of water quality indexes (DOC, UV254 and EEM) exhibited significant positive correlations with the fouling potential indexes of SE. Comparatively, only DOC was strongly positively correlated with the fouling potential of MW > 10 kDa component, while there was no significant correlation among these indexes as for MW < 10 kDa. Therefore, DOC of MW > 10 kDa was supposed to be a noticeable water index to monitor the membrane fouling variations in practical application.
Membrane fouling characteristics of critical fouling contributor
To systematically evaluate the membrane fouling characteristics and mechanisms of CFC, the plane and vertical morphological profiles of fouled membranes by different reclaimed water samples were observed and compared using field emission scanning electron microscope (FESEM). As displayed in Fig. 3, virgin RO membrane displayed rough surfaces with obvious ridge-and-valley structures. Membrane surfaces fouled by SE and MW > 10 kDa were completely covered by organics with smooth and flat surface morphology, while the profile of membrane fouled by MW < 10 kDa was quite similar to that of virgin RO membrane. As for the vertical morphological profiles, only one view of each sample was presented as representative, while the average fouling layer thickness was determined in several views. As shown in Fig. 3, membranes fouled by SE and MW > 10 kDa component exhibited compact and thick fouling layers, with thickness of 4.36 ± 0.83 and 4.50 ± 0.37 μm, respectively. However, the foulant layer thickness of MW < 10 kDa component was only 0.54 ± 0.06 μm, which was remarkably smaller than that of MW < 10 kDa, signifying little foulants retained on RO membrane surface.
The surface roughness of fouled membrane could also reflect the membrane fouling potential of tested water samples to some extent. With the accumulation of foulants on the membrane, the original rugged surface was gradually packed with foulants and became smoother, accordingly with decreased roughness. The surface roughness representing as Ra was determined by atomic force microscope (AFM). Ra of virgin RO membrane and membranes fouled by SE, MW > 10 kDa, and MW < 10 kDa were 74.7, 28.3, 24.4, and 58.5 nm, respectively (seen in Fig. 3). It was obvious to find that the roughness of membranes fouled by SE and MW > 10 kDa were significantly decreased compared with the virgin one, which could also account for the smooth plane surface morphology characterized by FESEM. In comparison, the roughness of membranes fouled by MW < 10 kDa showed a slight decrease.
In conclusion, morphological observation by FESEM and AFM were consistent with the membrane fouling performances of SE, MW > 10 kDa, and MW < 10 kDa in section “Molecular weight (MW) fractionation”. It could be further demonstrated that component with MW > 10 kDa in reclaimed water was the dominant membrane fouling contributor, which could be adequately identified as CFC in RO treatment.
Organic component analysis of critical fouling contributor
After RO separation, foulants in feed water could exist in permeate, concentrate and fouled RO membrane. The DOC concentration and volume of feed, permeate and concentrate were measured, and the fate of foulants in SE, MW > 10 kDa, and MW < 10 kDa were accordingly calculated according to the principle of mass conservation. As shown in Supplementary Fig. 4, foulants were mainly in the concentrate, and then the fouled membrane, and slightly in permeate. As for MW > 10 kDa, its mass proportion in concentrate, fouled membrane and permeate accounted for 72.0%, 20.1% and 7.9%, respectively. While MW < 10 kDa in fouled membrane only accounted for 4.7%, which was less than a quarter of MW > 10 kDa, signifying corresponding lower membrane fouling potential.
Three-dimensional excitation-emission matrix fluorescence spectra (EEM) was widely employed for the characterization of organics based on different intrinsic fluorescent chromophores48. The EEM spectrum was conventionally divided into five regions: aromatic proteins (region I and II), fulvic acids (region III), soluble microbial by-products (region IV), and humic acids (region V)49. The EEM fluorescence spectra of water samples (SE, MW > 10 kDa, and MW < 10 kDa solution) and detached foulants from RO membranes fouled by the three samples above were plotted. In Fig. 4a, c, a minor discrepancy was found between the EEM spectra of SE and MW < 10 kDa solution, containing organics in all five regions with strong fluorescence intensity. But fluorescent chromophores of MW > 10 kDa solution were completely concentrated in region I, II and IV with obviously lower fluorescent intensity, signifying aromatic proteins and soluble microbial by-products (Fig. 4b). However, as for the detached foulants from RO membrane fouled by SE and MW > 10 kDa, which were also concentrated in regions I, II, and IV (Fig. 4d, e), matched well with the EEM spectra of MW > 10 kDa solution. Comparatively, only slight fluorescent chromophores could be observed in the EEM spectra of detached foulants from RO membrane fouled by MW < 10 kDa (Fig. 4f). These results further demonstrated that MW > 10 kDa component was the dominant foulants on RO membrane and primarily contributed to fouling of reclaimed water. Xiao et al. investigated the correlation between EEM spectra and membrane fouling behaviors of organic matters and revealed that the region of Ex < 280 nm and Em = 280–330 nm was related to strong fouling propensity, which was almost overlapped with region I, II, and IV in this study, further verifying the high fouling potential of MW > 10 kDa component50.
As displayed in Fig. 5, the attenuated total reflection fourier transformed infrared spectroscopy (ATR-FTIR) spectrum of virgin RO membrane was consistent with polyamide membranes containing many sharp peaks between 500–1800 cm−1 51,52. The spectrum of membrane fouled by <10 kDa was quite similar to that of the virgin membrane, indicated slight fouling caused by MW < 10 kDa on RO membrane surface. In contrast, the spectra of membranes fouled by SE and MW > 10 kDa component exhibited similar regions of absorption, as well as that of MW > 10 kDa solution. Differently with the virgin RO membrane, many of the typical sharp absorption peaks between 500–1800 cm−1 could not be observed, indicating that polyamide membrane could not be detected through the fouling layer. And these results further revealed that MW > 10 kDa component served as CFC in RO membrane fouling process.
According to the previous literatures, the vibration bands at 1010–1040 cm−1 were assigned to polysaccharides or polysaccharide-like foulants (C–O, C=O)53,54. The peaks in the vicinity of 1540 cm−1 and 1650 cm−1 were associated with amides I (C=O) and amides II (N-H) groups of proteins or amino sugars, respectively53,54,55,56. The peaks near 1400 cm−1 were aliphatic C–H deformation, C–O stretching and O–H deformation of phenol, and near 2930 cm−1 were aliphatic C–H stretching53,57. The broad band of carboxyl and amide groups (O–H stretching and N-H stretching) was identified around 3300 cm−1. These results specifically suggested that the major organic compositions of MW > 10 kDa were proteins, polysaccharides, and aromatic compounds derived from humic-like compounds.
Based on aforesaid component analysis results, confocal laser scanning microscopy (CLSM) was adopted to investigate the vertical distribution of proteins and polysaccharides in fouling layers to gain a better understanding of the fouling development process. The CLSM slicing views of foulants were presented in Fig. 6. The proteins and polysaccharides were represented in green and red/blue, respectively. The bottom of each slice was the membrane surface side, and the fouling layer formation direction in each view was from the bottom to the top of the slice. It was inferred from the observation that proteins tended to adhere preferentially to the RO membrane surface, subsequently followed by polysaccharides, and finally proteins again. It seemed that polysaccharides were embedded into proteins, forming a protein-polysaccharide-protein “sandwich” fouling layer structure, which revealed proteins and polysaccharides had different adhesion propensities. Relatively different protein and polysaccharide intensities were found in membrane fouled by SE, MW > 10 kDa, and MW < 10 kDa, respectively, indicating their discrepancies in foulant layer and fouling development process.
Interaction energy determination of critical fouling contributor
The contact angle and zeta potential could reflect the hydrophilicity and surface charge of foulants, respectively, which were pivotal indexes affecting the fouling characteristics. As shown in Supplementary Table 1, the contact angle of membrane fouled by SE and MW > 10 kDa were larger than that of virgin RO membrane, indicating the significant hydrophobicity of CFC. But as for the MW < 10 kDa component, the reduced contact angle signified increased hydrophilicity of the fouled membrane instead. Liu et al. has reported that decreased contact angle as well as increased hydrophilicity would increase the energy barrier of acidic-basic (AB) interaction and prevented the adhesion of foulants on membrane surface, and thus hydrophilic substances obtained lower membrane fouling potential than hydrophobic ones58. Moreover, Kaur et al. demonstrated the decrease in the absolute value of zeta potential could reflect membrane fouling process59. ζ2 of membrane fouled by MW < 10 kDa reduced slightly compared with the virgin membrane, but decreased remarkedly as for SE and MW > 10 kDa component, which further indicated the fouling propensity of water samples and was in accordance with their fouling behaviors. Huisman et al. has also demonstrated that membrane fouling could be attributed to at least two important interactions: electrostatic or hydrophobic60. The hydrophobicity of membrane surface would increase when fewer charged groups existed, along with reduced absolute value of the zeta potential, indicating membrane fouling deterioration.
In order to further demonstrate the fouling mechanism of CFC, interfacial interaction energies involving foulant-membrane adhesion energy and foulant-foulant cohesion energy were determined and calculated through extended Derjaguin–Landau–Verwey–Overbeek (xDLVO) theory. A negative energy value referred to an attractive interaction and a positive value referred to a repulsive interaction on the contrary. The greater the absolute value of the interaction energy, the stronger the corresponding effect. As shown in Supplementary Table 2, the LW interaction energy (\(\Delta\)GLW) of three water samples including SE, MW > 10 kDa, and MW < 10 kDa were negative and quite similar, and the total interaction energy (\(\Delta\)GTOT) was mainly depended on the valued of AB interaction (\(\Delta\)GAB). \(\Delta\)GTOT of SE and MW > 10 kDa were negative signifying attractive interaction effects and strong fouling potential, while positive \(\Delta\)GTOT of MW < 10 kDa corresponded to repulsive force inversely. Figure 7 presents the distribution of the total foulant-membrane adhesion energy and foulant-foulant cohesion energy of these three water samples. The total attractive interaction of SE and MW > 10 kDa were quite similar (circled in red) and significantly different from the repulsion effect of MW < 10 kDa, which was consistent with their fouling performances and amply justified MW > 10 kDa was the CFC of membrane fouling.
Moreover, as for MW > 10 kDa component, foulant-foulant cohesion energy was greater than foulant-membrane adhesion energy in absolute value. It further suggested that initial fouling stage was attributed to attractive force between MW > 10 kDa component and RO membrane, and subsequently prominent attractive effects among MW > 10 kDa component led to the accumulation and aggregation of foulants on membrane surface. The molecular weight effect on the interaction of foulants and membrane was also reported in other studies. Chen et al. found EPSs with higher molecular weight had stronger interfacial interaction energy with membrane, which was consistent with this study61. Yin et al. reported that MW > 1 kDa component was dominant in seawater based on the interaction energy of foulant and membrane; the initial foulant-membrane interaction was the key driver of membrane fouling. Therefore, the fouling mechanism was varied by molecular weight as well as water qualities, which was worth further investigating43.
Implications and speculations of membrane fouling mechanisms of critical fouling contributor
Many studies were devoted to investigating the intrinsic structure in polyamide separation layer of RO membrane. “Ridge-and-valley” surface appearance has been reported62,63. In addition, recent literatures have paid attention to the three-dimensional and nanoscopic crumpled features on membrane surface, which was described as nodular and leave-like features (as shown in Supplementary Fig. 5), unraveling an approximate 30% voids and a porous structure with defects on the polyamide layer63,64,65. Considering the actual separation occurred at the frontside of the polyamide layer, it could be speculated that water sample flowed across crumpled and porous features reaching the interior voids, in which larger sized foulants might be retained or intercepted, and thus blocking the permeate pathways and causing fouling. According to the speculation of void retention effect, the fouling mechanism of large MW components on RO membrane surface could be considered as “apparent blocking” like porous membrane or size exclusion phenomenon. In order to better verify the fouling characteristics of different MW organic components during RO separation, a normalized intermediate blocking model was applied to simulate the separation process. As shown in Supplementary Fig. 6, the intermediate model exhibited superior determination coefficients for SE and MW > 10 kDa (0.991 ± 0.010 and 0.988 ± 0.012) under all circumstances, signifying precise fitting performances and indicating apparent “intermediate blocking” characteristics of CFC. Comparatively, the fitting results of MW < 10 kDa component were not promising with much lower R2, suggesting quite different fouling mechanism.
On one hand, higher MW components were more likely to be intercepted by ridge-and-valley surface and insert into the voids on membrane compared with lower ones, enhancing the difficulty of water permeation and thus causing flux decline. On the other hand, high MW compounds (>10 kDa) were proved to have strong attractive interactions with membranes and among themselves as demonstrated by xDLVO analysis, further strengthening the accumulation and aggregation of foulants on membrane surface.
Based on the aforementioned factors, the fouling mechanism of the CFC recognized in this study was speculated as shown in Fig. 8. Attributed to dual effects including advantageous ridge-and-valley surface appearance for interception and intrinsic attractive interactions, MW > 10 kDa component exhibited significant high fouling potential and remarkedly contributed to RO membrane fouling of reclaimed water. Furthermore, the MW study on organic fouling provided novel inspirations into the fouling mechanisms of RO membrane and was worthy of further exploration.
Computational fluid dynamics (CFD) simulation of critical fouling contributor
CFD simulation was adopted to demonstrate the filtration resistance induced by organic components with different MW. A micro-unit composed of modeled organics and polyamide layer assembled with nodular structures was used for fluid field simulation. Fluid velocity field of MW > 10 kDa and MW < 10 kDa components were shown in Fig. 9a, b. It could be observed that the flow velocity in the simulated flow filed presented higher values in the middle and lower values close to the boundary, owing to the narrowing of the flow channel caused by the existing of nodular structures. It was worth mentioning that the central flow velocity of MW > 10 kDa was significantly higher than that of MW < 10 kDa component, suggesting more narrowed flow channel as MW > 10 kDa component flowing across the membrane surface. Furthermore, fluid pressure field of MW > 10 kDa and MW < 10 kDa components were shown in Fig. 9c, d, and the outlet pressure was set as 0 Pa. Higher water velocity demanded higher inlet pressure, signifying higher pressure drop (ΔP) and filtration resistance. It could be observed that inlet pressure of MW > 10 kDa was remarkedly higher than that of MW < 10 kDa, demonstrating significant fouling potential of MW > 10 kDa component. The results of CFD simulation were consistent with above experimental results, providing strong evidence for MW > 10 kDa as the critical fouling contributor of reclaimed water in RO treatment process.
Methods
Water sample
The secondary effluent (SE) was collected from GaoBeiDian (GBD, 1,000,000 m3/d), XiaoHongMen (XHM, 600,000 m3/d), and BiShui (BS, 100,000 m3/d) wastewater reclamation plants (WWRPs, Beijing, China). Clean plastic buckets, which have been disinfected with 70% ethanol and then rinsed with ultrapure water, were used for water sample collection. When sampling, plastic buckets were firstly rinsed for 3 times with water sample, and then filled with water sample and sealed to avoid other contamination during the process. Water samples were transported to the lab and pretreated immediately on their arrival via prefiltration through 0.45-μm filters (Pall, USA) to remove suspended solids.
The water characteristics of samples were determined: Conductivity (SX713, Sanxin, China); Dissolved organic carbon (DOC; TOC-VCPH, Shimadzu, Japan); UV absorbance at 254 nm (UV254; DR6000, Hach, USA); Three-dimensional excitation-emission matrix fluorescence spectra (EEM; F-7000, Hitachi, Japan). The specific ultraviolet absorbance at 254 nm (SUVA) was calculated as the ratio of DOC to UV254 value. The corresponding water qualities of water samples from three WWRPs were listed in Table 1.
Molecular weight (MW) fractionation
Molecular weight cut-off (MWCO) ultrafilter membrane (PLGC07610, Millipore, USA) of 10 kDa was used to fractionate the pretreated SE into two fractions, namely the retentate (MW > 10 kDa) and the filtrate (MW < 10 kDa). The filtrate with MW < 10 kDa was collected for further analysis. The MW > 10 kDa retentate was re-dissolved into ultrapure water (Milli-Q system, Millipore Corp., USA) with original volume to prepare MW > 10 kDa solution. Biocide (FR110, Kurita, Japan) and antiscalant (hydroxyphosphono-acetic acid, Macklin, China) were added into the water samples with the dosage of 1 mL/L and 5 mg/L for organic system investigation. The conductivity of MW > 10 kDa solution was adjusted by NaCl (99.8%, Meryer, China) to the value of feed water.
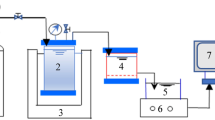
Cross-flow apparatus and fouling experiment
The cross-flow feeding reverse osmosis (RO) apparatus was adopted in this study. Aromatic polyamide composite RO membranes (LP100, Vontron, China) with recovery rate >75% and salt rejection efficiency >96% were used in the fouling experiments. RO membrane was fixed in the cross-flow module made of stainless steel, in which there was a magnetic stirrer below the membrane. The feeds were pumped into the modules by constant flow pumps (1 mL/min) and thoroughly stirred (200 rpm) before membrane separation. The module temperature (30 ± 1 °C) and transmembrane pressure (TMP, 1.2 Mpa) inside in operation were controlled through water bath and pressure regulators, respectively. The permeate and concentrate water of each module were collected separately, without returning to the feeds. The permeate water was collected in small plastic buckets placed on scales. The scale data were recorded during pretreatment and fouling experiments.
In fouling experiments, the RO membrane was pretreated in the RO module in turns of feeding ultrapure water (2 h), NaCl solution (500 mg/L, 2 h), and ultrapure water (4 h) sequentially. The salt rejection efficiency of each RO membrane was measured in the second feeding turn, and final salt rejection >90% was acceptable in this study. After the pretreatment, water samples were fed into the apparatus. The fouling experiments were stopped when the scale data maintained stable. The fouled RO membrane was carefully taken out of the module into a petri dish for further analysis.
Membrane permeate flux
The scale data were converted to membrane permeate flux (J, m3/(m2·s)) by Eq. (1):
Where A represented the effective membrane separation area (cm2), ρ was the permeate density (1 g/cm3), Δt was the time interval of data recording (5 min), and Δm was the permeate weight increment (g) within Δt. The normalized flux was determined and represented as J/J0. J0 was the initial flux in the first period of 5 min during the fouling experiment.
Filtration resistance analysis
According to Darcy’s law, the filtration resistance can be expressed as17,66:
ΔP was the transmembrane pressure (Pa), σ was the dynamic viscosity of permeate (Pa·s), and J represented permeate flux (m3/(m2·s)). RT, Rm, Rp, and Rf stood for total filtration resistance, intrinsic membrane filtration resistance, polarization filtration resistance and fouling filtration resistance, respectively. Rm, Rp and Rf were determined by feeding ultrapure water, salt solution and water samples, respectively.
Flux data fitting model
The modified intermediate blocking model, a two-parameter membrane filtration model, was proved effective and ideal for RO separation16,17,67, and was adopted in this study to fit the permeate flux data.
where Jpss and k were the two key parameters indicting fouling potential, which represented the normalized flux in steady state (m3/(m2·s)), and the blocked membrane area per unit of separation volume (s−1), respectively. k reflected the fouling rate in the early stage.
Characterization of fouled membranes
The plane and vertical profile of the fouled membrane surface were performed by field emission scanning electron microscope (FESEM; Geminisem 500, China) with a voltage of 15 kV to characterize the fouled membrane morphology foulant layer thickness. The surface roughness of the membrane was measured by atomic force microscope (AFM; SPA-300HV, Seiko, Japan) with a scan area of 10 × 10 μm. For each sample, three views were chosen and the roughness parameters (Ra) of each view were analyzed and reported in range (NanoScope Analysis 1.9).
The contact angles of fouled membranes were determined by a contact angle goniometer (OCA25, Dataphysics, Germany). The sessile drop method was adopted to measure contact angle with measurement time set as 5 s.
Foulants characterization
Foulants on membrane surface were detached by soaking with 50 ml of 0.01 M NaOH at 25 °C for 48 h. 0.01 M HCl (volume ration of NaOH: HCl = 15:13) was used to adjust solution pH=7 to prepare the solution of detached foulants. And the solution was pre-filtrated by 0.45-μm membrane for further DOC and EEM analysis.
The freeze-dried MW > 10 kDa component and foulants on fouled membranes were characterized by attenuated total reflection flourier transformed infrared spectroscopy (ATR-FTIR; IS50, Thermo, USA) for chemical functional group analysis, which provided a preliminary portrait of foulant composition.
The foulant distribution on membranes was further investigated by confocal laser scanning microscopy (CLSM; FV3000, Olympus, Japan). In the CLSM experiment, Calcofluor white (β-1,4 and β-1,3 polysaccharides), Con A conjugates (α-Mannopyranosyl, α-glucopyranosyl sugars), and FITC (protein, amino-sugars) targeting at polysaccharide and proteins were adopted. The staining procedure referred to68.
extended Derjaguin–Landau–Verwey–Overbeek (xDLVO) theory
The xDLVO theory has been used to reveal membrane fouling behaviors69,70. As introduced in xDLVO) theory, three kinds of interactions responsible for membrane fouling were van der Waals (LW) interaction, electrostatic (EL) interaction, and acidic-basic (AB) interactions, which could be calculated as follows:
where ΔGTOT, ∆GLW, ∆GAB, and ∆GEL represented per area unit of the total, LW, AB, and EL interaction energy (mJ/m2), respectively. γLW, γ+, and γ- were the surface tension (mJ/m2) of LW, electron acceptor, and electron donor, respectively. The specific subscript ‘l’, ‘m’, and ‘f’ represented liquid, membrane, and foulant, respectively. ζm and ζf represented surface potentials of membrane and foulants, respectively. The explanations and values of constant parameters were listed in Supplementary Table 3. Moreover, EL interaction energy was ignorable compared with the other two interactions, and thus ΔGTOT was directly calculated by the sum of ∆GLW and ∆GAB 34,69,71.
The surface tensions of membrane, foulants, and liquid were calculated by Eqs. (8)–(10)
where θ was contact angle; γAB was surface tension of AB. The γmLW, γm+, and γm− were determined through Eq. (8) by measuring three probe liquids with known surface tension parameters and different polarity, which were listed in Supplementary Table 4.
Computational fluid dynamics (CFD) simulation
COMSOL Multiphysics 6.0 was adopted in this study for CFD simulation to reveal the interactions between foulants in water sample and RO membrane. The model parameters of nodular and leave-like features on polyamide layer of RO membrane surface referred to the specific sizes reported by ref. 63, and the schematic diagrams of the constructed nodular structures were shown in Supplementary Fig. 7. These nodular structures were deformed into different classes on the basis of cylinder. In X-Y plane, the transverse dimensions of nodular structures were set as: upper diameter ≈lower diameter (67.7 × 66.9 nm), upper diameter > lower diameter (60.2 × 52.9 nm) and upper diameter < lower diameter (52.8 × 57.6 nm), respectively. Three vertical dimensions of nodular structures was set as 60, 118 and 178 nm. 21 nodular structures were installed with spacing of 35 nm, and the specific size parameters were listed in Supplementary Table 5. Besides, organic foulants were supposed as rigid cylinders, and the distributions of MW > 10 kDa and MW < 10 kDa were shown in Supplementary Fig. 8. The solution domain of CFD simulation was set as a cuboid (360 × 1360 × 280 nm). The inlet flow rate was set at 0.16 m/s, and the outlet pressure was set at 0 Pa. The hydrodynamic field was set as a transient process and the boundary condition was set as no slip.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Nixdorff, H., Noga, J., Amsalu, D., Springett, J. & Ashbolt, N. Improving the implementation of water and resource recovery in Canada. Water Reuse 11, 453–463 (2021).
Xu, Y. Q., Wu, Y. H., Tong, X., Luo, L. W. & Wang, H. B. How to select ideal model organic matters for membrane fouling research on water and wastewater treatment. Water Cycle 4, 55–59 (2023).
Li, H. B., Mo, Y. H., Wang, L. & Wang, X. Y. Feasibility of hydrophilic polyethylene separator for membrane bioreactor for wastewater treatment: Fouling behaviors and cleaning. Water Cycle 4, 79–86 (2023).
Wang, Q., Huang, N., Cai, H. Y., Chen, X. W. & Wu, Y. H. Water strategies and practices for sustainable development in the semiconductor industry. Water Cycle 4, 12–16 (2023).
Lee, H., Jin, Y. & Hong, S. Recent transitions in ultrapure water (UPW) technology: Rising role of reverse osmosis (RO). Desalination 399, 185–197 (2016).
Cai, H. Y. et al. Adsorption of neutral and negatively charged low-molecular-weight carbonyls in reverse osmosis permeates by ion-exchange resins. Water Cycle 3, 1–7 (2022).
Matin, A., Laoui, T., Falath, W. & Farooque, M. Fouling control in reverse osmosis for water desalination & reuse: current practices & emerging environment-friendly technologies. Sci. Total Environ. 765, 142721 (2021).
Nthunya, L. N. et al. Fouling, performance and cost analysis of membrane-based water desalination technologies: a critical review. J. Environ. Manag. 301, 113922 (2022).
Kucera, J. Biofouling of polyamide membranes: Fouling mechanisms, current mitigation and cleaning strategies, and future prospects. Membranes 9, 111 (2019).
Yu, T. et al. Microcoagulation improved the performance of the UF-RO system treating the effluent from a coastal municipal wastewater treatment plant: a pilot-scale study. Water Reuse 11, 177–188 (2021).
Malaeb, L. & Ayoub, G. M. Reverse osmosis technology for water treatment: state of the art review. Desalination 267, 1–8 (2011).
Tang, F. et al. Fouling characteristics of reverse osmosis membranes at different positions of a full-scale plant for municipal wastewater reclamation. Water Res. 90, 329–336 (2016).
Ang, W. S. & Elimelech, M. Protein (BSA) fouling of reverse osmosis membranes: implications for wastewater reclamation. J. Membr. Sci. 296, 83–92 (2007).
Li, Q., Xu, Z. & Pinnau, I. Fouling of reverse osmosis membranes by biopolymers in wastewater secondary effluent: Role of membrane surface properties and initial permeate flux. J. Membr. Sci. 290, 173–181 (2007).
Meng, X. et al. The structural and functional properties of polysaccharide foulants in membrane fouling. Chemosphere 268, 129364 (2021).
Tong, X. et al. The molecular structures of polysaccharides affect their reverse osmosis membrane fouling behaviors. J. Membr. Sci. 625, 118984 (2021).
Zhao, X. H. et al. Ozonation as an efficient pretreatment method to alleviate reverse osmosis membrane fouling caused by complexes of humic acid and calcium ion.Front. Environ. Sci. Eng. 13, 55 (2019).
Li, H., Xia, H. & Mei, Y. Modeling organic fouling of reverse osmosis membrane: From adsorption to fouling layer formation. Desalination 386, 25–31 (2016).
Filloux, E., Gallard, H. & Croue, J.-P. Identification of effluent organic matter fractions responsible for low-pressure membrane fouling. Water Res. 46, 5531–5540 (2012).
Ayache, C. et al. Impact of effluent organic matter on low-pressure membrane fouling in tertiary treatment. Water Res. 47, 2633–2642 (2013).
Jeong, G., Lee, H., Kim, C.-M. & Jang, A. Size-dependent transport and fouling formation of organic matters in a pilot-scale PFFO-RO hybrid system for real wastewater treatment. J. Clean. Prod. 361, 132233 (2022).
Ding, S., Yang, Y., Li, C., Huang, H. & Hou, L.-A. The effects of organic fouling on the removal of radionuclides by reverse osmosis membranes. Water Res. 95, 174–184 (2016).
Jarusutthirak, C. & Amy, G. Role of soluble microbial products (SMP) in membrane fouling and flux decline. Environ. Sci. Technol. 40, 969–974 (2006).
Khan, M. T. et al. How different is the composition of the fouling layer of wastewater reuse and seawater desalination RO membranes? Water Res. 59, 271–282 (2014).
Kong, F.-X. et al. Desalination and fouling of NF/low pressure RO membrane for shale gas fracturing flowback water treatment. Sep. Purif. Technol. 195, 216–223 (2018).
Kakuda, T., Naito, R., Suzuki, A., Hafuka, A. & Kimura, K. Quantitative and qualitative variations of biopolymers in a pilot-scale membrane bioreactor treating municipal wastewater throughout 3 years of operation. J. Environ. Manag. 328, 116863 (2023).
Tominaga, K., Nagai, R., Hafuka, A., Yu, W. & Kimura, K. Isolation of LC-OCD-quantified biopolymers from surface water: Significant differences between real biopolymers and model biopolymers. J. Membr. Sci. 658, 120714 (2022).
Le, C., Kunacheva, C. & Stuckey, D. C. “protein” measurement in biological wastewater treatment systems: a critical evaluation. Environ. Sci. Technol. 50, 3074–3081 (2016).
Le, C. & Stuckey, D. C. Colorimetric measurement of carbohydrates in biological wastewater treatment systems: A critical evaluation. Water Res. 94, 280–287 (2016).
Kennedy, M. D., Chun, H. K., Yangali, V. A. Q., Heijman, B. G. J. & Schippers, J. C. Natural organic matter (NOM) fouling of ultrafiltration membranes: fractionation of NOM in surface water and characterisation by LC-OCD. Desalination 178, 73–83 (2005).
Shen, Y., Zhao, W., Xiao, K. & Huang, X. A systematic insight into fouling propensity of soluble microbial products in membrane bioreactors based on hydrophobic interaction and size exclusion. J. Membr. Sci. 346, 187–193 (2010).
Yamamura, H., Okimoto, K., Kimura, K. & Watanabe, Y. Hydrophilic fraction of natural organic matter causing irreversible fouling of microfiltration and ultrafiltration membranes. Water Res. 54, 123–136 (2014).
Wang, S., Xiao, K. & Huang, X. Characterizing the roles of organic and inorganic foulants in RO membrane fouling development: The case of coal chemical wastewater treatment. Sep. Purif. Technol. 210, 1008–1016 (2019).
Bai, Y. et al. Critical minority fractions causing membrane fouling in reclaimed water: fouling characteristics, mechanisms and control strategies. Environ. Int. 173, 107818 (2023).
Jarusutthirak, C., Amy, G. & Croué, J.-P. Fouling characteristics of wastewater effluent organic matter (EfOM) isolates on NF and UF membranes. Desalination 145, 247–255 (2002).
Ren, L. X., Liu, C., Meng, T. & Sun, Y. X. Effects of micro-flocculation pretreatment on the ultrafiltration membrane fouling caused by different dissolved organic matters in treated wastewater. Water Reuse 11, 597–609 (2021).
Thanuttamavong, M., Yamamoto, K., Oh, J. I., Choo, K. H. & Choi, S. J. Rejection characteristics of organic and inorganic pollutants by ultra low-pressure nanofiltration of surface water for drinking water treatment. Desalination 145, 257–264 (2002).
Arabi, S. & Nakhla, G. Impact of molecular weight distribution of soluble microbial products on fouling in membrane bioreactors. Sep. Purif. Technol. 73, 391–396 (2010).
Hatziantoniou, D. & Howell, J. A. Influence of the properties and characteristics of sugar-beet pulp extract on its fouling and rejection behaviour during membrane filtration. Desalination 148, 67–72 (2002).
Song, Q. et al. The role of medium molecular weight organics on reducing disinfection by-products and fouling prevention in nanofiltration. Water Res. 215, 118263 (2022).
Ng, H. Y. & Elimelech, M. Influence of colloidal fouling on rejection of trace organic contaminants by reverse osmosis. J. Membr. Sci. 244, 215–226 (2004).
Yu, T. et al. Different bacterial species and their extracellular polymeric substances (EPSs) significantly affected reverse osmosis (RO) membrane fouling potentials in wastewater reclamation. Sci. Total Environ. 644, 486–493 (2018).
Yin, W., Li, X., Suwarno, S. R., Cornelissen, E. R. & Chong, T. H. Fouling behavior of isolated dissolved organic fractions from seawater in reverse osmosis (RO) desalination process. Water Res. 159, 385–396 (2019).
Yin, W., Ho, J. S., Cornelissen, E. R. & Chong, T. H. Impact of isolated dissolved organic fractions from seawater on biofouling in reverse osmosis (RO) desalination process. Water Res. 168, 115198 (2020).
Chen, C. et al. Unravelling temperature-dependent fouling mechanism in a pilot-scale anaerobic membrane bioreactor via statistical modelling. J. Membr. Sci. 644, 120145 (2022).
Kim, Y., Lee, S., Shon, H. K. & Hong, S. Organic fouling mechanisms in forward osmosis membrane process under elevated feed and draw solution temperatures. Desalination 355, 169–177 (2015).
Xu, B. et al. A review of temperature effects on membrane filtration. Membranes 14, 5 (2024).
Mao, Y. et al. Characterization of bacterial fluorescence: insight into rapid detection of bacteria in water. Water Reuse 11, 621–631 (2021).
Chen, W., Westerhoff, P., Leenheer, J. A. & Booksh, K. Fluorescence excitation-emission matrix regional integration to quantify spectra for dissolved organic matter. Environ. Sci. Technol. 37, 5701–5710 (2003).
Xiao, K. et al. Fluorescence quotient of excitation-emission matrices as a potential indicator of organic matter behavior in membrane bioreactors. Enviro. Sci. Water Res. Technol. 4, 281–290 (2018).
Beverly, S., Seal, S. & Hong, S. Identification of surface chemical functional groups correlated to failure of reverse osmosis polymeric membranes. J. Vac. Sci. Technol. 18, 1107–1113 (2000).
Kumar, M., Adham, S. S. & Pearce, W. R. Investigation of seawater reverse osmosis fouling and its relationship to pretreatment type. Environ. Sci. Technol. 40, 2037–2044 (2006).
Melián-Martel, N., Sadhwani, J. J., Malamis, S. & Ochsenkühn-Petropoulou, M. Structural and chemical characterization of long-term reverse osmosis membrane fouling in a full scale desalination plant. Desalination 305, 44–53 (2012).
Zhao, Y., Song, L. F. & Ong, S. L. Fouling behavior and foulant characteristics of reverse osmosis membranes for treated secondary effluent reclamation. J. Membr. Sci. 349, 65–74 (2010).
Belfer, S., Purinson, Y. & Kedem, O. Surface modification of commercial polyamide reverse osmosis membranes by radical grafting: an ATR-FTIR study. ACTA Polym. 49, 574–582 (1998).
Lee, W. et al. Evaluation of surface properties of reverse osmosis membranes on the initial biofouling stages under no filtration condition. J. Membr. Sci. 351, 112–122 (2010).
Tran, T., Bolto, B., Gray, S., Hoang, M. & Ostarcevic, E. An autopsy study of a fouled reverse osmosis membrane element used in a brackish water treatment plant. Water Res. 41, 3915–3923 (2007).
Liu, J. et al. Modelling the critical roles of zeta potential and contact angle on colloidal fouling with a coupled XDLVO-collision attachment approach. J. Membr. Sci. 623, 119048 (2021).
Kaur, G., Nakamura, K., Ogawa, K. & Wakui, K. Monitoring of MBR fouling properties by filtration resistance and zeta potential measured for both filtration and backwashing directions. J. Membr. Sci. 675, 121550 (2023).
Huisman, I. H., Prádanos, P. & Hernández, A. The effect of protein–protein and protein–membrane interactions on membrane fouling in ultrafiltration. J. Membr. Sci. 179, 79–90 (2000).
Kim, S. H., Kwak, S. Y. & Suzuki, T. Positron annihilation spectroscopic evidence to demonstrate the flux-enhancement mechanism in morphology-controlled thin-film-composite (TFC) membrane. Environ. Sci. Technol. 39, 1764–1770 (2005).
Pacheco, F. A., Pinnau, I., Reinhard, M. & Leckie, J. O. Characterization of isolated polyamide thin films of RO and NF membranes using novel TEM techniques. J. Membr. Sci. 358, 51–59 (2010).
Song, X. et al. Intrinsic nanoscale structure of thin film composite polyamide membranes: connectivity, defects, and structure-property correlation. Environ. Sci. Technol. 54, 3559–3569 (2020).
Song, X. et al. Unraveling the morphology–function relationships of polyamide membranes using quantitative electron tomography. ACS Appl. Mater. Interfaces 11, 8517–8526 (2019).
Li, Y. et al. Probing flow activity in polyamide layer of reverse osmosis membrane with nanoparticle tracers. J. Membr. Sci. 534, 9–17 (2017).
Zhang, M. et al. Effect of calcium ions on fouling properties of alginate solution and its mechanisms. J. Membr. Sci. 525, 320–329 (2017).
Tong, X. et al. Simulating and predicting the flux change of reverse osmosis membranes over time during wastewater reclamation caused by organic fouling. Environ. Int. 140, 105744 (2020).
Chen, M.-Y., Lee, D.-J., Yang, Z., Peng, X. F. & Lai, J. Y. Fluorecent staining for study of extracellular polymeric substances in membrane biofouling layers. Environ. Sci. Technol. 40, 6642–6646 (2006).
Lin, T., Lu, Z. J. & Chen, W. Interaction mechanisms and predictions on membrane fouling in an ultrafiltration system, using the XDLVO approach. J. Membr. Sci. 461, 49–58 (2014).
Yin, Z., Yeow, R. J. E., Ma, Y. & Chew, J. W. Link between interfacial interaction and membrane fouling during organic solvent ultrafiltration of colloidal foulants. J. Membr. Sci. 611, 118369 (2020).
Hu, M. L. et al. Facile preparation of antifouling nanofiltration membrane by grafting zwitterions for reuse of shale gas wastewater. Sep. Purif. Technol. 276, 119310 (2021).
Acknowledgements
This study was supported by National Key Research and Development Program of China (No. 2020YFC1806302), the Fundamental Research Funds for the Central Universities (2022NTST03), the Major Program of National Natural Science Foundation of China (No. 52293440, No. 52293442) and Science Fund for Creative Research Groups (No. 52221004).
Author information
Authors and Affiliations
Contributions
Yuan Bai: conceptualization, methodology, preparation, acquisition, analysis, writing—original draft. Yinhu Wu: supervision, conceptualization, funding acquisition, writing—review & editing. Ziwei Zhang: conceptualization, analysis, validation, writing—review & editing. Yu Mao: validation, writing—review & editing. Ruining Wang: methodology, writing—review & editing. Xin Tong: validation, methodology. Song Xue: validation, methodology. Haobin Wang: validation, methodology. Hongying Hu: supervision, funding acquisition, writing—review & editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bai, Y., Wu, Y., Zhang, Z. et al. Molecular weight insight into critical component contributing to reverse osmosis membrane fouling in wastewater reclamation. npj Clean Water 7, 62 (2024). https://doi.org/10.1038/s41545-024-00359-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41545-024-00359-w
This article is cited by
-
Characterization of Fouling Layers and Their Impact on RO Membrane Performance Degradation in Diverse Water Treatment Applications
Water, Air, & Soil Pollution (2025)
-
Towards healthy and economically sustainable communities through clean water and resource recovery
npj Clean Water (2024)