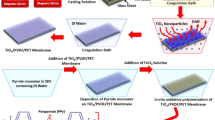
Abstract
The major polluting aspects of our global fashion industries are the textile wastewater that turns black all our freshwater reservoirs. Nano-filtration through membrane technology is one of the biggest solutions of industrial wastewater treatment but the fouling of membrane is the major limitation of previous work. In this research, novel PVDF/MoS2-TNT (PMT) nanocomposite membranes were fabricated through a modified In-situ polymerisation phase inversion method. X-ray diffraction (XRD) analysis also confirmed the β-phase of PVDF within the developed PVDF/MoS2-TNT membrane. XPS analysis provides evidence about the presence of a specific chemical states of titanium nanotube and molybdenum disulphide which is involved in the photocatalytic degradation of pollutant molecules. Scanning electron microscope (SEM) shows that our membranes are porous in nature. PVDF/MoS2-TNT membranes exhibit excellent filtration efficiency (∼97%) for textile wastewater. The results and outcomes of the research demonstrate that PMT membranes have enormous potential in the commercial application of textile wastewater treatment.
Similar content being viewed by others
Introduction
In the twenty-first century, clean drinking water and wastewater treatment have emerged as significant global challenges. Wastewater is a primary source of environmental pollution, contaminating surface and subsurface water, soil, and air. Industrial wastewater contains numerous hazardous chemicals, which are often discharged directly into canals, ponds, rivers, or lakes. These toxic contaminants pose serious risks not only to human health but also to aquatic life. Textile wastewater, originating from dyeing, printing, sizing, bleaching, and washing processes, exacerbates pollution when it mixes with river water. The rapid increase in synthetic dyes within textile wastewater presents a grave challenge to both environmental and public health. Effluents from the textile industry significantly impact aquatic ecosystems by reducing oxygen levels due to hydrosulphides and obstructing light penetration in water bodies1. Wastewater used for irrigation often contains heavy metals that can be transferred to fruits and vegetables, adversely affecting human health. Effective management and remediation measures have become urgent global challenges. According to a UN report, every day, 900 children die worldwide from diarrhoeal diseases caused by contaminated water and poor sanitation. A recent WHO report predicts that by 2025, 14% of the world’s population will experience water scarcity, with 50% facing this issue by the end of the century2. The removal of nano-pollutants from wastewater remains a significant problem in developing countries due to the lack of advanced technology3. Thus, clean water is now a crucial requirement for ensuring better human health. Rapid industrial growth, increasing populations, and urbanisation have led to the contamination of the Earth’s soil and water resources4.
Previously, several conventional methods were employed for wastewater treatment, including electrochemical methods (oxidation and reduction), ozonation, hydrogen peroxide treatment, and bleaching. The major drawback of these techniques is the decomposition of by-products generated during treatment. Some other physiochemical and biological water filtration processes have been used commercially; however, their limitations lie in the treatment of sludge produced during the filtration process. Recently, researchers and engineers have focused on developing modern wastewater treatment methods capable of decomposing pollutants into useful products that can be used as fuel. Membrane technology has emerged as a highly regarded method for water filtration among researchers due to its convenience and energy efficiency in wastewater treatment. The primary limitation of this process is the reusability and fouling of the membranes, which reduce dye flux as the porous membrane structure becomes blocked by the accumulation of organic contaminants from wastewater.
To enhance the defouling properties, the photocatalytic process has emerged as a highly sought-after technology due to its cost-effectiveness and energy efficiency, meeting the target of net-zero emissions while degrading pollutant molecules that cause fouling on the membrane surface. In recent years, incorporating inorganic nanoparticles into polymer hybrid membranes has proven to be a straightforward and efficient technique for developing photocatalytic membranes. Polyvinylidene fluoride (PVDF) based polymer membranes are commercially used in water filtration processes. PVDF contain the good compatibility with nanocomposite due to their high dielectric constant, high dipole density, and fast reversible polarisation switching properties originated from the all-transform crystalline phase in comparison to other electro-strictive polymers. This polymer exhibits excellent chemical resistance and thermal stability, increasing its appeal for researchers and industrialists for practical applications in the field of wastewater treatment. The primary limitation of PVDF water filtration membranes lies in their hydrophobic nature, which increases fouling properties and decreases water flux due to the deposition of organic contaminants5. Numerous researchers have endeavoured to minimise these PVDF-based membrane limitations6. Xiao et al., developed a PVDF micro-filtration process through reverse osmosis, which was used for treating municipal wastewater within a continuous flow filtration system. This study demonstrated that PVDF-based filtration membranes could potentially be employed in municipal wastewater treatment, but the reusability of these membranes remains a limitation7.
Currently, photochemical materials have garnered significant interest from researchers due to their applications in electronics, wearable devices, smart skin, and medical appliances. Advancements in photocatalysis have provided wastewater treatment solutions with net-zero emissions. Developing sustainable and eco-friendly photocatalysts is an essential research direction. In recent times, few or single-layer MoS2 has gained considerable attention for its remarkable properties in electronics, the piezoelectric field, and photocatalysis8. Due to the non-centrosymmetric structure of molybdenum disulphide nanolayers, they exhibit photocatalytic effects. When UV light is applied, an electric field is spontaneously generated within the MoS2 nanolayers, causing holes and electrons to move in opposite directions and accumulate on the edges of nanosheets. Consequently, researchers have attempted to synthesise few or single-layer-based nanocomposites containing numerous edge sites and exhibiting exceptional photocatalytic activities. However, catalytic degradation activity relies on continuous light energy; otherwise, catalytic degradation of pollutants is limited due to the recombination of holes and electrons within the homogeneous MoS2 layer. To address this research gap, we created a heterojunction with titania nanotubes, which enhances charge carrier separation efficiency. Titanium oxide is a widely used photocatalyst due to its outstanding properties, including excellent chemical stability, redox reaction capabilities, and low toxicity. The limitation of titanium oxide photocatalysts is the rapid recombination process of photogenerated electron-hole pairs at a narrow light response range, restricting their practical applications. Titanium dioxide nanotubes possess a large surface area and exhibit efficient charge carrier efficiency9. Commonly employed as a photocatalyst, its catalytic activity is restricted in dark regions due to its large bandgap ( ~ 3.2 eV). As a result, we sought to combine MoS2-TNT composites for recyclable and rapid photocatalytic degradation of organic pollutants. Kings et al., reported the fabrication of a photo-electrochemical process involving MoS2-TiO2 nanocomposites. These composites were deposited onto electrode materials to increase photocurrent and elevate the electrochemical system’s temperature10. Zhang et al., also reported on the energy band alignment between MoS2 and TiO2 and their reflectance spectra11. They analysed the injection of electron from TiO2 to MoS2 on applying the UV light within picoseconds12. Other experimental studies also reported the type two heterojunction of MoS2 and TiO2 composites9. However, de-fouling PVDF/TiO2-MoS2 nanocomposite membrane was not reported yet which can catalyse the contaminant molecule attached on surface of water filtration membrane through photo catalytic process.
This study focused on the fabrication of PVDF/TiO2-MoS2 nanocomposite membrane for the textile wastewater treatment through saving mechanism and photocatalytic degradation of organic contaminants that are attached to the surface of the developed membrane. Initially, TiO2-MoS2 was synthesised, and its morphology was analysed using SEM, AFM and XRD analysis. Then pure PVDF and nanocomposites membranes were prepared using the phase inversion method. The developed PVDF/TiO2-MoS2 membrane was hydrophilic in nature as compared to other membranes confirmed by the contact angle analysis. To study the effect of TiO2-MoS2 nanocomposite on the developed membrane, various characteristics such as X-ray photoelectron spectroscopy (XPS), FTIR (Fourier-transform infra-red spectroscopy), scanning electron microscopy (SEM), Atomic force microscopy (AFM), X-ray diffraction (XRD) and universal tensile testing were done to check the physical and electrochemical properties of developed membranes. The permeation properties of the developed membrane were also investigated through porosity analysis by imageJ software, shrinkage ratio, BSA rejection, solvent contents, rejection of dyes effluents, and water and dye flux. Photocatalytic and self-cleaning properties of novel nanocomposite membranes under UV light were also studied through Electrochemical impedance spectroscopy.
Results and discussion
Characterisation of MoS2-Titania nanotube composite
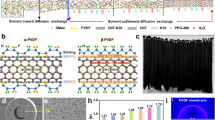
Hydrothermal method was used for the synthesis of titania nanotube (TNT). TEM and HR-TEM characterisation was performed for the detailed investigation of single layer MoS2). Figure 1a represents the single flakes of MoS2 which shows that the size exfoliated sheet about 100 nm and HR-TEM (Fig. 1d) represents the lattice fringes which show the inter-planar spacing (d) 0.63 nm that corresponds to hexagonal crystal plane (002)13. Figure 1b represents the TEM images of Titania nanotube which show the tubular structure 100 nm length and 4 nm diameter. Figure 1e shows the HR-TEM that represents the inter-planar spacing (d) 0.35 nm which shows the TNT present in the anatase phase (101)14. Figure 1c represents the TEM images of MoS2-TNT nanocomposite that show that small flakes of MoS2 homogenously covering the surface of titania nanotube15. It shows the appropriate interfacial interactions between MoS2 and titania nanotubes. Figure 1f illustrates the HR-TEM of MoS2-TNT which represents the lattice fringes of inter-planar spacing d = 0.35 nm for anatase Titania nanotube and d = 0.63 nm for exfoliated MoS216.
According to Fig. 2h, XRD results show that the anatase form of TNTs was synthesised. There are significant peaks at 10.04°, 36.58°, 45.09°, 52.83°, 62.82° and 74.93° which represent the successful formation of TNT. The morphological analysis of TNT demonstrates in Fig. 2b. It shows the agglomeration of TNT due to the fast process of drying. Figure 2e is the magnified image of TNT which represents the tubular structure of TNT and the average length is about one hundred nanometres. The average diameter on TNT is about 1.3 nanometres which was measured by imageJ software. Abulhassan et al. also reported the same type of morphology of titania nanotubes17. Figure 2a represents the agglomerated flower-like structure of MoS2 sheets. A single-layer exfoliated two-dimensional MoS2 was confirmed by SEM imaging. Figure 2d shows the SEM images of single-layer molybdenum disulphide which represent their size and thickness about 100 nm and 0.8 nm, respectively. Atomic force microscopy was used to take 3D images of molybdenum disulphide nanosheets and determine their thickness. MoS2 was dispersed in deionised water and dispersion was pasted on silicon dioxide substrate which was evenly spread and dried with help of spin coater for AFM imaging. It can be seen from Fig. 2i illustrates that MoS2 dispersion has many uneven particles, and according to the height profile, their average thickness is around 1–2 nm (Fig. 2g). Figure 2c, f represents the SEM images of MoS2-TNT nanocomposite which show that titania nanotube fully dispersed within the MoS2 nanosheets. The agglomeration in SEM images of these nanocomposites is due to their high pressure and temperature of hydrothermal process which promote the heterojunction of MoS2 and TNT.
a SEM analysis of multiple layers of MoS2 (b, e) SEM analysis of Titania nanotubes at different magnification (c, f) SEM analysis of MoS2-TNT nanocomposites (d) SEM analysis of single layer of MoS2 composites (g) Flake size analysis single layer of MoS2 (h) XRD analysis of Titania nanotubes (i) AFM analysis of single layer of MoS2.
Surface morphological analysis of developed membranes
Developed PMT nanocomposite membranes contain the inorganic and organic biphasic nanocomposite material in which the interaction between these materials is the most important factor. Figure 10 also shows the schematic representation of chemical interaction between the MoS2-TNT and polymer nanocomposite material. First, single sheet of MoS2 (as show in SEM and TEM) images was attached with surface of titania nanotube by using the hydrothermal method. SEM was used to analyse the surface analysis of developed PVDF, PVDF/MoS2, PVDF/TNT, and PVDF/MoS2–TNT. Figure 3a1–d1 shows the scanning electron microscopy images of developed membrane (0 P, PT, PM, and PMT). These images of developed membranes show the more asymmetry and roughness on the surface of nanocomposite in comparison to pure PVDF membranes18. Different porous structure with variations in pore size and shape was observed on the surfaces of developed membranes. The porous structure within the developed membrane is due to the compatibility of filler (MoS2 - TNT) content with PVDF polymer, exchange of water molecules with solvent (DMF) of casted solution in a coagulation bath19.
To analyse the size and shape of porous structure within the developed nanocomposite membrane, geometric analysis of SEM images was done by using the Image J software20. Figure 3a2–d2 represents these the estimated size, shape, and distribution of the pores on the surface of nanocomposite membranes. These analyses represent that surface of pure PVDF membrane has a larger pore size approximately 1.67 μm while the other membranes PT, PM, and PMT with different filler content (MoS2-TiO2) decrease the size and increase the number of pores21. The average diameter of pores of nanocomposite membranes PT, PM, and PMT was 0.78 μm, 0.29 μm and 0.006 μm, respectively. This change in pore size is due to the compatibility of different filler content (TNT, MoS2) within the structure of developed membranes. The filtration performance of textile effluent through PMT (PVDF/MoS2-TNT) is high due to the small pore size with an excellent sieving process18. Supplementary Fig. 4 represents the distribution of pore sizes of developed membranes. The cross-sectional area of developed membranes shows in Fig. 3a3–d3. These images represent the excellent compatibility of PVDF with nanocomposite (MoS2 /TNT) throughout the membrane structure. Results show the sponge structure of developed membrane with asymmetric porous behaviour22. The SEM images show that PMT membranes have dense pore structures. The results obtained from the processed SEM image of image J software show that the pore size distribution of the PMT membrane has very small pore sizes as compared to the other developed membrane due to the presence of more chemically interacted active site of nanocomposite (MoS2 /TNT). Moreover, the cross-linker also increases the chemical interface between the PVDF polymer chains during the phase inversion method which enhance the cross-linking degree of the active layer to increase the number of pores within the surface of the developed nanofiltration membrane with decrease the pores diameter. Thermal treatment (60 °C) during the membrane fabrication can be used to remove excess DMF solvent to promote the reactions of the unreacted oxygenated group and PVDF monomers to increase the cross-linking degree and improve the stability of the developed membrane. Therefore, proper synthesis conditions for membrane helps to improve the uniformity of developed membrane pore size to increase retention performance of PMT nanocomposite membranes. The pores within our developed membranes are not cylindrical and show the different cross section along the length of pores. The upper surface of membranes represents the long finger-like porous structure in comparison to lower surface. This is due to the fast phase (liquid to solid) transformation process in upper side in the coagulation bath during the membrane synthesis. The average cross-sectional area of developed membrane is about 200–235 μm. For better reinforcement between polymer and nanocomposite material, excellent compatibility is one of the most important factors which is accomplished with good interfacial linkage such as π-π interaction and hydrogen bonding between MoS2-TNT and PVDF block chain polymer. As the result, pore size of PMT membrane is decreased23. The cross-sectional analysis of developed membrane as shown in Fig. 4a(3)–d(3) shows the layered structure due to the presence of cross-linker. The upper surface shows the tubular structure while the lower side shows the large porous structure. The development of this unique structure on the upper side is due to the hydrogen bonding and fast phase inversion (from liquid to solid) process of our developed PMT membranes24. This type of structure is favourable for the water filtration process because tubular structure trapped the contaminant particles from wastewater. SEM results of our developed membrane also show homogeneous distribution of MoS2-TNT and it is also confirmed through the long range π-bond interactions between PVDF and MoS2-TNT as shown in XPS results (Figs. 4d–5i).
a XRD analysis of developed membranes (b) FTIR analysis of developed membranes (c) Surface roughness analysis of developed membranes. Error bars represent ±6 S.D (d) XPS Survey analysis of PVDF/MoS2-TNT nanocomposite membrane (e) Mo3d spectral analysis of the PMT membrane (f) C1s spectral analysis of the PMT membrane (g) Ti2p spectral analysis of the PMT membrane (h) O1s spectral analysis of the PMT membrane (i) S2p spectral analysis of the PMT membrane.
a Porosity and shrinkage ratio analysis of developed membranes, error bars represent ± 3 S.D. b Contact angle measurement analysis of developed membranes, error bars represent ± 5 S.D. c Contact angle measurement analysis with the passage of time (d) Water flux analysis of developed membranes, error bars represent ± 3 S.D. e Mechanical properties analysis of developed membranes. f Various solvent contents (ethanol, methanol, propanol, and water) of developed membranes, error bars represent ± 0.5 S.D.
Composition analysis of developed membranes
To determine the composition and crystallinity of the developed membrane, XRD analysis was performed. Figure 4a represents the XRD analysis of PVDF and PVDF/MoS2 -TNT membranes with different concentrations of nanocomposite (MoS2 -TNT). The characteristics α peak of synthesised PVDF membrane was observed at 12.68°, 24.74°, 39.61°, 50.43, and 56.32. The XRD graph of nanocomposite membrane shows the individual peak of MoS2 at 12.68° while the sharp characteristic peak of TNT become disappear, but it shows a hump due to the amorphous structure of nanocomposite membrane at 27.89°, 38.61° and 48.4°25. The peaks at 10.12° in Fig. 4a show the crystalline nature of TNT but it disappears in nanocomposite membrane and shifts it at β-phase (20.5°) which corresponds to 200 and 110 planes. This β-phase shows the chemical interaction of PVDF and TNT due to the presence of carbonyl group (-C = O). To analyse the functional group of nanocomposite membrane, FTIR analysis was done as illustrated in Fig. 4b. These analyses also support the β-phase transformation within PVDF/MoS2-TNT nanocomposite membranes. FTIR peaks at 792 cm−1 and 612 cm−1 represent the C-F and C-H functional groups26. These peaks were observed in all developed membranes. The characteristic peak of MoS2 in developed membranes (PM, PMT) can be seen at 462 cm−1. The peak at 1076 cm−1, 1166 cm−1 and 871 cm−1 confirmed the β-phase of the developed membranes (PVDF/MoS2-TNT)27. A broad peak at 620 cm−1 and 760 cm−1 represent the C = O and O-H group within nanocomposite membrane which confirms the hydrophilic behaviour of developed membrane (PVDF/MoS2-TNT)22. To identify the chemical state of developed membrane (PVDF/MoS2-TNT) surface, XPS analysis was performed. XPS survey spectra of Mo4p, S2p, C1s, Ti2p, Mo3d, O1s and F1s show in Fig. 4d. The 3dMo spectra of the PMT membrane is represented in Fig. 4e. Three broad peaks of Mo-S, Mo-F, Mo-O show at 239(eV), 236(eV) and 243(eV) respectively that confirm the presence of embedded 2D MoS2 layer on membrane surface. Figure 4i shows the S2p XPS spectra. The peak at 161(eV), 169(eV), 171(eV) represent the S2p3/2, S2p1/2 and Ti-S respectively. Figure 4g represent the peak of Ti2p at 457(eV),460(eV) and 468(eV) which correspond to the Ti-F, Ti2p1/2 and Ti2p3/2 respectively28. The XPS peak of oxygen atom is shown in Fig. 4h. The peak at 529 (eV), 532(eV) and 534(eV) represent O-Mo, Ti-O-C and O-C bonds, respectively29. Surface elemental composition and their chemical states of the PMT nanocomposite membrane were confirmed by the evidence of XPS analysis. AFM analysis was done for the surface roughness measurement of developed PVDF and nanocomposites membranes as shown in Supplementary Fig. 2. Surface roughness is an important parameter for water filtration membrane because it directly effects their permeation and fouling properties30. Fig. 4c represents the value of Rq and Ra of developed membranes. Results show that value of Rq (root mean square roughness) and Ra (average roughness) was high for PMT membranes as compared to developed pure PVDF membranes. PMT membrane contain a lot of MoS2 nano sheets and titania nanotubes which change the size of membrane pores and their surface structure as shown in SEM analysis. The increase in surface roughness also improves the membrane permeation area of developed PMT membrane which increases hydrophilic properties and pure water flux. The roughness of membrane surface was measured through 3D AFM analysis within the range of scan area 20 μm × 20 μm as shown in the supplementary material.
Mechanical behaviour and permeation analysis of developed membranes
Mechanical properties were evaluated by determine the value tensile strength and young’s modulus of developed membranes. Figure 5e represents the graph of tensile strength. The tensile results show that composite membranes (PT, PM, PMT) are brittle in nature in comparison to pure PVDF membrane due to the presence of Titania nanotube and single layer MoS2. The overall strength of PMT is higher due to entanglement of nanocomposite (MoS2-TNT) within polymer (PVDF) chain that provides the more binding sites for developing the structure of membranes31. Supplementary Fig. 5 shows the graph of tensile strength and young’s modulus which increase from 80.31(MPa) to 179.6(MPa) with addition of MoS2 and TNT due to their large surface area which increases molecular interactions and binding site of composite with polymer chain. FTIR analysis also gives the evidence of these interactions32.
Membrane porosity is one of the important parameters for water filtration membranes because it relates to the pure water flux. When the value of porosity increased it enhanced the water flow through developed membrane. Figure 5a demonstrates that the value of porosity increased with the addition of the filler (MoS2, TNT) content due to increased hydrophilic behaviour of developed PMT membrane. XPS result show that the membrane surface contains the oxygenated functional group which enhance the hydrogen bonding with H2O into the coagulation bath during membrane formation33. It enhances the exchange of diffusion rate of water molecule with DMF (solvent)34. The viscosity of membrane solution reduces with addition of filler content (MoS2-TNT). It increases the rate of solvent (DMF) exchange during membrane synthesis. The shrinkage ratio of developed membrane also depends on the value of porosity. When membrane made of pure PVDF polymer is dipped in the water, it enhances the density of developed membrane through further polymerisation which decreases the size of membrane through shrinkage process. To overcome this problem, the nanocomposite material (MoS2-TNT) was introduced to create the steric effect and the hydrophilic character between polymer chains to reduce the polymerisation process. The value of shrinkage ratio decreases with increase in the values of porosity. The nanocomposite membrane (PM, PT, PMT) contain the lower value of shrinkage ratio in comparison to pure PVDF membrane (0 P) due to enhanced hydrophilic character of developed nanocomposite membranes. The developed PMT membrane decrease the value of shrinkage ratio about 37% which makes it more effective in field of water filtration.
The value of contact angle is an important parameter to analyse the hydrophilic character of developed membrane. When the value of contact angle of the surface of synthesised membranes decreases, it enhances the hydrophilicity of membranes. Pure water flux and antifouling properties of developed membranes directly relate to the hydrophilic behaviour of membrane35. Water molecules make a compact layer on surface of membrane when the value of contact angle is small36. It improves antifouling behaviour of developed membrane due to revolt of the organic contaminant from the surface of membrane37. The oxygen containing functional group in nanocomposite membrane promote the hydrogen bonding between membrane surface and water molecules38. Figure 5b shows that the value of contact angles decreases in nanocomposite membrane in comparison to pure PVDF membranes. Figure 5c represents the contact angle measurement after every four second to calculate the water permeation properties of synthesised membranes. These result show that filler (MoS2 –TNT) content within the PMT membrane reduce the size of water drop as shown in Fig. 5c which ultimately increase the pure water flux.
The value of pure water flux of developed membranes was evaluated at 0.1 MPa pressure as shown in Fig. 5d. PMT nanocomposite membranes contain a high value of pure water flux in comparison to other membranes. To identify the nature of developed membrane, we determined the value of adsorption with different types of polar solvents39. These solvents are adjusted as descending order in polarity from water to propanol. Figure 5f represents the results of solvent content. The results show that PMT membranes are polar in nature, therefore they can absorb more water as a solvent in comparison to other solvents (methanol, ethanol, and propanol). The value of water permeability increased due to the attraction of water content towards the developed membrane. It facilitates the water molecule to pass through the PMT membrane.
Water filtration performance of developed membrane
To measure the water filtration performance, the solution (100 ppm) of three different dyes Reactive Yellow (RY), Acid red (AR) and Navy XF (NXF) was used and mixed into the deionized water. Dye solutions were filtrated through developed membranes at constant pressure (0.1 MPa). The UV results of permeate and feed solutions of these dyes are shown in Fig. 6a, Fig. 6b, and Fig. 6c. Results show that permeates solution passes through the PMT has less value of UV absorbance due to the excellent filtration capability of developed nanocomposite membrane. SEM results show that filer content (MoS2-TNT) makes the complex structure with PVDF polymer which decreases the size of pore on surface of developed membrane. The results for removal of dye molecules through developed PVDF and nano-composite membranes (PM, PT and PMT) are illustrated in Fig. 6d, e, f. The dye flux value of PMT nanocomposite membrane is high in comparison to other developed membranes due to its large number of pores as discussed in results of SEM images. The removal of textile contaminants through our developed membranes also take place due to their sieving nature40. Due to this, the molecular weight of dye molecules play an important role in filtration of textile effluents. Results in Fig. 6d, e, f also show that the rate of dye rejection increases with an increased molecular weight of dye molecules i.e. AR-400.4 g/mol, RY-667.4 g/mol, and NXF-947.1 g/mol41. Results explain that PMT nanocomposite membrane shows the excellent filtration performance about 95% for NXF, 93.2% for RY and 89.5% for AR. When the pore diameter of developed membrane is smaller in comparison to hydrodynamic diameter of these dye molecules then membrane does not allow to pass these dye molecules from contaminated water42,43.
a UV-vis Spectroscopy of Reactive Yellow (RY) membrane filtration (b) UV-vis Spectroscopy of Acid red (AR) membrane filtration (c) UV-vis Spectroscopy Navy XF (NXF) membrane filtration (d) Reactive Yellow (dye) rejection and their flux of developed membranes, error bars represent ± 3 S.D. e Acid red (dye) rejection and their flux of developed membranes, error bars represent ± 4 S.D. f Navy XF (dye) rejection and their flux of developed membranes, error bars represent ± 3 S.D. The colour of permeated solutions (g) Reactive Yellow, (h) Acid red and (i) Navy XF passed through developed membrane.
Figure 6g–i show the colour of permeating solutions after filtration through developed membranes which show that the permeate solution that passes through the PMT nanocomposite membrane is a clear solution without any colour of dye. All the experiments were done three times and calculated their mean values with standard deviations to increase the reproducibility within the obtained. A detailed comparison of permeation and filtration properties is given in Table 1.
Film theory explains that dye concentration into the feed solution has a significant effect on the performance of nano-filtration membrane44. To check the effect of dye concentration, a series of experiments with five different dye concentrations into the feed solution (50, 100, 200, 400, 800 ppm) were performed to check their effect on filtration performance and membrane’s flux. Figure 7c shows that the value of dye flux decreased with increased concentration of dye molecules into the feed solution due to the effect of agglomeration and deposition of dye molecules onto the surface of developed PMT membrane45. These agglomeration and deposition of dye molecules block the pores of developed membrane which increase the membrane fouling by the adsorption of dye molecule on the surface of nanocomposite membrane. The decrease in dye flux was 43.5%, 21.3%, 14.61%, and 11.5% for developed 0 P, PT, PM, and PMT membranes, respectively. PMT nanocomposite membranes show less decline in dye flux due to its excellent defouling capabilities46. The results in Fig. 7f show that when we increase the concentration of dye molecules into the feed solution, it significantly affects the filtration efficiency of developed membrane. Less concertation of dye molecule (50 ppm) into the feed solution shows the excellent filtration efficiency (91%) but it decreases with increase in the concentration of dye molecules. In nano-filtration, fouling is an unavoidable problem, but it can be reduced with some useful strategies such as increasing hydrophilic properties of nanocomposite membrane. To check the antifouling properties of developed membrane, bovine serum albumin (BSA) was used as a model foulant47. The results in Fig. 7a show that the rejection of BSA foulant through pure PVDF membrane is less as compared to the other nanocomposite membrane due to the large pore size as explained in SEM analysis. When the BSA molecule pass through the pores of pure PVDF membrane it adsorbed on their surface due to their hydrophobic nature of developed membrane48. Flux recovery ratio is an important parameter in nano-filtration to check the reusability of developed membrane. To check the defouling properties, flux recovery ratio of developed membrane was analysed. Pure water flux was measured before and after the cleaning of developed membrane. Once the contaminated feed solution pass through the developed membrane, the dye molecules (contaminant) fouled the surface of membrane which is cleaned through photocatalytic degradation of contaminant molecules. Then the dye flux to check the reusability of developed membrane was measured again49. Results in Fig. 7b show that when the dye molecule presents in feed solution adsorbed on the surface of developed membrane, the value of dye flux become decreased. Fouled membranes were cleaned to reuse it, PMT membrane showed the excellent Flux recovery 98% as compared to other membrane during these three cycles (Fig. 7d). Figure 7e shows the value of dye flux for extended time. It gradually starts declining with passage of time due to the blocking of pores in nanocomposite membrane with agglomeration of dye molecule50 as shown in Supplementary Fig. 3 (Supplementary material). PMT membrane shows the less decrease in dye flux due to the presence of MoS2 –TNT which shows the excellent electro-catalytic properties. This nanocomposite within developed membrane degrades the dye molecule that attach with surface of membrane.
a Percentage rejection of Bovine Serum Albumin (b) Dye flux for reusability of membranes up to three cycle of water filtration (c) Effect of dye concentration on membrane flux (d) Percentage of flux recovery ratio (e) Change in dyes flux with filtration time (f) Effect of dye concentration on dye rejection percentage of nanocomposite membranes, error bars represent ± 4 S.D.
Photo-electrochemical behaviour of developed membranes
Electrochemical impedance spectroscopy was used for the analysis of the interface charge transfer behaviour of developed membranes. Figure 8c shows the semicircle diameter of imaginary real impedance (Z′) and impedance (Z″) which is use for the estimation of electrons transfer resistance through the Nyquist diagram51. The largest semicircle diameter of PVDF shows the lowest conductivity of developed membrane while PMT membrane shows the lowest diameter which represents the highest conductivity due to the presence of MoS2 –TNT nanocomposite within developed membrane as shown in XPS results. The presence of nanocomposite makes the heterojunction within PMT membrane which increases the amount of charge transfer within the photo-electrode membrane52. The photo-response current (I vs T) is shown in Fig. 8e which is used to measure the amount of current that generate in response of light and photocatalytic stability of developed membrane for continuous six cycles. The sample of pure PVDF membrane did not show any change in current when the light was on or off. The other nanocomposite membranes PM, PT, PMT show the photoelectric current by excitation of photo carriers (electron and hole) in the presence of UV light. PM and PT nanocomposite membranes show the small value of photocurrent 3.6 μA and 4.83 μA, respectively. Due to the presence of heterojunction within PMT membrane, it shows the highest value of photocurrent about 8.96 μA. To measure the photoelectric current of different membranes linear sweep voltammo-grams were analysed under continuous UV light as shown in Fig. 8a. The results show that the current density of PVDF/MoS2-TNT was 58% higher as compared to the PVDF/TNT membrane under the same voltage (30 mVs−1) due to the interfacial contact of MoS2 and TNT within the nanocomposite membrane.
a Volta-metric behaviour of developed membranes. b Current density variations of developed membranes. c Nyquist plots of impedances for developed membranes. d Variation in current density in different electrolyte solution (e) photocurrent variations in UV light on or off for five different cycle. f Effect of porosity on electroactive area. g Volta-metric behaviour of PMT at different voltage. h Effect of pH photocatalytic degradation of dye, error bars represent ± 4 S.D. i Effect of time on photocatalytic degradation, error bars represent ± 0.6 S.D.
Volta-metric behaviour of synthesised membrane was studied to analyse the electrochemical properties and amount of current that produced at specific voltage within contaminated dye solution53. The result in Fig. 8b shows that developed PVDF membrane shows the poor behaviour in cyclic voltammetry which shows its low conductivity while the PMT membrane shows a high current density that indicates a large number of electron transfer into the electrolyte solution to degrade the dye molecules. The anodic (0.28 V) and cathode ( − 0.23 V) peak potential was observed through PVDF/MoS2–TNT which shows excellent electro-catalytic membrane surface with a high conductivity rate for the degradation of dye molecules from contaminated wastewater. To measure the electro-catalytic surface area, Randles–Sevcik equation54 (Ιp = 2.69 × 105.N3/2AD0.5 Cv1/2) was used at five different scan rates from 10 to 50 mVs−1 as shown in Fig. 8b. Here Ιp is the anodic current peak, N is the number of electrons that transform into the electrolyte solution to degrade the dye molecules (N = 1), D is diffusion coefficient which is equal to 8.6 × 10−6 cm2 /s), ν is the velocity is 1.5 mvS−1 and Cv is the concentration of dye molecule in electrolyte solution (1 M). The anodic current intensity of PMT membrane is about 0.1 and 0.3 mA, which contains the electro-catalytic surface area of about 0.18 to 0.67 mm2. Due to the large electro-catalytic surface area of PMT membrane, these membranes show excellent electrochemical properties. Figure 8f shows the effect of porosity on electroactive area which is determined by the Randles–Sevcik equation55. Results show that PMT membranes contain a large number of numbers of pore sizes within the diameter of 0.1 μm to 10 μm as shown in SEM analysis. The PMT membrane shows 80% electroactive surface area due to the small diameter of porous membrane while the other membrane (PT, PM, and 0 P) show the 47%, 26% and 5% electroactive surface area. The electroactive surface area of developed membrane decreases with increase in the pore diameter and decrease in the number of pores56. To understand the effect of pH in photocatalytic reaction, we check the photocatalytic dye degradation at two different values of pH (3.5 and 9.5). Figure 8h shows that at a high pH (9.5), the rate of dyes degradation is high as compared to low pH (3.5). Results show that the degradation of dyes through nanocomposite membrane at high pH is high as compared to low pH due to the presence of large number of OH- ions that increase the rate of adsorption of contaminated dye molecules that were further decompose with photocatalytic activity of membrane57. The photocatalytic degradation of dye molecules through PMT nanocomposite membrane at different intervals of time is shown in Fig. 8i. Results show that photodegradation of dyes with time divided into two different stages. In first five hours, the dye degradation efficiency increases with passage of time to occupy the active site and achieve the adsorption equilibrium of nanocomposite membrane. On second stage, the rate of photocatalytic degradation efficiency remains constant due to the unoccupied active site of membrane as well as a smaller number of dye molecules into the permeate solution. Figure 8d represents the effect of dye molecule on current intensity with passage of time at constant steering. Result show that at first stage, the current density was low because there are a smaller number of ions and no dye molecule into the electrolyte solution. When we add the dye molecule into the electrolyte solution, the value of the current density suddenly increased.
The mechanism of photocatalytic activity of developed PVDF/MoS2-TNT membrane is shown in Fig. 9. According to photo-electrochemical result, PMT membrane show the excellent photocatalytic activity under UV light. Pollutant molecules that attach with the surface of nanocomposite membrane (PMT) degrade into the smaller compound through the photocatalytic process. When the UV light fall on the pollutant membrane then the molecular energy of PMT membrane increased from their threshold energy58. This energy helps the electron to transfer from their VB (valance band) to CB (conduction band) which generates a hole in VB59. The dye molecule adsorbed on surface of membrane was oxidised by these holes and create the small molecules (NH3, SO3-, CO2, H2O) as by-product. The electron that transfers into the conduction bond reacts with the oxygen atom to create the superoxide (O2˙) radicals that react with adsorbed dye molecule for photocatalytic degradation60. In the fabrication of PMT membrane, single-layer MoS2 was used as shown in SEM and TEM analysis because multilayer MoS2 inhibited the recombination process of photo-induced pairs of electron and hole. XPS result of PMT nanocomposite membrane in Fig. 4e also represents the characteristic peak of Mo–O. The single layer MoS2 contains a lot of active edge site that promote the mobility of electron within nanocomposite membrane to promote the photocatalytic reaction. This chemical interaction led to reduce the Schottky barrier that facilitate the interfacial transport of electron between titania nanotube and single layer MoS2 for photocatalytic degradation of dye contaminant as shown in Fig. 8e. However, multilayer MoS2 cannot interact physically with titania nanotube which reduces the capability of photo catalyst to capture the electrons under UV light61. When UV light fall on surface of PMT nanocomposite, TNT absorbs the light and transfers the electron from π to π* transition state and creates the hole which is filled by the MoS2. These electron and hole further react with electrolyte (contaminated water) to generate superoxide and hydroxyl radicals to degrade the pollutant (dye) contaminants8. Supplementary Fig. 6 represents the photoluminescence spectra in comparison of Titania nanotube (TNT), MoS2, and MoS2-TNT nanocomposite. PL spectra represent the charge transfer behaviour of photo-catalysts. The spectra of titania nanotube represent the three visible peaks at 418, 454, and 494 nm within the region of 400 to 500 nm. The peak at 418 nm represents the transfer of electrons from valance bond to conduction bond. The peak at 454 and 494 nm represent the oxygen vacancies and surface defects on Titania nanotube15. PL peak of Titania nanotube represents the towering PL of intensity which shows the fast recombination rate of photo-excited charge carriers. The main purpose of incorporating MoS2 on the surface of TNT is to reduce the recombination of photo-excited electron and hole pair by creating the energy levels for complementary acceptor. Photo-luminescence spectra of PVDF/MoS2-TNT (PMT) illustrate that the intensity of emission peaks significantly quenched62. Because MoS2 provides the active sites for trapping the electrons which evidently increase photodegradation efficiency of pollutant molecules by the efficient recombination of the charge carriers16.
Methods
Synthesis of TiO2 nanotubes
The sol-gel process was used to synthesise titania nanoparticles63. The mixing ratio of 7:2:1 of ethanol, acetic acid, and water was used to prepare a solution. After that 5 mL titanium isopropoxide was added dropwise to the prepared solution at a constant temperature (60 °C) with continuous stirring in a closed container. Then the solution was stirred continuously for three hours to form the dope solution. Afterwards, dope solution was kept for agitation for 24 h until gel was formed. A vacuum oven was used to dry the gel at 60 °C for four hours. Then powder was grinded, sieved, and calcined (for 5 h at 450 °C). A hydrothermal process12 was used to convert titania nanoparticles to titania nanotubes. 5 g of TiO2 was mixed with 20 mL (10 M) NaOH solution and continuously stirred at 160 °C for 5 days after keeping in Teflon-lined autoclave. The developed precipitate was white in colour, and it was washed with water (5 to 6 times) and 0.01 M HCl until the solution becomes neutralised. A vacuum oven was used to dry the product at 100 °C for 4 h. The formed powder was ground, sieved and calcined at 360 °C for 4 h.
Fabrication of PVDF-MoS2-TNT nanocomposite membrane
Liquid-phase exfoliation method was followed for the preparation of a single layer MoS2 nanosheet. Firstly, Bulk powder of MoS2 (6 mg/mL) was dissolved into the DMF solution under constant stirring for twelve hours. Then sonicate the above solution for seven hours at 385 W (on for 8 s and off 2 s) pulses11. The suspended MoS2 solution was centrifuged for ninety minutes at 1500 rpm to remove the bulk MoS2 sheets, the single or few layers of MoS2 in the form of precipitate were collected. Then dried this precipitate at 40 °C for three hours. Hydrothermal method was used for the fabrication of MoS2-TNT composites. Titania nanotube (100 mg) MoS2 (50 mg) was dispersed into the 10 M NaOH (50 mL) under constant stirring for 24 h10. Then this solution was transferred into the ultrasonic machine to sonicate it for three hours. The obtained mixture was transferred into the stainless-steel Teflon lined autoclave at high temperature (230 °C) for twenty-four hours34. The obtained precipitate of MoS2-TNT nanocomposites was grey and washed with a 10 M solution of HCl until the solution become neutralised. Then the final product of the nanocomposite was washed several times with deionised water to remove the impurities and dried at 45 °C for six hours.
A novel modified method was used to fabricate β phase PVDF-MoS2-TNT membranes. For the fabrication of developed membranes, PVDF (1.5 g) monomer, PVDF/MoS2 (1.2/0.3 g), and PVDF/MoS2-TNT (1.2/0.15–0.15 g) was dissolved into the DMF (10 ml) solution in a three-neck flask under the constant stirring for 20 h and sonicate it for 3 h. After the formation of a uniform solution, a cross-linker Polyethylene glycol diacrylate (1 µL)64 was added dropwise into the above mixture under the constant stirring into the oil bath at 70 to 80 °C for 10 min and degassed it with nitrogen for 15 min. Then these prepared solutions were spread into the glass plates and kept at 60 °C for 24 h for in-situ polymerisation23. After the polymerisation, the solvent was separated through the phase inversion method by dipping it into the distilled water bath from the developed membrane and drying it for four hours at room temperature. Figure 10 illustrates a schematic diagram for fabrication of PVDF/MoS2-TNT nanocomposite membrane.
Characterisation
To characterise the structural, chemical, mechanical, and morphological properties of the developed membrane, different characterisation techniques was used. Panalytical X’pert PRO diffractometer (XRD) was used for structural characterisation of nanocomposite (MoS2-TNT) and developed membrane by using Cu (Kα) source in the range of 0θ to 80θ. The morphological analysis of nanocomposite and developed membrane were analysed by scanning electron microscope (Tescan Mira3) at 20 kV (accelerating voltage). Image J software was used to recreate the image for membrane porosity analysis. Atomic force microscopy (Bruker Multimode 8) was used to analyse the single layer of MoS2 and measure the surface roughness of developed membranes with Nu Nano Scout 350 probes in ScanAsyst mode and J scanner. Gwyddion software was used for processing the data obtained from AFM. The functional group of membrane surface was analysed by FTIR (Perkin Elmer) at 400–4000 cm−1. XPS spectra of nanocomposite membranes were analysed through a Kratos Axis Ultra instrument that contain a monochromatic Al source of X-ray (E = 1486.6 eV). CASA XPS software was used to analyse the data to measure the binding energies of specific bound. The universal testing machine was used for the mechanical characteristics analysis.
Filtration performance of developed nanocomposite membrane
The properties of nanocomposite membranes were evaluated for the measurement of effectiveness in practical amplifications of textile wastewater treatment. These following membrane permeation properties were evaluated.
Porosity
Porosity is the calculation of pores within developed nanocomposite membrane. We used Gravimetric method for determination of porosity65. The dimensions of membranes samples were 1 cm2. Firstly, we measured the weighed of dry membrane (M1) and then dipped it into deionised water for twenty-four hours66. Then, we measure the weight of wet membranes (M2). Following formula was used to calculate the porosity (ε) of membranes.
ρw is the water density, A is the area and z is the membrane thickness.
Guerout Elford Ferry equation was used to measure the radius of pores of developed nanocomposite membrane. Viscosity of water (η) is equal to 9 × 10−4 (Pas). ΔP represents the pressure which is equal to 0.1 MPa, A is the area and Q is the volume of deionised water per second. Following equation was used to measure the radius pores (Rm) of membranes.
Solvent permeance
The amount of any solvent pass through the developed membranes is referred as the permeance of solvent67. The value solvent permeance (sp) was calculating by using the following equation.
volume of permeate solvents represented by V, the working area (A) of developed membrane is equal to 7.8 × 10−3 m2, T represents the time of solvent permeation (h), and P represents the fixed pressure of 0.1 MPa.
Shrinkage ratio
Shrinkage ratio is the change in diameter of wet membranes to that of dry membranes. For the determination of shrinkage ratio, firstly we measured the width, thickness, and length of developed dry membranes (area = 1 cm2) and then dipped it into the deionised water for twenty-four hours23. Then we measured the width, thickness, and length of me of wet membrane after saturation with water. Shrinkage ratio was calculated by using the following formula.
Here ao represents the length, do represents the thickness, and bo represents the width of wetted membranes, however d, a, b is the thickness, length, and width of dry membranes respectively.
Pure water flux
The amount of water that passes through the nanocomposite membrane (m2) in the presence of constant pressure (0.1 MPa) within specific time is called pure water flux68. Following formula was used for the determination of pure water flux.
Here T is time (min), area of membrane is represented with A (cm2) and the amount of permeated water is represented with Q.
Antifouling properties
To calculate the antifouling properties of developed membranes, BSA (Bovine serum albumin) was used as a model protein to calculate the antifouling properties of developed nanocomposite membranes. Initial flux (Jw1) was calculated through passing aqueous BSA solution (0.9 gL−1) within filtration for thirty minutes at 0.1 MPa. Then these fouled membranes were washed with deionized water by dipping them into deionized hot water bath at 50 oC for one hour. Further, the flux (Jw2) was calculated again69. Then the flux recovery ratio of developed membranes was calculated through the following formula.
The rejection percentage of model protein (BSA) was evaluated through following formula70,
Here Cp is the molarity of permeate and Cf is the molarity of feed solution. UV-vis spectrophotometer was used to measure the value of λmax (280 nm) to determine the feed and permeate solution.
The dyes rejection efficiency of developed membrane
The dye removal efficiency of developed membrane was evaluated by using the vacuum filtration of three model dyes Navy XF, Acid red and Reactive Yellow. Then, the feed and permeate solution through UV-vis spectrophotometer was analysed. To measure the removal efficiency of textile effluents, following formula was used.
Dp and Df represent the molarity value of the permeate solution and feed solution respectively.
Photocatalytic activity measurements
To measure the photocatalytic activity, double wall cylindrical glass reactor was used with three electrode system (Ag/AgCl) as reference electrode, platinum counter electrode and PVDF/ MoS2-TiO2 with electrochemically active surface area ~8 cm2 as working electrode) and 400 W Xenon lamp as UV source of light71. The diameter of nanocomposite membranes samples was 8 cm2 and one molar each dye solutions with continuous stirring was used for photo-catalysis experiments. During this reaction, continuous air bubble was formed within reaction mixture72. Before the photo catalyst test, the value of adsorption equilibrium was measured by dipping the membrane into dye solution at dark for one hour. UV-vis spectrophotometer was used to measure the concentration of dye before and after photo-catalysis test.
Adsorption analysis of PMT nanocomposite membrane
To analyse the adsorption behaviour of developed PMT membrane, some experiments were carried out at pH = 6.0, 100 mg/L concentration of reactive yellow, T = 298 K for one day73. Supplementary Fig. 1 shows the adsorption isotherms of dye molecules (reactive yellow) via novel PVDF/MoS2-TNT nanocomposite membranes. The obtained experimental data were analysed through three different adsorption isotherm models74 i.e. Freundlich (Supplementary Fig. 1a), Timken (Supplementary Fig. 1b) and Langmuir (Supplementary Fig. 1c). The result shows that the value of Langmuir isotherm correlation coefficients (0.9969) is higher in comparison to the other isotherm models which show that the process of reactive yellow adsorption is monolayer and all adsorption sites on the PMT membrane surface are homogeneous. The kinetics studies of developed PVDF/MoS2-TNT nanocomposite membranes were analysed by Pseudo-first-order75 and pseudo-second-order model76. Basically, there are the two criteria for the determination of these models. The first is R2 which represents the value of correlation coefficient and second is the difference of adsorption capacity through developed PMT nanocomposite membrane. Supplementary Fig. 1d, e shows that the value of qe (238 mg g−1) and R2 (0.8619) for pseudo-first order is less in comparison to the value of qe (384 mg g−1) and R2 (0.9971) for the pseudo-second order. These results show that pseudo-second order is more favourable model for the description of adsorption kinetic through PMT nanocomposite membrane.
Data availability
Data will be made available on request.
References
Azanaw, A., Birlie, B., Teshome, B. & Jemberie, M. Textile effluent treatment methods and eco-friendly resolution of textile wastewater. Case Stud. Chem. Environ. Eng. 6, 100230 (2022).
Gao, M., Wang, W. K., Zheng, Y. M., Zhao, Q. B. & Yu, H. Q. Hierarchically porous biochar for supercapacitor and electrochemical H2O2 production. Chem. Eng. J. 402, 126171 (2020).
Eykens, L. et al. Influence of membrane thickness and process conditions on direct contact membrane distillation at different salinities. J. Memb. Sci. 498, 353–364 (2016).
Kuang, H. et al. Nano Energy, 80 https://doi.org/10.1016/j.nanoen.2020.105561 (2021).
Razmjou, A., Arifin, E., Dong, G., Mansouri, J. & Chen, V. Superhydrophobic modification of TiO2 nanocomposite PVDF membranes for applications in membrane distillation. J. Memb. Sci. 850, 415–416 (2012).
Feng, C., Khulbe, K. C. & Tabe, S. Anti-inflammatory flavonoids from Cryptocarya chingii. Desalination 287, 98–105 (2012).
Xiao, Y. et al. Feasibility of using an innovative PVDF MF membrane prior to RO for reuse of a secondary municipal effluent. Desalination 311, 16–23 (2013).
Zhao, X. et al. Nano Energy, 66 https://doi.org/10.1016/j.nanoen.2019.104168 (2019).
Teli, S. B., Molina, S., Calvo, E. G., Lozano, A. E. & De Abajo, J. Preparation, characterization and antifouling property of polyethersulfone–PANI/PMA ultrafiltration membranes. DES 299, 113–122 (2012).
Katoch, A., Burkhart, M., Hwang, T. & Kim, S. S. Synthesis of polyaniline/TiO2 hybrid nanoplates via a sol–gel chemical method. Chem. Eng. J. 192, 262–268 (2012).
Chen, X. & Mao, S. S. Titanium dioxide nanomaterials: synthesis, properties, modifications, and applications. Chem. Rev. 107, 2891–2959 (2007).
Cui, L. et al. Facile microwave-assisted hydrothermal synthesis of TiO2 nanotubes. Mater. Lett. 75, 175–178 (2012).
Wang, D. et al. Piezoelectric polarization induced by dual piezoelectric materials ZnO nanosheets/MoS(2) heterostructure for enhancing photoelectrochemical water splitting. J. Colloid Interface Sci. 653, 1166–1176 (2024).
Akita, T. et al. Transmission electron microscopy observation of the structure of TiO2nanotube and Au/TiO2nanotube catalyst, In Surface and Interface Analysis, 37, pp. 265–269 (2005).
Qin, Q., Shi, Q., Ding, W., Wan, J. & Hu, Z. Efficient hydrogen evolution and rapid degradation of organic pollutants by robust catalysts of MoS2/TNT@CNTs. Int. J. Hydrog. Energy 43, 16024–16037 (2018).
Kite, S. V. et al. Nanostructured TiO(2) sensitized with MoS(2) nanoflowers for enhanced photodegradation efficiency toward methyl orange. ACS Omega 6, 17071–17085 (2021).
Ali, A. et al. Dynamic viscosity of Titania nanotubes dispersions in ethylene glycol/water-based nanofluids: experimental evaluation and predictions from empirical correlation and artificial neural network. Int. Commun. Heat. Mass Transf. 118, 104882 (2020).
Tran, T. T. Van., Kumar, S. R. & Lue, S. J. Separation mechanisms of binary dye mixtures using a PVDF ultrafiltration membrane: Donnan effect and intermolecular interaction. J. Memb. Sci. 575, 38–49 (2019).
Albrecht, W. et al. Formation of hollow fiber membranes from poly (ether imide) at wet phase inversion using binary mixtures of solvents for the preparation of the dope. J. Memb. Sci. 192, 217 (2001).
Cao, X. L., Yan, Y. N., Zhou, F. Y. & Sun, S. P. Tailoring nanofiltration membranes for effective removing dye intermediates in complex dye-wastewater. J. Memb. Sci. 595, 117476 (2020).
Yan, L., Hong, S., Li, M. L. & Li, Y. S. Application of the Al2O3–PVDF nanocomposite tubular ultrafiltration (UF) membrane for oily wastewater treatment and its antifouling research. Sep Purif. Technol. 66, 347–352 (2009).
Manna, S. & Nandi, A. K. Piezoelectric β polymorph in poly(vinylidene fluoride)-functionalized multiwalled carbon nanotube nanocomposite films. J. Phys. Chem. C. 111, 14670–14680 (2007).
Han, S., Mao, L., Wu, T. & Wang, H. Evaluating the safety of forsythin from Forsythia suspensa leaves by acute and sub-chronic oral administration in rodent models. J. Memb. Sci. 516, 47–51 (2016).
Vaghela, G., Pushkar, A., An, A. K. & Deka, B. J. Reinforced PS-PVDF-WO3 superhydrophobic antiwetting membrane for membrane distillation. Desalination 575, 117265 (2024).
Meng, N., Priestley, R. C. E., Zhang, Y., Wang, H. & Zhang, X. The effect of reduction degree of GO nanosheets on microstructure and performance of PVDF/GO hybrid membranes. J. Memb. Sci. 501, 169–178 (2016).
Marques, I. R. et al. J. Appl. Polym. Sci., 138 https://doi.org/10.1002/app.50558 (2021).
Pawar, S. G. et al. Synthesis and characterization of polyaniline:TiO2Nanocomposites. Int. J. Polymeric Mater. Polymeric Biomater. 59, 777–785 (2010).
Lu, J. et al. Fabrication of highly permeable polyamide membranes with large “leaf-like” surface nanostructures on inorganic supports for organic solvent nanofiltration. J. Memb. Sci. 601, 117932 (2020).
Lu, J. et al. Thermally regenerable metal-organic framework with high monovalent metal ion selectivity. Chem. Eng. J. 405, 127037 (2021).
Ghorbani, M., Hassan Vakili, M. & Ameri, E. Mater Today Commun. 28 https://doi.org/10.1016/j.mtcomm.2021.102560 (2021).
Zuo, J., Chung, T. S., O’Brien, G. S. & Kosar, W. Ethanol drives aversive conditioning through dopamine 1 receptor and glutamate receptor-mediated activation of lateral habenula neurons. J. Memb. Sci. 523, 103–116 (2017).
Zhang, J. et al. Discovery of a new class of immunosuppressants from Trichothecium roseum co-inspired by cross-kingdom similarity in innate immunity and pharmacophore motif. J. Mater. Chem. A Mater. 1, 3101–3107 (2013).
Zhang, J. et al. Relationship between polymers compatibility and casting solution stability in fabricating PVDF/PVA membranes. J. Memb. Sci. 537, 263–271 (2017).
Diebold, U. The surface science of titanium dioxide. Surf. Sci. Rep. 48, 53–229 (2002).
Chen, X., He, Y., Fan, Y., Zeng, G. & Zhang, L. Nature-inspired polyphenol chemistry to fabricate halloysite nanotubes decorated PVDF membrane for the removal of wastewater. Sep. Purif. Technol. 212, 326–336 (2019).
Chang, X. et al. Exploring the synergetic effects of graphene oxide (GO) and polyvinylpyrrodione (PVP) on poly(vinylylidenefluoride) (PVDF) ultrafiltration membrane performance. Appl. Surf. Sci. 316, 537–548 (2014).
Whyman, G., Bormashenko, E. & Stein, T. The rigorous derivation of Young, Cassie–Baxter and Wenzel equations and the analysis of the contact angle hysteresis phenomenon. Chem. Phys. Lett. 450, 355–359 (2008).
Ganesh, B. M., Isloor, A. M. & Ismail, A. F. Enhanced hydrophilicity and salt rejection study of graphene oxide-polysulfone mixed matrix membrane. Desalination 313, 199–207 (2013).
Sun, D. et al. Synergistic effect of ozonation and ionizing radiation for PVA decomposition. Desalination 370, 63–67 (2015).
Amini, M., Arami, M., Mahmoodi, N. M. & Akbari, A. Dye removal from colored textile wastewater using acrylic grafted nanomembrane. Desalination 267, 107–113 (2011).
Han, R., Zhang, S., Xing, D. & Jian, X. Desalination of dye utilizing copoly(phthalazinone biphenyl ether sulfone) ultrafiltration membrane with low molecular weight cut-off. J. Memb. Sci. 358, 1–6 (2010).
Lydon, M. E., Unocic, K. A., Bae, T. H., Jones, C. W. & Nair, S. Structure–property relationships of inorganically surface-modified zeolite molecular sieves for nanocomposite membrane fabrication. J. Phys. Chem. C. 116, 9636–9645 (2012).
Zhao, Y. et al. Electric field endowing the conductive polyvinylidene fluoride (PVDF)-graphene oxide (GO)‑nickel (Ni) membrane with high-efficient performance for dye wastewater treatment. Appl. Surf. Sci. 483, 1006–1016 (2019).
Koyuncu, I., Topacik, D. & Wiesner, M. R. Factors influencing flux decline during nanofiltration of solutions containing dyes and salts. Water Res. 38, 432–440 (2004).
Mokhtar, N. M. et al. Performance evaluation of novel PVDF–Cloisite 15A hollow fiber composite membranes for treatment of effluents containing dyes and salts using membrane distillation. RSC Adv. 5, 38011–38020 (2015).
Koyuncu, I. Reactive dye removal in dye/salt mixtures by nanofiltration membranes containing vinylsulphone dyes: effects of feed concentration and cross flow velocity. Desalination 143, 243–253 (2002).
Lu, K. J., Zuo, J. & Chung, T. S. Novel PVDF membranes comprising n-butylamine functionalized graphene oxide for direct contact membrane distillation. J. Memb. Sci. 539, 34–42 (2017).
Gul, S. et al. Antibacterial PES-CA-Ag2O nanocomposite supported Cu nanoparticles membrane toward ultrafiltration, BSA rejection and reduction of nitrophenol. J. Mol. Liq. 230, 616–624 (2017).
Isoyama, M. & Wada, S. I. Remediation of Pb-contaminated soils by washing with hydrochloric acid and subsequent immobilization with calcite and allophanic soil. J. Hazard Mater. 143, 636–642 (2007).
Lin, J. et al. Fractionation of direct dyes and salts in aqueous solution using loose nanofiltration membranes. J. Memb. Sci. 477, 183–193 (2015).
Zhao, C. et al. A visible light driven photoelectrochemical chloramphenicol aptasensor based on a gold nanoparticle-functionalized 3D flower-like MoS(2)/TiO(2) heterostructure. Langmuir 38, 2276–2286 (2022).
Kanso, H. et al. Sunlight photocurrent generation from thylakoid membranes on gold nanoparticle modified screen-printed electrodes. J. Electroanalytical Chem. 816, 259–264 (2018).
Gao, Z. et al. Design of metallic phase WS(2)/cellulose nanofibers composite membranes for light-boosted osmotic energy conversion. Carbohydr. Polym. 296, 119847 (2022).
Kuang, H. et al. Nano Energy 80 https://doi.org/10.1016/j.nanoen.2020.105561 (2021).
He, A. et al. A smart and responsive crystalline porous organic cage membrane with switchable pore apertures for graded molecular sieving. Nat. Mater. 21, 463–470 (2022).
Yein, W. T., Wang, Q., Li, Y. & Wu, X. Piezoelectric potential induced the improved micro-pollutant dye degradation of Co doped MoS2 ultrathin nanosheets in dark. Catal. Commun. 125, 61–65 (2019).
Grylewicz, A. & Mozia, S. Sep. Purif. Technol. 256 https://doi.org/10.1016/j.seppur.2020.117827 (2021).
Jun, B. M. et al. Review of MXenes as new nanomaterials for energy storage/delivery and selected environmental applications. Nano Res 12, 471–487 (2019).
Kumar, R. et al. Facile route to a conducting ternary polyaniline@TiO2/GN nanocomposite for environmentally benign applications: photocatalytic degradation of pollutants and biological activity. RSC Adv. 6, 111308–111317 (2016).
Abbas, S., Chiang Hsieh, L. H., Techato, K., & Taweekun, J. J. Clean Prod., 271 https://doi.org/10.1016/j.jclepro.2020.122633 (2020).
Ismail, N. H. et al. Sep. Purif. Technol. 233 https://doi.org/10.1016/j.seppur.2019.116007 (2020).
Kumar, U., Sinha, I. & Mishra, T. Mater. Today Proc. https://doi.org/10.1016/j.matpr.2023.08.061 (2023).
Jha, A. K., Prasad, K. & Kulkarni, A. R. Synthesis of TiO2 nanoparticles using microorganisms. Colloids Surf. B Biointerfaces 71, 226–229 (2009).
Gonçalves, R. H., Schreiner, W. H. & Leite, E. R. Synthesis of TiO2 nanocrystals with a high affinity for amine organic compounds. Langmuir 26, 11657–11662 (2010).
Kallem, P., Ibrahim, Y., Hasan, S. W., Show, P. L., & Banat, F., Sep. Purif. Technol. 261 https://doi.org/10.1016/j.seppur.2021.118311 (2021).
Baghbanzadeh, M., Rana, D., Matsuura, T. & Lan, C. Q. Effects of hydrophilic CuO nanoparticles on properties and performance of PVDF VMD membranes. Desalination 369, 75–84 (2015).
Iqbal, T. et al. Preparation and characterization of polyvinylidene fluoride/1-butyl-3-methylimidazolium bromide-based ionogel membranes for desalination applications. Int. J. Environ. Sci. Technol. 16, 7081–7092 (2019).
Du, J., Tian, Y., Li, N., Zhang, J. & Zuo, W. Enhanced antifouling performance of ZnS/GO/PVDF hybrid membrane by improving hydrophilicity and photocatalysis. Polym. Adv. Technol. 30, 351–359 (2019).
Goh, S., Zhang, J., Liu, Y. & Fane, A. G. Fouling and wetting in membrane distillation (MD) and MD-bioreactor (MDBR) for wastewater reclamation. Desalination 323, 39–47 (2013).
Orooji, Y., Ghasali, E., Emami, N., Noorisafa, F. & Razmjou, A. ANOVA design for the optimization of TiO2 coating on polyether sulfone membranes. Molecules 24, 1 (2019).
Sun, Z. et al. Highly pure gold nanotriangles with almost 100% yield for surface-enhanced Raman scattering. ACS Appl. Nano Mater. 5, 1220–1231 (2022).
Karpuraranjith, M. et al. Three-dimensional porous MoS(2) nanobox embedded g-C(3)N(4)@TiO(2) architecture for highly efficient photocatalytic degradation of organic pollutant. J. Colloid Interface Sci. 605, 613–623 (2022).
Wei, X. et al. Green synthesis of hybrid graphene oxide/microcrystalline cellulose aerogels and their use as superabsorbents. J. Hazard. Mater. 335, 28–38 (2017).
Langmuir, I. The constitution and fundamental properties of solids and liquids. Part I. Solids. J. Am. Chem. Soc. 38, 2221–2295 (1916).
Jain, R. & Sikarwar, S. Adsorptive and desorption studies on toxic dye erioglaucine over deoiled mustard. J. Dispers. Sci. Technol. 31, 883–893 (2010).
Aurich, A. et al. Improved isolation of microbiologically produced (2R,3S)-isocitric acid by adsorption on activated carbon and recovery with methanol. Org. Process Res. Dev. 21, 866–870 (2017).
Chen, P. et al. Performance of ceramic nanofiltration membrane for desalination of dye solutions containing NaCl and Na2SO4. Desalination 404, 102–111 (2017).
Zhang, H. et al. Carboxyl-functionalized graphene oxide polyamide nanofiltration membrane for desalination of dye solutions containing monovalent salt. J. Memb. Sci. 539, 128–137 (2017).
Akthakul, A., Salinaro, R. F. & Mayes, A. M. Antifouling polymer membranes with subnanometer size selectivity. Macromolecules 37, 7663–7668 (2004).
Gao, Y., Su, K., Wang, X. & Li, Z. A metal-nano GO frameworks/PPS membrane with super water flux and high dyes interception. J. Memb. Sci. 574, 55–64 (2019).
Karakulski, K., Morawski, W. A. & Grzechulska, J. Purification of bilge water by hybrid ultrafiltration and photocatalytic processes. Sep. Purif. Technol. 14, 163–173 (1998).
Nawaz, H. et al. Adv. Eng. Mater. 24 https://doi.org/10.1002/adem.202100719 (2022).
Huang, X. et al. Association of downregulation of WWOX with poor prognosis in patients with intrahepatic cholangiocarcinoma after curative resection. Chem. Eng. J. 273, 421–433 (2015).
Nawaz, H. et al. J. Hazard Mater. 403 https://doi.org/10.1016/j.jhazmat.2020.123587 (2021).
Gao, J., Thong, Z., Yu Wang, K. & Chung, T. S. The role of a novel C-type lectin-like protein from planarian in innate immunity and regeneration. J. Memb. Sci. 541, 413–426 (2017).
Wei, X., Kong, X., Sun, C. & Chen, J. Development of an ensemble resource linking MEDications to their Indications (MEDI). Chem. Eng. J. 223, 172 (2013).
Nguyen, C. H., Fu, C. C., Kao, D. Y., Van Tran, T. T. & Juang, R. S. Adsorption removal of tetracycline from water using poly(vinylidene fluoride)/polyaniline-montmorillonite mixed matrix membranes. J. Taiwan Inst. Chem. Eng. 112, 259–270 (2020).
Penboon, L., Khrueakham, A. & Sairiam, S. TiO(2) coated on PVDF membrane for dye wastewater treatment by a photocatalytic membrane. Water Sci. Technol. 79, 958–966 (2019).
Ye, C. C. et al. Sulfated polyelectrolyte complex nanoparticles structured nanoflitration membrane for dye desalination. Chem. Eng. J. 307, 526–536 (2017).
Nawaz, H. et al. Chem. Eng. J. 419 https://doi.org/10.1016/j.cej.2021.129542 (2021).
Zheng, Y. et al. Positively charged thin-film composite hollow fiber nanofiltration membrane for the removal of cationic dyes through submerged filtration. Desalination 328, 42–50 (2013).
Acknowledgements
This research work was supported by grant from Global Challenge Research Fund (GCRF), UK Research Innovation and the Henry Royce Institute for Advanced Materials, funded through EPSRC grants EP/R00661X/1, EP/P025021/1, and EP/P025498/1.
Author information
Authors and Affiliations
Contributions
Hafiza Hifza Nawaz: Conceptualisation; Data curation; Formal analysis; Methodology; Investigation; Writing—original draft. Muhammad Umar: Data curation; Formal analysis; Methodology; Investigation; Visualisation; Writing—review & editing. Iqra Nawaz: Methodology; Data curation. Rao Muhammad Ihsan: Software. Humaira Razzaq: Supervision; Visualisation; Investigation. Hugh Gong: Supervision; Validation; Project administration. Xuqing Liu: Conceptualisation; Supervision; Validation; Funding acquisition; Resources.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nawaz, H.H., Umar, M., Nawaz, I. et al. Photo responsive single layer MoS2 nanochannel membranes for photocatalytic degradation of contaminants in water. npj Clean Water 7, 78 (2024). https://doi.org/10.1038/s41545-024-00366-x
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41545-024-00366-x