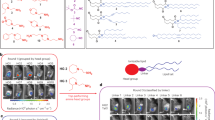
Abstract
Targeting the delivery of vaccines to dendritic cells (DCs) is challenging. Here we show that, by mimicking the fast and strong antigen processing and presentation that occurs during the rejection of xenotransplanted tissue, xenogeneic cell membrane-derived vesicles exposing tissue-specific antibodies can be leveraged to deliver peptide antigens and mRNA-encoded antigens to DCs. In mice with murine melanoma and murine thymoma, xenogeneic vesicles encapsulating a tumour-derived antigenic peptide or coated on lipid nanoparticles encapsulating an mRNA coding for a tumour antigen elicited potent tumour-specific T-cell responses that inhibited tumour growth. Mice immunized with xenogeneic vesicle-coated lipid nanoparticles encapsulating an mRNA encoding for the spike protein of severe acute respiratory syndrome coronavirus 2 elicited titres of anti-spike receptor-binding domain immunoglobulin G and of neutralizing antibodies that were approximately 32-fold and 6-fold, respectively, those elicited by a commercialized mRNA–lipid nanoparticle vaccine. The advantages of mimicking the biological recognition between immunoglobulin G on xenogeneic vesicles and fragment crystallizable receptors on DCs may justify the assessment of the safety risks of using animal-derived biological products in humans.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The data supporting the results in this study are available within the paper and its Supplementary Information. The raw and analysed datasets generated during the study are available for research purposes from the corresponding authors on reasonable request. Source data are provided with this paper.
References
Rappuoli, R., Mandl, C. W., Black, S. & De Gregorio, E. Vaccines for the twenty-first century society. Nat. Rev. Immunol. 11, 865–872 (2011).
Chen, S. et al. Nanotechnology-based mRNA vaccines. Nat. Rev. Methods Primers 3, 63 (2023).
Barbier, A. J., Jiang, A. Y., Zhang, P., Wooster, R. & Anderson, D. G. The clinical progress of mRNA vaccines and immunotherapies. Nat. Biotechnol. 40, 840–854 (2022).
Wculek, S. K. et al. Dendritic cells in cancer immunology and immunotherapy. Nat. Rev. Immunol. 20, 7–24 (2020).
Steinman, R. M. & Banchereau, J. Taking dendritic cells into medicine. Nature 449, 419–426 (2007).
Pulendran, B. & Ahmed, R. Immunological mechanisms of vaccination. Nat. Immunol. 12, 509–517 (2011).
Pollard, A. J. & Bijker, E. M. A guide to vaccinology: from basic principles to new developments. Nat. Rev. Immunol. 21, 83–100 (2021).
De Koker, S. et al. Designing polymeric particles for antigen delivery. Chem. Soc. Rev. 40, 320–339 (2011).
Guerrini, G., Magrì, D., Gioria, S., Medaglini, D. & Calzolai, L. Characterization of nanoparticles-based vaccines for COVID-19. Nat. Nanotechnol. 17, 570–576 (2022).
Baden, L. R. et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 384, 403–416 (2021).
Chaudhary, N., Weissman, D. & Whitehead, K. A. mRNA vaccines for infectious diseases: principles, delivery and clinical translation. Nat. Rev. Drug Discov. 20, 817–838 (2021).
Wang, Z. et al. Exosomes decorated with a recombinant SARS-CoV-2 receptor-binding domain as an inhalable COVID-19 vaccine. Nat. Biomed. Eng. 6, 791–805 (2022).
Kim, J. et al. Injectable, spontaneously assembling, inorganic scaffolds modulate immune cells in vivo and increase vaccine efficacy. Nat. Biotechnol. 33, 64–72 (2015).
Huang, W.-C. et al. A malaria vaccine adjuvant based on recombinant antigen binding to liposomes. Nat. Nanotechnol. 13, 1174–1181 (2018).
He, L. et al. Presenting native-like trimeric HIV-1 antigens with self-assembling nanoparticles. Nat. Commun. 7, 12041 (2016).
Gong, N. et al. Proton-driven transformable nanovaccine for cancer immunotherapy. Nat. Nanotechnol. 15, 1053–1064 (2020).
Yin, Q. et al. A TLR7-nanoparticle adjuvant promotes a broad immune response against heterologous strains of influenza and SARS-CoV-2. Nat. Mater. 22, 380–390 (2023).
Lee, J. et al. In vivo fate and intracellular trafficking of vaccine delivery systems. Adv. Drug Deliv. Rev. 186, 114325 (2022).
Rohner, E., Yang, R., Foo, K. S., Goedel, A. & Chien, K. R. Unlocking the promise of mRNA therapeutics. Nat. Biotechnol. 40, 1586–1600 (2022).
Chen, J. et al. Lipid nanoparticle-mediated lymph node-targeting delivery of mRNA cancer vaccine elicits robust CD8+ T cell response. Proc. Natl Acad. Sci. USA 119, e2207841119 (2022).
Krauson, A. J., Casimero, F. V. C., Siddiquee, Z. & Stone, J. R. Duration of SARS-CoV-2 mRNA vaccine persistence and factors associated with cardiac involvement in recently vaccinated patients. npj Vaccines 8, 141 (2023).
Lee, J., Woodruff, M. C., Kim, E. H. & Nam, J.-H. Knife’s edge: balancing immunogenicity and reactogenicity in mRNA vaccines. Exp. Mol. Med. 55, 1305–1313 (2023).
Trougakos, I. P. et al. COVID-19 mRNA vaccine-induced adverse effects: unwinding the unknowns. Trends Mol. Med. 28, 800–802 (2022).
Zin Tun, G. S., Gleeson, D., Al-Joudeh, A. & Dube, A. Immune-mediated hepatitis with the Moderna vaccine, no longer a coincidence but confirmed. J. Hepatol. 76, 747–749 (2022).
Bachmann, M. F. & Jennings, G. T. Vaccine delivery: a matter of size, geometry, kinetics and molecular patterns. Nat. Rev. Immunol. 10, 787–796 (2010).
Wang, W. et al. Dual-targeting nanoparticle vaccine elicits a therapeutic antibody response against chronic hepatitis B. Nat. Nanotechnol. 15, 406–416 (2020).
Xia, Y. et al. Exploiting the pliability and lateral mobility of Pickering emulsion for enhanced vaccination. Nat. Mater. 17, 187–194 (2018).
Kuai, R., Ochyl, L. J., Bahjat, K. S., Schwendeman, A. & Moon, J. J. Designer vaccine nanodiscs for personalized cancer immunotherapy. Nat. Mater. 16, 489–496 (2017).
Liu, C. et al. A nanovaccine for antigen self-presentation and immunosuppression reversal as a personalized cancer immunotherapy strategy. Nat. Nanotechnol. 17, 531–540 (2022).
Conniot, J. et al. Immunization with mannosylated nanovaccines and inhibition of the immune-suppressing microenvironment sensitizes melanoma to immune checkpoint modulators. Nat. Nanotechnol. 14, 891–901 (2019).
Salomon, R. et al. Bispecific antibodies increase the therapeutic window of CD40 agonists through selective dendritic cell targeting. Nat. Cancer 3, 287–302 (2022).
Wang, H. et al. Metabolic labeling and targeted modulation of dendritic cells. Nat. Mater. 19, 1244–1252 (2020).
Bros, M. et al. The protein corona as a confounding variable of nanoparticle-mediated targeted vaccine delivery. Front. Immunol. 9, 1760 (2018).
Mo, J., Xie, Q., Wei, W. & Zhao, J. Revealing the immune perturbation of black phosphorus nanomaterials to macrophages by understanding the protein corona. Nat. Commun. 9, 2480 (2018).
Vu, V. P. et al. Immunoglobulin deposition on biomolecule corona determines complement opsonization efficiency of preclinical and clinical nanoparticles. Nat. Nanotechnol. 14, 260–268 (2019).
Reddy, S. T. et al. Exploiting lymphatic transport and complement activation in nanoparticle vaccines. Nat. Biotechnol. 25, 1159–1164 (2007).
Du, Y., Zhou, H., Su, G., Ma, M. & Liu, Y. Protein corona-driven nanovaccines improve antigen intracellular release and immunotherapy efficacy. J. Control. Release 345, 601–609 (2022).
Sykes, M. & Sachs, D. H. Progress in xenotransplantation: overcoming immune barriers. Nat. Rev. Nephrol. 18, 745–761 (2022).
Jaycox, J. R., Dai, Y. & Ring, A. M. Decoding the autoantibody reactome. Science 383, 705–707 (2024).
Anand, R. P. et al. Design and testing of a humanized porcine donor for xenotransplantation. Nature 622, 393–401 (2023).
Baker, K., Rath, T., Lencer, W. I., Fiebiger, E. & Blumberg, R. S. Cross-presentation of IgG-containing immune complexes. Cell. Mol. Life Sci. 70, 1319–1334 (2013).
Baker, K. et al. Neonatal Fc receptor for IgG (FcRn) regulates cross-presentation of IgG immune complexes by CD8−CD11b+ dendritic cells. Proc. Natl Acad. Sci. USA 108, 9927–9932 (2011).
Giodini, A., Rahner, C. & Cresswell, P. Receptor-mediated phagocytosis elicits cross-presentation in nonprofessional antigen-presenting cells. Proc. Natl Acad. Sci. USA 106, 3324–3329 (2009).
Clatworthy, M. R. et al. Immune complexes stimulate CCR7-dependent dendritic cell migration to lymph nodes. Nat. Med. 20, 1458–1463 (2014).
Rogers, N. J., Mirenda, V., Jackson, I., Dorling, A. & Lechler, R. I. Costimulatory blockade by the induction of an endogenous xenospecific antibody response. Nat. Immunol. 1, 163–168 (2000).
Wu, F. et al. Fluorescence imaging of the lymph node uptake of proteins in mice after subcutaneous injection: molecular weight dependence. Pharm. Res. 29, 1843–1853 (2012).
Roseboom, I. C., Rosing, H., Beijnen, J. H. & Dorlo, T. P. C. Skin tissue sample collection, sample homogenization, and analyte extraction strategies for liquid chromatographic mass spectrometry quantification of pharmaceutical compounds. J. Pharm. Biomed. Anal. 191, 113590 (2020).
Hu, C.-M. J. et al. Nanoparticle biointerfacing by platelet membrane cloaking. Nature 526, 118–121 (2015).
Fang, R. H., Kroll, A. V., Gao, W. & Zhang, L. Cell membrane coating nanotechnology. Adv. Mater. 30, 1706759 (2018).
Zhai, Y. et al. T lymphocyte membrane-decorated epigenetic nanoinducer of interferons for cancer immunotherapy. Nat. Nanotechnol. 16, 1271–1280 (2021).
Elechalawar, C. K., Hossen, M. N., McNally, L., Bhattacharya, R. & Mukherjee, P. Analysing the nanoparticle–protein corona for potential molecular target identification. J. Control. Release 322, 122–136 (2020).
Brasel, K., De Smedt, T., Smith, J. L. & Maliszewski, C. R. Generation of murine dendritic cells from flt3-ligand-supplemented bone marrow cultures. Blood 96, 3029–3039 (2000).
Quah, B. J. C., Warren, H. S. & Parish, C. R. Monitoring lymphocyte proliferation in vitro and in vivo with the intracellular fluorescent dye carboxyfluorescein diacetate succinimidyl ester. Nat. Protoc. 2, 2049–2056 (2007).
Li, A. W. et al. A facile approach to enhance antigen response for personalized cancer vaccination. Nat. Mater. 17, 528–534 (2018).
Shin, M. D. et al. COVID-19 vaccine development and a potential nanomaterial path forward. Nat. Nanotechnol. 15, 646–655 (2020).
Aldén, M. et al. Intracellular reverse transcription of Pfizer BioNTech COVID-19 mRNA vaccine BNT162b2 in vitro in human liver cell line. Curr. Issues Mol. Biol. 44, 1115–1126 (2022).
Herrmann, I. K., Wood, M. J. A. & Fuhrmann, G. Extracellular vesicles as a next-generation drug delivery platform. Nat. Nanotechnol. 16, 748–759 (2021).
Du, Y. et al. Intranasal administration of a recombinant RBD vaccine induced protective immunity against SARS-CoV-2 in mouse. Vaccine 39, 2280–2287 (2021).
Neerukonda, S. N., Vassell, R., Weiss, C. D. & Wang, W. Measuring neutralizing antibodies to SARS-CoV-2 using lentiviral spike-pseudoviruses. Methods Mol. Biol. 2452, 305–314 (2022).
Porrett, P. M. et al. First clinical-grade porcine kidney xenotransplant using a human decedent model. Am. J. Transplant. 22, 1037–1053 (2022).
Montgomery, R. A. et al. Results of two cases of pig-to-human kidney xenotransplantation. N. Engl. J. Med. 386, 1889–1898 (2022).
Moazami, N. et al. Pig-to-human heart xenotransplantation in two recently deceased human recipients. Nat. Med. 29, 1989–1997 (2023).
Griffith, B. P. et al. Genetically modified porcine-to-human cardiac xenotransplantation. N. Engl. J. Med. 387, 35–44 (2022).
Patience, C., Takeuchi, Y. & Weiss, R. A. Infection of human cells by an endogenous retrovirus of pigs. Nat. Med. 3, 282–286 (1997).
Łopata, K., Wojdas, E., Nowak, R., Łopata, P. & Mazurek, U. Porcine endogenous retrovirus (PERV)—molecular structure and replication strategy in the context of retroviral infection risk of human cells. Front. Microbiol. 9, 730 (2018).
Denner, J. Recent progress in xenotransplantation, with emphasis on virological safety. Ann. Transplant. 21, 717–727 (2016).
Acknowledgements
This work was supported by the National Key Research & Development Program of China (grant nos. 2021YFA1201000, 2023YFC2605000, 2024YFA1208301, 2024YFA1212400), National Natural Science Foundation of China (NSFC) (grant nos. 32030060, 82430067, GG9100007028, 22477118). The authors also appreciate the support by the Science Fund for Creative Research Groups of Nature Science Foundation of Hebei Province (B2021201038). This work was also supported by the NSFC (grant no. 82104105), China Postdoctoral Science Foundation 2021M693966. We thank D. Zhang and J. Lian (Core Facility, Center of Biomedical Analysis, Tsinghua University) for technical support with whole LN immunolabeling, tissue clearing, imaging and computational analysis.
Author information
Authors and Affiliations
Contributions
J.W., N.G. and X.-J.L. conceived and designed the experiments. J.W., N.G., Y.Z., H.X., Y.J., W.L., X.L., F.X. and B.X. performed the experiments. J.W., X.-J.L., Y.Y. and S.S. analysed the results. J.C., Y.Y., X.L., W.G., S.S., J.Z. and A.Z. provided suggestions and technical support. J.L. provided support for the use of light-sheet microscopy for LN imaging and contributed to the discussion and guidance on the characterization of the vaccine. J.W., N.G. and X.-J.L. wrote the manuscript. N.G. and X.-J.L. supervised the entire project. All authors discussed the results and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
J.W., N.G. and X.-J.L. have filed a patent application related to this study. The other authors declare no competing interests.
Peer review
Peer review information
Nature Biomedical Engineering thanks Guangjun Nie and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 IgG modified liposome targets DCs.
a, Illustration of the preparation of liposomes (LIP) and IgG-modified liposomes (LIP-IgG). b, Hydrodynamic diameter and Zeta-potential of LIP and LIP-IgG. c, Quantitative analysis of the uptake of FITC labeled LIP- or LIP-IgG by different cells (3T3, HUVEC, RAW 264.7, DC2.4) using flow cytometry. d, Quantitative analysis of the uptake of LIP, LIP-IgG, or Fc-fragment modified Lip (LIP-Fc) by DC2.4 cells. e-f, DC2.4 cells were pretreated with various concentrations of IgG (e) or Fc fragment (f) and then FITC labeled LIP-IgG were added. After 2 h incubation, mean fluorescence intensity (MFI) of DC2.4 cells were determined using flow cytometry. Data are presented as mean ±s.d., n = 3 independent biological samples per group, statistical differences in c were determined using two-way ANOVA with Tukey’s test for multiple comparison.
Extended Data Fig. 2 IgG binding to XMVs increases DC uptake.
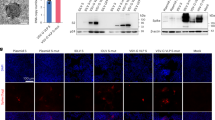
Mouse subcutaneous tissue homogenate was prepared and centrifuged to remove cells. The supernatant was collected and exploited to treat XMVs (30 min, 37 °C). After that, the XMVs were collected by centrifugation and washed with PBS. a, A representative TEM image of XMV after stained with an anti-IgG antibody-conjugated to gold bead. Scare bar, 50 nm. Liquid chromatography tandem mass spectrometry (LC-MS/MS) was used to identify and quantify the components of the protein corona. Classifications of components of the proteins (b) and their isoelectric point (pI) (c) of the top 21 abundant proteins. d, Gating strategies for analyzing antibody binding to XMVs. e, The binding of IgG, IgA, or IgM to XMVs after XMVs were incubated with subcutaneous tissue homogenate, analyzed using a CytoFLEX nanoflow cytometry. f, XMVs from different cell lines (PED, Vero) were used to prepare XMV-Ag. Mouse IgG was mixed with XMV-Ag for 30 min to form IgG-XMV-Ag. Various XMV-Ag, AUV-Ag (AUVs were derived from mouse RBC cells), or Lip- Ag were used to treat DC2.4 cells. After 2 h, The uptake of the antigen peptide by DC2.4 were determined using flow cytometry. g, XMV-Ag were preincubated with different amounts of IgG and then were added to DC2.4 cells. After 2 h, MFI of DC2.4 cells were determined using flow cytometry. h, XMV-Ag or XMV-Ag with IgG-preincubation were used to treat DC2.4 cells in the absence or present of FcR blockade. i, BMDCs were incubated with the XMV-Ag for 24 h and the presentation of SIINFEKL peptide on DC surface were stained using a H-2 K(b) OVA (SIINFEKL) antibody. After that, the cells were observed using a confocal microscopy. Green, Ag/MHC-I; blue, nuclei. Data are presented as mean ±s.d. and statistical differences in f were determined using one-way ANOVA with Tukey’s test for multiple comparison.
Extended Data Fig. 3 XMV-Ag targets DCs and promotes DC maturation in vivo.
Mice were subcutaneously injected with FITC labeled XMV-Ag at 0 h. After 24 h, the LNs were collected and the percentages of cellular uptake of FITC-XMV-Ag in LNs were determined using flow cytometry. a. Flow cytometry analysis of cellular uptake of XMV-Ag in LNs. Representative flow dot plots (left) and the percentages of XMV-Ag uptake by various cells in LNs (right) are shown. b. Gating strategy for flow cytometry analysis of DC subtypes such as cDC1(CD317-B220-CD11c+MHC-II+XCR1+), cDC2(CD317-B220-CD11c+MHC-II+CD172+) and pDC (CD317+B220+) in LNs. Mice were treated with XMV-Ag for 24 h and the percentages of CD11c+ CD40+ DCs (c) and CD11c+ CD86+ DCs (d) in LNs were determined using flow cytometry. Data are presented as mean ± s.d. Data in a, c-d, n = 3 independent biological samples per group.
Extended Data Fig. 4 XMV-Ag induces T-cell immune responses.
a, Flow cytometry analysis of OVA tetramer+CD8+ T cells in total blood immune cells. b, Percentages of IFN-γ+ cells in splenic CD8+CD44+ T cells from mice treated with three vaccinations of XMV-Ag, AUV-Ag, LIP-Ag, free Ag, or PBS. Representative flow dot plots (c-d) and quantifications (e-f) of memory cells in CD4+ or CD8+ T cells, naive T cells in CD4+ or CD8+ T cells in spleen cells after three-doses of XMV-Ag, AUV-Ag, LIP-Ag, free Ag, or PBS. Data are presented as mean ±s.d.(n = 4), statistical differences were determined using one-way ANOVA with Tukey’s test for multiple comparison.
Supplementary information
Source data
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, J., Zhang, Y., Jia, Y. et al. Targeting vaccines to dendritic cells by mimicking the processing and presentation of antigens in xenotransplant rejection. Nat. Biomed. Eng 9, 201–214 (2025). https://doi.org/10.1038/s41551-025-01343-6
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41551-025-01343-6
This article is cited by
-
Clinical relevance of extracellular vesicles in cancer — therapeutic and diagnostic potential
Nature Reviews Clinical Oncology (2025)