Abstract
In pulmonary arterial hypertension (PAH), a phenotypic switch in pulmonary arterial smooth muscle cells (PASMCs) that is primarily caused by aberrant gene regulatory networks can lead to dysregulated vascular remodelling, heart failure or death. No curative therapies for PAH are currently available, presumably because of a lack of viral vectors specifically targeting PASMCs. Here we show that a highly mobile and PASMC-tropic adeno-associated virus variant developed via directed evolution overcomes physical barriers that inhibit its transfer from bronchial airways to vascular layers, ultimately boosting therapeutic efficacy in murine models of PAH. Intratracheal administration of the adeno-associated virus variant carrying a transgene for fibroblast growth factor 12—a key factor regulating the PASMC phenotype—suppressed pulmonary vascular remodelling, prevented the development of PAH in mice and reversed established PAH in rats. The variant’s mobility and enhanced tropism for PASMCs may enable curative treatments for PAH.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
The data supporting the results in this study are available within the paper and its Supplementary Information. Source data are provided with this paper.
References
Farber, H. W. & Loscalzo, J. Pulmonary arterial hypertension. N. Engl. J. Med. 351, 1655–1665 (2004).
Schermuly, R. T., Ghofrani, H. A., Wilkins, M. R. & Grimminger, F. Mechanisms of disease: pulmonary arterial hypertension. Nat. Rev. Cardiol. 8, 443–455 (2011).
Thompson, A. R. & Lawrie, A. Targeting vascular remodeling to treat pulmonary arterial hypertension. Trends Mol. Med. 23, 31–45 (2017).
Morrell, N. W. et al. Altered growth responses of pulmonary artery smooth muscle cells from patients with primary pulmonary hypertension to transforming growth factor-β1 and bone morphogenetic proteins. Circulation 104, 790–795 (2001).
Dorai, H., Vukicevic, S. & Sampath, T. K. Bone morphogenetic protein-7 (osteogenic protein-1) inhibits smooth muscle cell proliferation and stimulates the expression of markers that are characteristic of SMC phenotype in vitro. J. Cell. Physiol. 184, 37–45 (2000).
Lagna, G. et al. Control of phenotypic plasticity of smooth muscle cells by bone morphogenetic protein signaling through the myocardin-related transcription factors. J. Biol. Chem. 282, 37244–37255 (2007).
Hu, L. et al. An emerging strategy for targeted therapy of pulmonary arterial hypertension: vasodilation plus vascular remodeling inhibition. Drug Discov. Today 27, 1457–1463 (2022).
Sung, Y. K., Yuan, K. & de Jesus Perez, V. A. Novel approaches to pulmonary arterial hypertension drug discovery. Expert Opin. Drug Discov. 11, 407–414 (2016).
Pullamsetti, S. S., Mamazhakypov, A., Weissmann, N., Seeger, W. & Savai, R. Hypoxia-inducible factor signaling in pulmonary hypertension. J. Clin. Invest. 130, 5638–5651 (2020).
Yeo, Y. et al. FGF12 (fibroblast growth factor 12) inhibits vascular smooth muscle cell remodeling in pulmonary arterial hypertension. Hypertension 76, 1778–1786 (2020).
Harper, R. L., Reynolds, A. M., Bonder, C. S. & Reynolds, P. N. BMPR2 gene therapy for PAH acts via Smad and non-Smad signalling. Respirology 21, 727–733 (2016).
Pousada, G. et al. Molecular and functional characterization of the BMPR2 gene in pulmonary arterial hypertension. Sci. Rep. 7, 1923 (2017).
Austin, E. D. & Loyd, J. E. The genetics of pulmonary arterial hypertension. Circ. Res. 115, 189–202 (2014).
Hadri, L. et al. Therapeutic efficacy of AAV1.SERCA2a in monocrotaline-induced pulmonary arterial hypertension. Circulation 128, 512–523 (2013).
Strauss, B. et al. Intra-tracheal gene delivery of aerosolized SERCA2a to the lung suppresses ventricular arrhythmias in a model of pulmonary arterial hypertension. J. Mol. Cell. Cardiol. 127, 20–30 (2019).
Song, S.-H. et al. Fibroblast growth factor 12 is a novel regulator of vascular smooth muscle cell plasticity and fate. Arterioscler. Thromb. Vasc. Biol. 36, 1928–1936 (2016).
Somlyo, A. P. & Somlyo, A. V. Signal transduction and regulation in smooth muscle. Nature 372, 231–236 (1994).
Pickles, R. J. Physical and biological barriers to viral vector–mediated delivery of genes to the airway epithelium. Proc. Am. Thorac. Soc. 1, 302–308 (2004).
Halbert, C. L. et al. Expression of human α1-antitrypsin in mice and dogs following AAV6 vector-mediated gene transfer to the lungs. Mol. Ther. 18, 1165–1172 (2010).
Li, W., Zhang, L., Wu, Z., Pickles, R. J. & Samulski, R. J. AAV-6 mediated efficient transduction of mouse lower airways. Virology 417, 327–333 (2011).
Travaglini, K. J. et al. A molecular cell atlas of the human lung from single-cell RNA sequencing. Nature 587, 619–625 (2020).
Braciale, T. J., Sun, J. & Kim, T. S. Regulating the adaptive immune response to respiratory virus infection. Nat. Rev. Immunol. 12, 295–305 (2012).
Kucharz, K. et al. Post-capillary venules are the key locus for transcytosis-mediated brain delivery of therapeutic nanoparticles. Nat. Commun. 12, 4121 (2021).
Kim, Y. C. et al. Gelling hypotonic polymer solution for extended topical drug delivery to the eye. Nat. Biomed. Eng. 4, 1053–1062 (2020).
Yu, M. et al. Rapid transport of deformation-tuned nanoparticles across biological hydrogels and cellular barriers. Nat. Commun. 9, 2607 (2018).
Wu, Z., Miller, E., Agbandje-McKenna, M. & Samulski, R. J. α2,3 and α2,6 N-linked sialic acids facilitate efficient binding and transduction by adeno-associated virus types 1 and 6. J. Virol. 80, 9093–9103 (2006).
Shirk, R. A., Church, F. C. & Wagner, W. D. Arterial smooth muscle cell heparan sulfate proteoglycans accelerate thrombin inhibition by heparin cofactor II. Arterioscler. Thromb. Vasc. Biol. 16, 1138–1146 (1996).
Aguero, J. et al. Intratracheal gene delivery of SERCA2a ameliorates chronic post-capillary pulmonary hypertension: a large animal model. J. Am. Coll. Cardiol. 67, 2032–2046 (2016).
Kotterman, M. A. & Schaffer, D. V. Engineering adeno-associated viruses for clinical gene therapy. Nat. Rev. Genet. 15, 445–451 (2014).
Wang, D., Tai, P. W. L. & Gao, G. Adeno-associated virus vector as a platform for gene therapy delivery. Nat. Rev. Drug Discov. 18, 358–378 (2019).
Li, C. & Samulski, R. J. Engineering adeno-associated virus vectors for gene therapy. Nat. Rev. Genet. 21, 255–272 (2020).
Carpentier, A. C. et al. Effect of alipogene tiparvovec (AAV1-LPLS447X) on postprandial chylomicron metabolism in lipoprotein lipase-deficient patients. J. Clin. Endocrinol. 97, 1635–1644 (2012).
Maguire, A. M. et al. Safety and efficacy of gene transfer for Leber’s congenital amaurosis. N. Engl. J. Med. 358, 2240–2248 (2008).
George, L. A. et al. Hemophilia B gene therapy with a high-specific-activity factor IX variant. N. Engl. J. Med. 377, 2215–2227 (2017).
Mendell, J. R. et al. Single-dose gene-replacement therapy for spinal muscular atrophy. N. Engl. J. Med. 377, 1713–1722 (2017).
Bisserier, M. et al. Regulation of the methylation and expression levels of the BMPR2 gene by SIN3a as a novel therapeutic mechanism in pulmonary arterial hypertension. Circulation 144, 52–73 (2021).
Claude, C. et al. Inhalable delivery of AAV-based MRP4/ABCC4 silencing RNA prevents monocrotaline-induced pulmonary hypertension. Mol. Ther. Methods Clin. Dev. 2, 14065 (2015).
Jiang, H. et al. Proteomic analysis reveals that Xbp1s promotes hypoxic pulmonary hypertension through the p-JNK MAPK pathway. J. Cell. Physiol. 237, 1948–1963 (2022).
Duncan, G. A. et al. An adeno-associated viral vector capable of penetrating the mucus barrier to inhaled gene therapy. Mol. Ther. Methods Clin. Dev. 9, 296–304 (2018).
Fernandes, D. J., McConville, J. F., Stewart, A. G., Kalinichenko, V. & Solway, J. Can we differentiate between airway and vascular smooth muscle? Clin. Exp. Pharmacol. Physiol. 31, 805–810 (2004).
Moiseenko, A. et al. Origin and characterization of alpha smooth muscle actin-positive cells during murine lung development. Stem Cells 35, 1566–1578 (2017).
Abe, K. et al. Haemodynamic unloading reverses occlusive vascular lesions in severe pulmonary hypertension. Cardiovasc. Res. 111, 16–25 (2016).
Dalkara, D. et al. In vivo-directed evolution of a new adeno-associated virus for therapeutic outer retinal gene delivery from the vitreous. Sci. Transl. Med. 5, 189ra176 (2013).
Jobe, A. H. et al. Surfactant enhances adenovirus-mediated gene expression in rabbit lungs. Gene Ther. 3, 775–779 (1996).
Weiss, D. J. et al. Transient increase in lung epithelial tight junction permeability: an additional mechanism for enhancement of lung transgene expression by perfluorochemical liquids. Mol. Ther. 8, 927–935 (2003).
Asbury, G. R. & Hill, H. H. Jr Separation of amino acids by ion mobility spectrometry. J. Chromatogr. A 902, 433–437 (2000).
Schuster, B. S. et al. Overcoming the cystic fibrosis sputum barrier to leading adeno-associated virus gene therapy vectors. Mol. Ther. 22, 1484–1493 (2014).
Huang, L.-Y. et al. Characterization of the adeno-associated virus 1 and 6 sialic acid binding site. J. Virol. 90, 5219–5230 (2016).
Wu, Z. et al. Single amino acid changes can influence titer, heparin binding, and tissue tropism in different adeno-associated virus serotypes. J. Virol. 80, 11393–11397 (2006).
Nelson, D. & Cox, M. Principles of Biochemistry 4th edn (W.H. Freeman, 2005).
Venkatakrishnan, B. et al. Structure and dynamics of adeno-associated virus serotype 1 VP1-unique N-terminal domain and its role in capsid trafficking. J. Virol. 87, 4974–4984 (2013).
Kozlowski, L. P. IPC—Isoelectric Point Calculator. Biol. Direct 11, 55 (2016).
Chockalingam, K., Blenner, M. & Banta, S. Design and application of stimulus-responsive peptide systems. Protein Eng. Des. Sel. 20, 155–161 (2007).
Yu, M. et al. The investigation of protein diffusion via H-cell microfluidics. Biophys. J. 116, 595–609 (2019).
Johnson, F. B., Ozer, H. L. & Hoggan, M. D. Structural proteins of adenovirus-associated virus type 3. J. Virol. 8, 860–863 (1971).
Rose, J. A. et al. Structural proteins of adenovirus-associated viruses. J. Virol. 8, 766–770 (1971).
Heck, A. et al. Adeno-associated virus capsid assembly is divergent and stochastic. Nat. Commun. 12, 1642 (2021).
Koerber, J. T., Maheshri, N., Kaspar, B. K. & Schaffer, D. V. Construction of diverse adeno-associated viral libraries for directed evolution of enhanced gene delivery vehicles. Nat. Protoc. 1, 701–706 (2006).
Jang, J. H. et al. An evolved adeno-associated viral variant enhances gene delivery and gene targeting in neural stem cells. Mol. Ther. 19, 667–675 (2011).
Cho, M. et al. Safety and efficacy evaluations of an adeno-associated virus variant for preparing IL10-secreting human neural stem cell-based therapeutics. Gene Ther. 26, 135–150 (2019).
Kang, M. H. et al. A lung tropic AAV vector improves survival in a mouse model of surfactant B deficiency. Nat. Commun. 11, 3929 (2020).
Boumahdi, S. et al. SOX2 controls tumour initiation and cancer stem-cell functions in squamous-cell carcinoma. Nature 511, 246–250 (2014).
Lin, H.-T. et al. Application of droplet digital PCR for estimating vector copy number states in stem cell gene therapy. Hum. Gene Ther. Methods 27, 197–208 (2016).
Bienkowska-Haba, M. et al. Human papillomavirus genome copy number is maintained by S-phase amplification, genome loss to the cytosol during mitosis, and degradation in G1 phase. J. Virol. 97, e01879–22 (2023).
Del Gaudio, D. et al. Increased MECP2 gene copy number as the result of genomic duplication in neurodevelopmentally delayed males. Genet. Med. 8, 784–792 (2006).
Filipe, V., Hawe, A. & Jiskoot, W. Critical evaluation of nanoparticle tracking analysis (NTA) by NanoSight for the measurement of nanoparticles and protein aggregates. Pharm. Res. 27, 796–810 (2010).
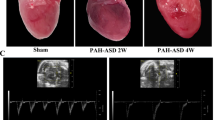
Park, J.-B. et al. Assessment of inflammation in pulmonary artery hypertension by 68Ga-mannosylated human serum albumin. Am. J. Respir. Crit. Care Med. 201, 95–106 (2020).
Brittain, E. et al. Echocardiographic assessment of the right heart in mice. J. Vis. Exp. 81, e50912 (2013).
Acknowledgements
This research was supported by the National Research Foundation funded by the Ministry of Science and ICT (NRF-2018M3A9H2019045, NRF-2019M3A9H1032791, RS-2023-00219962, RS-2024-00438808 and RS-2024-00451880) and the National Institute of Food and Drug Safety Evaluation (21173MFDS562). This work was also supported by Samsung Research Funding and Incubation Center of Samsung Electronics under project number SRFC-MA2202-08; Korea Drug Development Fund funded by Ministry of Science and ICT, Ministry of Trade, Industry and Energy, and Ministry of Health and Welfare (RS-2023-00217737, Republic of Korea); and a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: RS-2024-00438444). Yoojin Kim gratefully acknowledges support from the Basic Science Research Program (NRF-2020R1A6A3A01100408) and the Sejong Science Fellowship (RS-2024-00395068) supported by the National Research Foundation funded by the Korean government (Ministry of Science and ICT). We would like to thank H. Kang, Y. S. Cho and D.-H. Yeom for their help in animal experiments and O. M. Kwon and N. R. Park for their help in animal echocardiography.
Author information
Authors and Affiliations
Contributions
W.S. and J.-H.J. conceived and supervised the experiments. Yoojin Kim and Y.Y. performed the majority of the experiments and M.K., Y.-W.S., J.K., S.K., K.L.K., S.O., H.L., H.-W.P., Yunha Kim, D.L. and S.J.L. contributed to the experiments. Yoojin Kim and S.O. constructed the AAV library pool. Yoojin Kim, Y.Y., S.K. and S.O. contributed to the AAVp2CV in vivo selection, including AAV packaging and AAV variants evaluation. Yoojin Kim, Y.Y., Yunha Kim and D.L. conducted the intratracheal surgeries. Yoojin Kim, Y.Y. and K.L.K. assessed the AAV off-targeting by genome quantification and observation of transgene expression. Yoojin Kim and J.K. performed the immunohistochemistry. Yoojin Kim and J.-H.J. performed the AAV trajectory analysis. Y.Y., Yoojin Kim, K.L.K., Yunha Kim, D.L. and M.K. contributed to the preparation of PAH disease models. Y.Y., M.K. and W.S. conducted the haemodynamic assay and Fulton’s index analysis of the PAH models. C.K. and H.C. contributed to the analysis of data obtained from the AAV selection procedure. C.S.P. and S.-P.L. contributed to the echocardiography measurement and interpretation. Yoojin Kim, Y.Y., W.S. and J.-H.J. wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Biomedical Engineering thanks Lahouaria Hadri, Roger Hajjar and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 In vitro transduction efficiency.
(a) Transduction efficiencies (GFP %) obtained by the four vectors for HEK293T cells (MOI 1 × 104) and HUVECs (MOI 5 × 104) cultured on tissue culture plates (48 h post-infection). AAV2 vector was used as a positive control. (b) GFP intensity obtained by the two vectors in HUVECs (MOI 5 × 104).
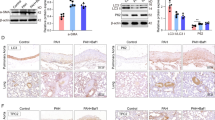
Extended Data Fig. 2 AAVp2CV-FGF12 gene transfer enhances the phosphorylation of MEF2a and the expression of its downstream target genes in PAH mice.
Mice received an intratracheal injection of either saline or AAV and were exposed to chronic hypoxia and weekly injection of SU5416 (Hyp+ SU) for 3 weeks for PAH induction. Mice maintained under normoxia served as the normal control. (a) Protein levels of p-MEF2a, MEF2a, and FGF12 in lung tissues as determined by western blot analysis. The band intensities of p-MEF2a were normalized to those of MEF2a and expressed relative to those of the normal controls (n = 4 biological replicates). (b) mRNA levels of MEF2a target genes related to the cell cycle (Ccne1, Mcm6) and contractile SMC markers (Lmod1, Myocd) as evaluated by real-time RT‒PCR (n = 3–4 biological replicates). All data are presented as the mean ± SEM. (one-way ANOVA with Bonferroni post hoc analysis).
Extended Data Fig. 3 Mutagenic analysis of the role of two mutations on AAVp2CV in generating mobile properties.
(a) Trajectory of three mutants: AAV1-647A, AAV1-647V, and AAV1-647Y was observed (PBS at pH 7.4). (b) The MSD of 647 site mutants was calculated and normalized to that of AAV1-647V. (c) Trajectory of three mutants: AAV1-430R, AAV1-430C, and AAV1-430E (PBS at pH 7.4). (d) MSD of 430 site mutants was measured and normalized to that of AAV1-430C. All data (n = 10) are presented as the mean ± STD.
Extended Data Fig. 4
Trajectories of AAVp2CV under different pH environments and comparison of MSD (n = 10).
Supplementary information
Supplementary Information
Supplementary figures.
Table
Source data for supplementary figures.
Source data
Source Data Fig. 1
Source data.
Source Data Fig. 2
Source data.
Source Data Fig. 3
Source data.
Source Data Fig. 6
Source data.
Source Data Fig. 7
Source data.
Source Data Fig. 8
Source data.
Source Data Fig. 8
Full-length unprocessed western blots.
Source Data Extended Data Fig. 1
Source data.
Source Data Extended Data Fig. 2
Source data.
Source Data Extended Data Fig. 2
Full-length unprocessed western blots.
Source Data Extended Data Fig. 3
Source data.
Source Data Extended Data Fig. 4
Source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kim, Y., Yeo, Y., Kim, M. et al. A highly mobile adeno-associated virus targeting vascular smooth muscle cells for the treatment of pulmonary arterial hypertension. Nat. Biomed. Eng 9, 1418–1436 (2025). https://doi.org/10.1038/s41551-025-01379-8
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41551-025-01379-8