Abstract
Alveolar epithelial regeneration is essential for recovery from devastating lung diseases. This process occurs when type II alveolar pneumocytes (AT2 cells) proliferate and transdifferentiate into type I alveolar pneumocytes (AT1 cells). We used genome-wide analysis of chromatin accessibility and gene expression following acute lung injury to elucidate repair mechanisms. AT2 chromatin accessibility changed substantially following injury to reveal STAT3 binding motifs adjacent to genes that regulate essential regenerative pathways. Single-cell transcriptome analysis identified brain-derived neurotrophic factor (Bdnf) as a STAT3 target gene with newly accessible chromatin in a unique population of regenerating AT2 cells. Furthermore, the BDNF receptor tropomyosin receptor kinase B (TrkB) was enriched on mesenchymal alveolar niche cells (MANCs). Loss or blockade of AT2-specific Stat3, Bdnf or mesenchyme-specific TrkB compromised repair and reduced Fgf7 expression by niche cells. A TrkB agonist improved outcomes in vivo following lung injury. These data highlight the biological and therapeutic importance of the STAT3–BDNF–TrkB axis in orchestrating alveolar epithelial regeneration.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
ATAC-seq and scRNA-seq data that support the findings of this study have been deposited in the Gene Expression Omnibus under accession code GSE132535. All other data supporting the findings of this study are available from the corresponding author on reasonable request. Source data are provided with this paper.
References
Matthay, M. A., Robriquet, L. & Fang, X. Alveolar epithelium: role in lung fluid balance and acute lung injury. Proc. Am. Thorac. Soc. 2, 206–213 (2005).
Guillot, L. et al. Alveolar epithelial cells: master regulators of lung homeostasis. Int. J. Biochem. Cell Biol. 45, 2568–2573 (2013).
Tyrrell, C., McKechnie, S. R., Beers, M. F., Mitchell, T. J. & McElroy, M. C. Differential alveolar epithelial injury and protein expression in pneumococcal pneumonia. Exp. Lung Res. 38, 266–276 (2012).
Herold, S., Becker, C., Ridge, K. M. & Budinger, G. R. Influenza virus-induced lung injury: pathogenesis and implications for treatment. Eur. Respir. J. 45, 1463–1478 (2015).
Herold, S. et al. Lung epithelial apoptosis in influenza virus pneumonia: the role of macrophage-expressed TNF-related apoptosis-inducing ligand. J. Exp. Med. 205, 3065–3077 (2008).
Matthay, M. A., Ware, L. B. & Zimmerman, G. A. The acute respiratory distress syndrome. J. Clin. Invest. 122, 2731–2740 (2012).
Thompson, B. T., Chambers, R. C. & Liu, K. D. Acute respiratory distress syndrome. N. Engl. J. Med. 377, 1904–1905 (2017).
Martin, T. R., Hagimoto, N., Nakamura, M. & Matute-Bello, G. Apoptosis and epithelial injury in the lungs. Proc. Am. Thorac. Soc. 2, 214–220 (2005).
Herridge, M. S. et al. One-year outcomes in survivors of the acute respiratory distress syndrome. N. Engl. J. Med. 348, 683–693 (2003).
Matthay, M. A. & Wiener-Kronish, J. P. Intact epithelial barrier function is critical for the resolution of alveolar edema in humans. Am. Rev. Resp. Dis. 142, 1250–1257 (1990).
Ingbar, D. H. & Matthay, R. A. Pulmonary sequelae and lung repair in survivors of the adult respiratory distress syndrome. Crit. Care Clin. 2, 629–665 (1986).
Guan, W. et al. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 382, 1708–1720 (2020).
Bhatraju, P. K. et al. Covid-19 in critically ill patients in the Seattle region—case series.N. Engl. J. Med. 382, 2012–2022(2020).
Konigshoff, M., Saglani, S., Marsland, B. J. & Eickelberg, O. Rebuilding a diseased lung: repair and regeneration. Eur. Respir. J. 41, 497–499 (2013).
Kotton, D. N. & Morrisey, E. E. Lung regeneration: mechanisms, applications and emerging stem cell populations. Nat. Med. 20, 822–832 (2014).
Evans, M. J., Cabral, L. J., Stephens, R. J. & Freeman, G. Renewal of alveolar epithelium in the rat following exposure to NO2. Am. J. Pathol. 70, 175–198 (1973).
Barkauskas, C. E. et al. Type 2 alveolar cells are stem cells in adult lung. J. Clin. Invest. 123, 3025–3036 (2013).
Nabhan, A. N., Brownfield, D. G., Harbury, P. B., Krasnow, M. A. & Desai, T. J. Single-cell Wnt signaling niches maintain stemness of alveolar type 2 cells. Science 359, 1118–1123 (2018).
Zacharias, W. J. et al. Regeneration of the lung alveolus by an evolutionarily conserved epithelial progenitor. Nature 555, 251–255 (2018).
Jain, R. et al. Plasticity of Hopx+ type I alveolar cells to regenerate type II cells in the lung. Nat. Commun. 6, 6727 (2015).
Wang, Y. et al. Pulmonary alveolar type I cell population consists of two distinct subtypes that differ in cell fate. Proc. Natl Acad. Sci. USA 115, 2407–2412 (2018).
Kim, C. F. et al. Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell 121, 823–835 (2005).
Liu, Q. et al. Lung regeneration by multipotent stem cells residing at the bronchioalveolar-duct junction. Nat. Genet. 51, 728–738 (2019).
Salwig, I. et al. Bronchioalveolar stem cells are a main source for regeneration of distal lung epithelia in vivo.EMBO J. 38, e102099 (2019).
Zuo, W. et al. p63+Krt5+ distal airway stem cells are essential for lung regeneration. Nature 517, 616–620 (2015).
Ray, S. et al. Rare SOX2+ airway progenitor cells generate KRT5+ cells that repopulate damaged alveolar parenchyma following influenza virus infection. Stem Cell Rep. 7, 817–825 (2016).
Vaughan, A. E. et al. Lineage-negative progenitors mobilize to regenerate lung epithelium after major injury. Nature 517, 621–625 (2015).
Nolen-Walston, R. D. et al. Cellular kinetics and modeling of bronchioalveolar stem cell response during lung regeneration. Am. J. Physiol. Lung Cell. Mol. Physiol. 294, L1158–L1165 (2008).
Yee, M. et al. Alternative progenitor lineages regenerate the adult lung depleted of alveolar epithelial type 2 cells. Am. J. Respir. Cell Mol. Biol. 56, 453–464 (2017).
Xi, Y. et al. Local lung hypoxia determines epithelial fate decisions during alveolar regeneration. Nat. Cell Biol. 19, 904–914 (2017).
Mason, R. J. & Williams, M. C. Type II alveolar cell. Defender of the alveolus. Am. Rev. Resp. Dis. 115, 81–91 (1977).
Fehrenbach, H. Alveolar epithelial type II cell: defender of the alveolus revisited. Respir. Res. 2, 33–46 (2001).
Crapo, J. D. et al. Morphometric characteristics of cells in the alveolar region of mammalian lungs. Am. Rev. Resp. Dis. 128, S42–S46 (1983).
Takahashi, K. & Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126, 663–676 (2006).
Rodolfa, K. T. & Eggan, K. A transcriptional logic for nuclear reprogramming. Cell 126, 652–655 (2006).
Buenrostro, J. D., Giresi, P. G., Zaba, L. C., Chang, H. Y. & Greenleaf, W. J. Transposition of native chromatin for fast and sensitive epigenomic profiling of open chromatin, DNA-binding proteins and nucleosome position. Nat. Methods 10, 1213–1218 (2013).
Barkauskas, C. E. et al. Lung organoids: current uses and future promise. Development 144, 986–997 (2017).
Kadyk, L. C., DeWitt, N. D. & Gomperts, B. Proceedings: regenerative medicine for lung diseases: a CIRM Workshop Report. Stem Cells Transl. Med. 6, 1823–1828 (2017).
Paris, A. J. et al. Neutrophils promote alveolar epithelial regeneration by enhancing type II pneumocyte proliferation in a model of acid-induced acute lung injury.Am. J. Physiol. Lung Cell. Mol. Physiol. 311, L1062–L1075 (2016).
Heinz, S. et al. Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Mol. Cell 38, 576–589 (2010).
Kimura, S. et al. The T/ebp null mouse: thyroid-specific enhancer-binding protein is essential for the organogenesis of the thyroid, lung, ventral forebrain, and pituitary. Genes Dev. 10, 60–69 (1996).
Yuan, B. et al. Inhibition of distal lung morphogenesis in Nkx2.1−/− embryos. Dev. Dynam. 217, 180–190 (2000).
Li, S. et al. Foxp1/4 control epithelial cell fate during lung development and regeneration through regulation of anterior gradient 2. Development 139, 2500–2509 (2012).
Shu, W. et al. Foxp2 and Foxp1 cooperatively regulate lung and esophagus development. Development 134, 1991–2000 (2007).
Ghahary, A. & Ghaffari, A. Role of keratinocyte–fibroblast cross-talk in development of hypertrophic scar. Wound Repair Regen. 15, S46–S53 (2007).
Zepp, J. A. et al. Distinct mesenchymal lineages and niches promote epithelial self-renewal and myofibrogenesis in the lung. Cell 170, 1134–1148.e10 (2017).
Schutte, H. et al. Bronchoalveolar and systemic cytokine profiles in patients with ARDS, severe pneumonia and cardiogenic pulmonary oedema. Eur. Respir. J. 9, 1858–1867 (1996).
Zhong, Z., Wen, Z. & Darnell, J. E. Jr Stat3: a STAT family member activated by tyrosine phosphorylation in response to epidermal growth factor and interleukin-6. Science 264, 95–98 (1994).
Zemans, R. L. & Matthay, M. A. Bench-to-bedside review: the role of the alveolar epithelium in the resolution of pulmonary edema in acute lung injury. Crit. Care 8, 469–477 (2004).
Matthay, M. A., Folkesson, H. G. & Clerici, C. Lung epithelial fluid transport and the resolution of pulmonary edema. Physiol. Rev. 82, 569–600 (2002).
Riemondy, K. A. et al. Single cell RNA sequencing identifies TGFβ as a key regenerative cue following LPS-induced lung injury.JCI Insight 5, e123637 (2019).
Leibrock, J. et al. Molecular cloning and expression of brain-derived neurotrophic factor. Nature 341, 149–152 (1989).
Chen, B. et al. Autocrine activity of BDNF induced by the STAT3 signaling pathway causes prolonged TrkB activation and promotes human non-small-cell lung cancer proliferation. Sci. Rep. 6, 30404 (2016).
Tang, Q. P. et al. STAT3 signal that mediates the neural plasticity is involved in willed-movement training in focal ischemic rats. J. Zhejiang Univ. Sci. B 17, 493–502 (2016).
Zhang, J. X. et al. Unique genome-wide map of TCF4 and STAT3 targets using ChIP-seq reveals their association with new molecular subtypes of glioblastoma. Neuro. Oncol. 15, 279–289 (2013).
Hixson, K. M., Cogswell, M., Brooks-Kayal, A. R. & Russek, S. J. Evidence for a non-canonical JAK/STAT signaling pathway in the synthesis of the brain’s major ion channels and neurotransmitter receptors. BMC Genomics 20, 677 (2019).
Cazorla, M. et al. Identification of a low-molecular weight TrkB antagonist with anxiolytic and antidepressant activity in mice. J. Clin. Invest. 121, 1846–1857 (2011).
Jang, S. W. et al. A selective TrkB agonist with potent neurotrophic activities by 7,8-dihydroxyflavone. Proc. Natl Acad. Sci. USA 107, 2687–2692 (2010).
Hogan, B. Stemming lung disease? N. Engl. J. Med. 378, 2439–2440 (2018).
Weiss, D. J. Concise review: current status of stem cells and regenerative medicine in lung biology and diseases. Stem Cells 32, 16–25 (2014).
Yang, J. & Jia, Z. Cell-based therapy in lung regenerative medicine. Regen. Med. Res. 2, 7 (2014).
Kang, M. & Thebaud, B. Stem cell biology and regenerative medicine for neonatal lung diseases. Pediatr. Res. 83, 291–297 (2018).
Hokuto, I. et al. Stat-3 is required for pulmonary homeostasis during hyperoxia. J. Clin. Invest. 113, 28–37 (2004).
Quinton, L. J. et al. Alveolar epithelial STAT3, IL-6 family cytokines, and host defense during Escherichia coli pneumonia. Am. J. Respir. Cell Mol. Biol. 38, 699–706 (2008).
Severgnini, M. et al. Activation of the STAT pathway in acute lung injury. Am. J. Physiol. Lung Cell. Mol. Physiol. 286, L1282–L1292 (2004).
Liu, X. STAT3 activation inhibits human bronchial epithelial cell apoptosis in response to cigarette smoke exposure. Biochem. Biophys. Res. Commun. 353, 121–126 (2007).
Tadokoro, T. et al. IL-6/STAT3 promotes regeneration of airway ciliated cells from basal stem cells. Proc. Natl Acad. Sci. USA 111, E3641–E3649 (2014).
Giard, D. J. et al. In vitro cultivation of human tumors: establishment of cell lines derived from a series of solid tumors. J. Natl Cancer Inst. 51, 1417–1423 (1973).
Chao, M. V. Neurotrophins and their receptors: a convergence point for many signalling pathways. Nat. Rev. Neurosci. 4, 299–309 (2003).
Chapman, H. A. et al. Integrin α6β4 identifies an adult distal lung epithelial population with regenerative potential in mice. J. Clin. Invest. 121, 2855–2862 (2011).
Li, L. et al. The functional organization of cutaneous low-threshold mechanosensory neurons. Cell 147, 1615–1627 (2011).
Luikart, B. W., Nef, S., Shipman, T. & Parada, L. F. In vivo role of truncated TrkB receptors during sensory ganglion neurogenesis. Neuroscience 117, 847–858 (2003).
Tan, C. L. et al. Warm-sensitive neurons that control body temperature. Cell 167, 47–59.e15 (2016).
Rios, M. et al. Conditional deletion of brain-derived neurotrophic factor in the postnatal brain leads to obesity and hyperactivity. Mol. Endocrinol. 15, 1748–1757 (2001).
Takeda, N. et al. Hopx expression defines a subset of multipotent hair follicle stem cells and a progenitor population primed to give rise to K6+ niche cells. Development 140, 1655–1664 (2013).
Chen, Y. et al. Thermal stress induces glycolytic beige fat formation via a myogenic state. Nature 565, 180–185 (2019).
Madisen, L. et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat. Neurosci. 13, 133–140 (2010).
Moh, A. et al. Role of STAT3 in liver regeneration: survival, DNA synthesis, inflammatory reaction and liver mass recovery. Lab. Invest. 87, 1018–1028 (2007).
Das, S., MacDonald, K., Chang, H. Y. & Mitzner, W. A simple method of mouse lung intubation. J. Vis. Exp. 73, e50318 (2013).
Paris, A. J. et al. Using selective lung injury to improve murine models of spatially heterogeneous lung diseases. PLoS ONE 14, e0202456 (2019).
Alder, J. K. et al. Telomere dysfunction causes alveolar stem cell failure. Proc. Natl Acad. Sci. USA 112, 5099–5104 (2015).
Zacharias, W. & Morrisey, E. Isolation and culture of human alveolar epithelial progenitor cells. Nat. Protoc. Exchange http://dx.doi.org/10.1038/protex.2018.015 (2018).
Buenrostro, J. D., Wu, B., Chang, H. Y. & Greenleaf, W. J. ATAC-seq: a method for assaying chromatin accessibility genome-wide. Curr. Protoc. Mol. Biol. 109, 21.29.1–29.29.9 (2015).
Langmead, B., Trapnell, C., Pop, M. & Salzberg, S. L. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 10, R25 (2009).
Zhang, Y. et al. Model-based analysis of ChIP-Seq (MACS). Genome Biol. 9, R137 (2008).
Ramírez, F. et al. deepTools2: a next generation web server for deep-sequencing data analysis. Nucleic Acids Res. 44, W160–W165 (2016).
Butler, A., Hoffman, P., Smibert, P., Papalexi, E. & Satija, R. Integrating single-cell transcriptomic data across different conditions, technologies, and species. Nat. Biotechnol. 36, 411–420 (2018).
Matute-Bello, G. et al. An official American Thoracic Society workshop report: features and measurements of experimental acute lung injury in animals. Am. J. Respir. Cell Mol. Biol. 44, 725–738 (2011).
Nick, J. A. et al. Selective suppression of neutrophil accumulation in ongoing pulmonary inflammation by systemic inhibition of p38 mitogen-activated protein kinase. J. Immunol. 169, 5260–5269 (2002).
Acknowledgements
We acknowledge S. M. Albelda, J. S. Brenner and A. E. Vaughan for help with preparing this manuscript, as well as the Pathology, Flow Cytometry, Nucleic Acid and PCR cores and the Center for Applied Genomics at the Children’s Hospital of Philadelphia Research Institute. This work was supported by the Parker B. Fellowship Program (A.J.P.) and multiple grants from the National Institutes of Health (K08 HL136698 (A.J.P.), R01 AI121321 (M.D.W.), R01 AI099479 (G.S.W.) and R01 DK114054 (G.S.W.)). We also thank the patients and families who donated lung tissue to support this research.
Author information
Authors and Affiliations
Contributions
A.J.P. conceived of and co-performed the ATAC-seq experiment, performed all of the acid-induced lung injury experiments, analysed the histological data and co-wrote the manuscript. K.E.H. analysed the ATAC-seq and scRNA-seq data. J.H.O. assisted with the data analysis and co-wrote the manuscript. D.C.A. co-performed the ATAC-seq experiment. S.A.T. performed the influenza injury experiments. J.A.Z. performed the scRNA-seq of mesenchymal cells and assisted with the murine alveolar organoid experiments. W.J.Z. assisted with the murine and human alveolar organoid experiments. J.B.K. performed the survival analysis and assisted with isolating the human AT2 cells. M.C.B. identified the human histological samples. M.C.B. and M.M.K. isolated the human AT2 cells. A.R.S. assisted with performing the murine and human organoid experiments. S.J., A.S. and D.B.F. analysed the mean linear intercept and alveolar wall thickness of the murine lung specimens. N.D. and P.W. bred the mice, genotyped the mice, administered the tamoxifen and isolated the murine AT2 cells and fibroblasts. P.W. cultured the MRC5 cells and set up the murine and human organoid experiments. E.C. procured the human lung tissue. G.S.W. blindly scored the histological specimens. L.C.E., M.F.B. and E.E.M. assisted with the experimental design and with preparing the manuscript. M.D.W. and G.S.W. designed, conceived of and supervised the experiments and co-wrote the manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors have applied for a provisional patent in the United States related to this work. A.J.P. and G.S.W. are listed as co-inventors on this application.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Sorting Strategies for Isolating AT2 cells.
Lungs from C57BL/6 mice were digested into a single-cell suspension. We collected EpCAM positive cells (APC) that were PDPN, CD45, CD34, CD31 and SCA1 negative (all PE).
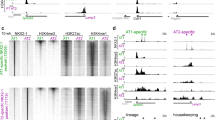
Extended Data Fig. 2 STAT3 is activated following sterile and infectious lung injuries.
Cells staining positive for proSFTPC+ and pSTAT3+ are highlighted with a white box while proSFTPC + pSTAT3- cells are highlighted with a yellow box. Areas that were felt to be indeterminate for cell type or pSTAT3 status are highlighted in blue. Scale bars, 50μm.
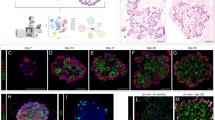
Extended Data Fig. 3 Loss of Stat3 diminished alveolar organoid formation.
a, PCR shows the expected 802bp product when corn oil is administered to SftpcCreERT2:Stat3LoxP/LoxP mice and the recombined 514bp product when tamoxifen is administered to SftpcCreERT2:Stat3LoxP/LoxP mice. The gels in this figure represent n=2 mice per condition (corn oil and tamoxifen). This experiment was repeated 4 times with similar results. b, AT2 cells from tamoxifen treated SftpcCreERT2-Rosa26tdTomato and SftpcCreERT2-Rosa26tdTomato:Stat3LoxP/LoxP mice were isolated and co-cultured with PDGFRα mesenchymal cells in transwells containing Matrigel for four weeks (n=4 wells per condition). Organoid forming efficiency was significantly decreased in the absence of STAT3 signaling in AT2 cells (p=2.2x10-8). Data is shown as the mean +/- SEM. Statistical significance was determined using a two-tailed Student’s t-test. Scale bars, 2.5mm.
Extended Data Fig. 4 Loss of Stat3 in AT2 cells does not alter distal lung morphology.
SftpcCreERT2:Stat3LoxP/LoxP mice were given tamoxifen or vehicle and euthanized three months later. Representative H&E stained histological specimens are shown. The Mean linear intercept was unchanged by the loss of Stat3 in these unchallenged mice (n=4 mice per group). Data is shown as the mean +/- SEM. Statistical significance was determined with a two-tailed Student’s t-test. Scale bars, 500μm.
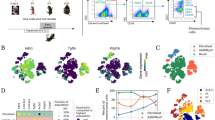
Extended Data Fig. 5 Single-cell expression of TrkB, Ccnd1, Tm4sf1 and Sftpc in isolated AT2 cells.
Plots show relative expression of TrkB, Ccnd1, Tm4sf1 and Sftpc in AT2 cells isolated isolated from uninjured mice and mice 24h after acid-induced lung injury.
Extended Data Fig. 6 Pathways analysis of isolated AT2 cells reveals multiple subsets of cells with multiple functions.
Pathways analysis of differentially expressed genes in the clusters shown in Fig. 4b.
Extended Data Fig. 7 AT2 cells express Bdnf after acid-induced lung injury.
BdnfCre-Rosa26tdTomato were subjected to acid-induced lung injury (n=4 mice per group). One day after injury the mice were euthanized, and the lungs were probed for Tomato (red) and proSFTPC (green). Autofluorescence delineates tissue structure. Scale bar, 25μm.
Extended Data Fig. 8 Loss of TrkB in mesenchymal cells worsens outcomes following sterile and infectious lung injuries.
Lungs from PdgfraEGFP mice were digested into a single-cell suspension either before or 24h after acid-induced lung injury (n=4/group). We first gated on PDGFRα+ and then quantified the percent of GFP+ cells within that population.
Extended Data Fig. 9 Loss of TrkB in mesenchymal cells worsens outcomes following sterile and infectious lung injuries.
a, H&E staining and ATS lung injury scores of tamoxifen and corn oil exposed PdgfrαCreERT2:TrkBLoxP/LoxP mice 24 hours after acid-induced lung injury (n=4 mice per group). b-d, H&E (b), PDPN (c) and Krt5 (d) staining of tamoxifen and corn oil exposed PdgfrαCreERT2:TrkBLoxP/LoxP mice 21 days after infection with PR8 influenza (n=3 mice per group). Statistical analysis was performed with a two-tailed Student’s t-test or ANOVA, where appropriate. For panels (a) and (d), data is shown as the mean +/- SEM. Statistical significance was determined with a two-tailed Student’s t-test (a) and (d). Scale bars, 100μm.
Extended Data Fig. 10 The TrkB agonist 7,8-DHF is unable to rescue mice with an AT2-specific Stat3 deletion.
Survival curves for Tamoxifen-exposed SftpcCreERT2:Stat3LoxP/LoxP mice that had been infected with intranasal PR8 influenza (5x10-5 HAU/mouse) and were given intraperitoneal injections of 7,8-DHF or vehicle every other day (n=5 mice per group). The data analyzed using the Log-rank (Mantel-Cox) test and is not statistically significant.
Supplementary information
Supplementary Tables
Table 1. Table of STAT3 binding sites. Table 2. Table of antibody concentrations. Table 3. Table of RT-PCR primer sequences. Table 4. Table of PCR primer sequences.
Source data
Source Data Fig. 1
Statistical source data
Source Data Fig. 2
Statistical source data
Source Data Fig. 3
Statistical source data
Source Data Fig. 4
Statistical source data
Source Data Fig. 5
Statistical source data
Source Data Fig. 6
Statistical source data
Source Data Fig. 7
Statistical source data
Source Data Fig. 8
Statistical source data
Source Data Extended Data Fig. 3
Statistical source data
Source Data Extended Data Fig. 3
Unprocessed gels and statistical source data
Source Data Extended Data Fig. 4
Statistical source data
Source Data Extended Data Fig. 9
Statistical source data
Source Data Extended Data Fig. 10
Statistical source data
Rights and permissions
About this article
Cite this article
Paris, A.J., Hayer, K.E., Oved, J.H. et al. STAT3–BDNF–TrkB signalling promotes alveolar epithelial regeneration after lung injury. Nat Cell Biol 22, 1197–1210 (2020). https://doi.org/10.1038/s41556-020-0569-x
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41556-020-0569-x
This article is cited by
-
Activation of the circAGFG1/miR-195-5p/PD-L1 axis induces lung injury in sepsis
Human Cell (2025)
-
Co-culture of human AT2 cells with fibroblasts reveals a MUC5B phenotype: insights from an organoid model
Molecular Medicine (2024)
-
METTL3-m6A methylation inhibits the proliferation and viability of type II alveolar epithelial cells in acute lung injury by enhancing the stability and translation efficiency of Pten mRNA
Respiratory Research (2024)
-
Unlocking lung regeneration: insights into progenitor cell dynamics and metabolic control
Cell Regeneration (2024)
-
Lung injury-induced activated endothelial cell states persist in aging-associated progressive fibrosis
Nature Communications (2024)