Abstract
Nutrient stress represents an important barrier for anti-tumour immunity, and tumour interstitial fluid often contains metabolites that hinder immune function. However, it is difficult to isolate the effects of tumour nutrient stress from other suppressive factors. Thus, we used a chemically defined cell culture medium based on the metabolomic profile of tumour interstitial fluid: tumour interstitial fluid medium (TIFM). Culture of CD8+ T cells in TIFM limited cell expansion and impaired CD8+ T cell effector functions upon restimulation, suggesting that tumour nutrient stress alone is sufficient to drive T cell dysfunction. We identified phosphoethanolamine (pEtn), a phospholipid intermediate, as a driver of T cell dysfunction. pEtn dampened T cell receptor signalling by depleting T cells of diacylglycerol required for T cell receptor signal transduction. The reduction of pEtn accumulation in tumours improved intratumoural T cell function and tumour control, suggesting that pEtn accumulation plays a dominant role in immunosuppression in the tumour microenvironment.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
RNA sequencing data that support the findings of this study have been deposited in the Gene Expression Omnibus (GEO) under accession code GSE235214. Metabolomics and lipidomics datasets have been deposited and are available via Metabolomics Workbench under study number ST003664 at https://dev.metabolomicsworkbench.org:22222/data/DRCCMetadata.php?Mode=Study&StudyID=ST003664&Access=IqaQ1644. Data in Extended Data Fig. 5a were reanalysed from ref. 31, and data in Extended Data Fig. 6j were reanalysed from ref. 46. Source data are provided with this paper. All other data supporting the findings of this study are available from the corresponding author on reasonable request.
References
Pagès, F. et al. Immune infiltration in human tumors: a prognostic factor that should not be ignored. Oncogene 29, 1093–1102 (2010).
Dahlin, A. M. et al. Colorectal cancer prognosis depends on T-cell infiltration and molecular characteristics of the tumor. Mod. Pathol. 24, 671–682 (2011).
Ribas, A. & Wolchok, J. D. Cancer immunotherapy using checkpoint blockade. Science 359, 1350–1355 (2018).
Ai, L. et al. Research status and outlook of PD-1/PD-L1 inhibitors for cancer therapy. Drug Des. Dev. Ther. 14, 3625–3649 (2020).
Sambi, M., Bagheri, L. & Szewczuk, M. R. Current challenges in cancer immunotherapy: multimodal approaches to improve efficacy and patient response rates. J. Oncol. 2019, 4508794 (2019).
Anderson, K. G., Stromnes, I. M. & Greenberg, P. D. Obstacles posed by the tumor microenvironment to T cell activity: a case for synergistic therapies. Cancer Cell 31, 311–325 (2017).
Verma, N. K. et al. Obstacles for T-lymphocytes in the tumour microenvironment: therapeutic challenges, advances and opportunities beyond immune checkpoint. EBioMedicine 83, 104216 (2022).
Blank, C. U. et al. Defining ‘T cell exhaustion’. Nat. Rev. Immunol. 19, 665–674 (2019).
Scharping, N. E. et al. Mitochondrial stress induced by continuous stimulation under hypoxia rapidly drives T cell exhaustion. Nat. Immunol. 22, 205–215 (2021).
Xia, A., Zhang, Y., Xu, J., Yin, T. & Lu, X. J. T cell dysfunction in cancer immunity and immunotherapy. Front. Immunol. 10, 1719 (2019).
Scharping, N. E. & Delgoffe, G. M. Tumor microenvironment metabolism: a new checkpoint for anti-tumor immunity. Vaccines 4, 46 (2016).
Watson, M. J. & Delgoffe, G. M. Fighting in a wasteland: deleterious metabolites and antitumor immunity. J. Clin. Invest. 132, e148549. (2022).
Wilfahrt, D. & Delgoffe, G. M. Metabolic waypoints during T cell differentiation. Nat. Immunol. 25, 206–217 (2024).
Buck, M. D., O’Sullivan, D. & Pearce, E. L. T cell metabolism drives immunity. J. Exp. Med. 212, 1345–1360 (2015).
Chapman, N. M., Boothby, M. R. & Chi, H. Metabolic coordination of T cell quiescence and activation. Nat. Rev. Immunol. 20, 55–70 (2020).
Pavlova, N. N., Zhu, J. & Thompson, C. B. The hallmarks of cancer metabolism: still emerging. Cell Metab. 34, 355–377 (2022).
Reinfeld, B. I. et al. Cell programmed nutrient partitioning in the tumor microenvironment. Nature 593, 282–288 (2021).
Grzywa, T. M. et al. Myeloid cell-derived arginase in cancer immune response. Front. Immunol. 11, 536322 (2020).
Platten, M., Wick, W. & Van Den Eynde, B. J. Tryptophan catabolism in cancer: beyond IDO and tryptophan depletion. Cancer Res. 72, 5435–5440 (2012).
Hezaveh, K. et al. Tryptophan-derived microbial metabolites activate the aryl hydrocarbon receptor in tumor-associated macrophages to suppress anti-tumor immunity. Immunity 55, 324–340 (2022).
Schaaf, M. B., Garg, A. D. & Agostinis, P. Defining the role of the tumor vasculature in antitumor immunity and immunotherapy. Cell Death Dis. 9, 115 (2018).
Leone, R. D. et al. Glutamine blockade induces divergent metabolic programs to overcome tumor immune evasion. Science 366, 1013–1021 (2019).
Li, J. et al. Targeting metabolism in cancer cells and the tumour microenvironment for cancer therapy. Molecules 25, 4831 (2020).
Sullivan, M. R. et al. Quantification of microenvironmental metabolites in murine cancers reveals determinants of tumor nutrient availability. eLife 8, e44235 (2019).
Saab, J. J. A. et al. Pancreatic tumors exhibit myeloid-driven amino acid stress and upregulate arginine biosynthesis. eLife 12, e81289 (2023).
Raynor, J. L. & Chi, H. Nutrients: signal 4 in T cell immunity. J. Exp. Med. 221, e20221839 (2024).
Rodriguez, P. C., Ochoa, A. C. & Al-Khami, A. A. Arginine metabolism in myeloid cells shapes innate and adaptive immunity. Front. Immunol. 8, 93 (2017).
Rodriguez, P. C. et al. l-arginine consumption by macrophages modulates the expression of CD3 zeta chain in T lymphocytes. J. Immunol. 171, 1232–1239 (2003).
Crump, N. T. et al. Chromatin accessibility governs the differential response of cancer and T cells to arginine starvation. Cell Rep. 35, 109101 (2021).
Geiger, R. et al. l-Arginine modulates T cell metabolism and enhances survival and anti-tumor activity. Cell 167, 829–842.e13 (2016).
Abbott, K. L. et al. Metabolite profiling of human renal cell carcinoma reveals tissue-origin dominance in nutrient availability. eLife 13, RP95652 (2024).
Neish, W. J. & Rylett, A. Accumulation of phosphoethanolamine in the livers of rats injected with hepatocarcinogens. Br. J. Cancer 14, 737–745 (1960).
Osawa, T. et al. Phosphoethanolamine accumulation protects cancer cells under glutamine starvation through downregulation of PCYT2. Cell Rep. 29, 89–103 (2019).
Courtney, A. H., Lo, W. L. & Weiss, A. TCR signaling: mechanisms of initiation and propagation. Trends Biochem. Sci. 43, 108–123 (2018).
Leney-Greene, M. A., Boddapati, A. K., Su, H. C., Cantor, J. R. & Lenardo, M. J. Human plasma-like medium improves T lymphocyte activation. iScience 23, 100759 (2020).
MacPherson, S. et al. Clinically relevant T cell expansion media activate distinct metabolic programs uncoupled from cellular function. Mol. Ther. Methods Clin. Dev. 24, 380–393 (2022).
Tighanimine, K. et al. A homoeostatic switch causing glycerol-3-phosphate and phosphoethanolamine accumulation triggers senescence by rewiring lipid metabolism. Nat. Metab. 6, 323–342 (2024).
Saab, R. Senescence and pre-malignancy: how do tumors progress? Semin. Cancer Biol. 21, 385–391 (2011).
Wang, B., Kohli, J. & Demaria, M. Senescent cells in cancer therapy: friends or foes? Trends Cancer 6, 838–857 (2020).
Deng, Y., Wu, L. Ding, Q. & Yu, H. AGXT2L1 is downregulated in carcinomas of the digestive system. Oncol. Lett. https://doi.org/10.3892/ol.2020.11645 (2020).
Ding, Q. et al. AGXT2L1 is down-regulated in heptocellular carcinoma and associated with abnormal lipogenesis. J. Clin. Pathol. 69, 215–220 (2016).
Vodnala, S. K. et al. T cell stemness and dysfunction in tumors are triggered by a common mechanism. Science 363, eaau0135 (2019).
Kataoka, S. et al. The costimulatory activity of Tim-3 requires Akt and MAPK signaling and its recruitment to the immune synapse. Sci. Signal 14, eaba0717 (2021).
Matyash, V., Liebisch, G., Kurzchalia, T. V., Shevchenko, A. & Schwudke, D. Lipid extraction by methyl-tert-butyl ether for high-throughput lipidomics. J. Lipid Res. 49, 1137–1146 (2008).
Pino, L. K. et al. The Skyline ecosystem: Informatics for quantitative mass spectrometry proteomics. Mass Spectrom. Rev. 39, 229–244 (2020).
Peralta, R. M. et al. Dysfunction of exhausted T cells is enforced by MCT11-mediated lactate metabolism. Nat. Immunol. 25, 2297–2307 (2024).
Acknowledgements
We thank C. J. Workman for the assistance with synapse imaging, S. G. Wendell and S. J. Mullett for the help with lipidomics analysis and L. C. Hermida for the help with RNA sequencing analysis. A.M. was supported by the Brinson Foundation and the Cancer Research Foundation. We thank L. Becker for sharing interstitial fluid samples from B16F10 murine tumours. We also thank M. V. Heiden and M. Sullivan for sharing interstitial fluid samples from BrafCA; PTENfl/fl; Tyr-CreER and MMTV-Cre; Brca1fl/fl; Trp53+/− tumour-bearing mice. We thank funding sources for this work, including the Cancer Research Institute Lloyd J. Old STAR Award (CRI3447) awarded to G.M.D., Mark Foundation for Cancer Research’s Emerging Leader Award (19-040-ELA) awarded to G.M.D., The Pittsburgh Foundation (MR2023-134140) awarded to G.M.D., NIAID R01AI171483 awarded to G.M.D., NIAID R01AI166598 awarded to G.M.D., NCI R01CA277473 awarded to G.M.D., T32AI089443 awarded to D.W., L70CA294410 awarded to D.W., Brinson Foundation Junior Investigator Award to A.M. and Cancer Research Foundation Young Investigator Award to A.M.
Author information
Authors and Affiliations
Contributions
D.W. and Y.W. designed and performed the majority of experiments, analysed data and wrote the manuscript. They are listed alphabetically by last name on the manuscript, and they provided equal contribution. P.J. conducted and analysed lipidomics analysis. K.L. designed overexpression vectors. C.C. performed and analysed metabolomics. B.C. performed cell culture and flow cytometry experiments. B.X. generated overexpressing tumour cells and aided in tumour experiments. R.M.P. performed and analysed microscopy experiments. E.R.S. performed some of the cell culture studies. W.G.G. measured tumours blinded and generated tumour growth curves. R.A. and H.S. collected and processed data. D.B.R. assisted with tumour growth and tumour-lymphocyte analysis. A.M. and G.M.D. jointly oversaw and directed the research, analysed data, obtained research funding, and wrote and edited the manuscript.
Corresponding authors
Ethics declarations
Competing interests
G.M.D. declares competing financial interests and has submitted patents targeting exhausted T cells that are licensed or pending and is entitled to a share in net income generated from licensing of these patent rights for commercial development. G.M.D. consults for and/or is on the scientific advisory board of BlueSphere Bio, Novasenta, Xyphos and Kalivir Immunotherapeutics; has grants from Novasenta, Astellas, RemplirBio and Kalivir; and owns stock in Novasenta, BlueSphere Bio and RemplirBio. The other authors declare no competing interests.
Peer review
Peer review information
Nature Cell Biology thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
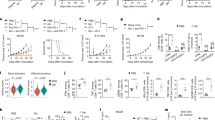
Extended Data Fig. 1 Shorter exposure to TIFM has effect on T cell inhibitory receptor function and Granzyme B expression.
A) %PD1hi cells from Fig. 1c. Data are ±SEM from four independent experiments (n = 8 mice/group (p-value 0.0018). B-E) Cells from Fig. 1d-f analyzed for B) Normalized IFNγ MFI±SEM C) Normalized TNF MFI ±SEM D) %Granzyme Bhi cells and E) %IL-2hi cells. Data quantified from four independent experiments (n = 8-9 mice/group, B) p = 0.1918, C) p = 0.3385 D) p < 0.0001 E) p = 0.5219). Statistics were calculated using a two-tailed paired t-test. B) p-value F) Timeline Schematic of experiments in G-J G) Normalized PD-1 MFI (mean fluorescence intensity) ±SEM from four independent experiments (n = 8-9 mice/group, p-value RPMI vs. T3 p = 0.0010, T1 vs. T3 p = 0.0061). H) Day 7 OT-I CD8+ T cells were analyzed for IFNγ+TNF+ co-expression after 6 hours αCD3/αCD28 restimulation. Data quantified show %IFNγ+TNF+ events among Live CD8+ T cells ±SEM from four independent experiments (n = 8 mice/group). I-J) Day 7 OT-I T cells were analyzed for expression of I) IL-2 or J) Granzyme B after 6 hours of αCD3/αCD28 restimulation. Data quantified show normalized MFI ±SEM from four independent experiments (n = 8-9 mice/group, J) p-value RPMI vs. T3 p < 0.0001, RPMI vs. T3, p = 0.0026). K) Day 7 OT-I CD8+ T cells were co-cultured with B16-OVA cells at a 1:1 effector:target ratio in an Agilent xCELLigence RTCA DP system to monitor target cell killing. XY plot on the left shows %Cytolysis ±SEM, and Area under the curve values are quantified from three independent experiments (n = 5-6 mice/group, p values RPMI vs. T1 p = 0.9897, RPMI vs. T3, p = 0.0179, T1 vs. T3, p = 0.0224). Statistical analysis for all indicated comparisons in F-K) was determined using a one-way ANOVA with Tukey’s multiple comparisons test. L-O) Cells from Fig. 1h-j were restimulated with αCD3/αCD28 in RPMI or TIFM for 6 hours. After restimulation, cells were analyzed for L) Normalized IFNγ MFI of Live IFN+ CD8+ Cells, M) Normalized TNF MFI of Live TNF+CD8+ cells N) % of Granzyme Bhi cells and J) % of IL-2hi cells. (n = 8 mice/group from three independent experiments). For L-O), statistics were calculated using a two-tailed paired t-test (p-values L) p = 0.0268 M) p < 0.0001, N) p < 0.0001, O) p = 0.0006) Source numerical data are available in source data.
Extended Data Fig. 2 Differences in metabolite concentrations between TIF and plasma.
Fold change of the metabolite concentrations measured in the TIF relative to those in the plasma of mice bearing PDAC tumor, highlighted metabolites are marked by color to indicate metabolites that are depleted (red) and enriched (blue) in the tumor. Source numerical data are available in source data.
Extended Data Fig. 3 TIFM +Arginine and RPMI supplemented with phosphoethanolamine alone have effector dysfunction.
A) Data related to Fig. 2c. Data from Fig. 2c were analyzed for % of PD-1hi cells from three independent experiments (n = 5 mice/group, RPMI vs. TIFM p = 0.0499, RPMI vs. TIFM+Arg p = 0.190, TIFM vs. TIFM+Arg p = 0.0954) B-E) Data from Fig. 2d-f were analyzed for B) Normalized IFNγ MFI of Live IFN+ CD8+ Cells, C) Normalized TNF MFI of Live TNF+CD8+ cells D) % of IL-2hi cells and E) % of Granzyme Bhi cells. Data are compiled from three independent experiments (n = 5 mice/group, p-values B) RPMI vs. TIFM+Arg p = 0.0478, TIFM vs. TIFM+Arg, p = 0.0398, C) RPMI vs. TIFM+Arg, p = 0.0025, TIFM vs. TIFM+Arg, p = 0.0453, D) RPMI + TIFM+Arg p = 0.0151, E) RPMI vs. TIFM p < 0.0001, RPMI vs. TIFM+Arg p = 0.0002, TIFM vs. TIFM+Arg, p = 0.0002). F-I) Data from Fig. 3i-k were analyzed for F) Normalized IFNγ MFI of Live IFN+ CD8+ Cells, G) Normalized TNF MFI of Live TNF+CD8+ cells H) % of IL-2hi cells and I) % of Granzyme Bhi cells. Data from I-L are quantified from 3 independent experiments (n = 6 mice/group, p-values, F) RPMI vs. RPMI+pEtn p = 0.0627, RPMI vs. TIFM p = 0.0424, RPMI+pEtn vs. TIFM p = 0.0030, G) RPMI vs. RPMI+pEtn p = 0.0336, RPMI vs. TIFM p = 0.0856, RPMI+pEtn vs. TIFM p = 0.8107, H) RPMI vs. RPMI+pEtn p = 0.0137, RPMI vs. TIFM p = 0.9164, RPMI+pEtn vs. TIFM p = 0.2426 I) RPMI vs. RPMI+pEtn p = 0.0018, RPMI vs. TIFM p < 0.0001, RPMI+pEtn vs. TIFM p = 0.0055). Statistical analysis for all indicated comparisons in this figure was determined using a one-way ANOVA with Tukey’s multiple comparisons test. Source numerical data are available in source data.
Extended Data Fig. 4 pEtn or TIFM treatment effects on CD8+ T cells can be reversed by Pcyt2-KO or pEtn addback, respectively.
A-C) OT-I CD8 + T cells were activated and cultured as in Fig. 1a) in RPMI, TIFM, or TIFM formulated without pEtn and supplemented with RPMI levels of Arginine. (TIFM -pEtn +Arg) for 5 days. At the end of culture, cells were restimulated and analyzed for A) Granzyme B MFI B) IL-2 MFI and C) %IFNγ+TNF+ cells. Bar plots show quantified normalized MFI or %+ ±SEM on the left of each flow cytogram, data are from 3 independent experiments (n = 6 mice/group, p-values A) RPMI vs. TIFM p = 0.0014, TIFM vs. TIFM+Arg-pEtn p = 0.0458, RPMI vs. TIFM-pEtn+Arg p = 0.5720 B) RPMI vs. TIFM p = 0.0389, TIFM vs. TIFM+Arg-pEtn p = 0.0315 RPMI vs. TIFM-pEtn+Arg p = 0.9741 C) RPMI vs. TIFM p = 0.0019, TIFM vs. TIFM+Arg-pEtn p = 0.0210, RPMI vs. TIFM-pEtn+Arg p = 0.1116). Statistical analysis for all indicated comparisons in A-C) was determined using a one-way analysis of variance (ANOVA) with Tukey’s multiple comparisons test. Source numerical data are available in source data.
Extended Data Fig. 5 The TIF contains an altered metabolic milieu from that of the plasma.
A) Data analyzed from previous reports31. Previously generated data pEtn concentration (µM) of human patient plasma or renal cell tumor tumor interstitial fluid (TIF) (n = 27-46 samples/group, p < 0.0001). B) Choline and phosphocholine levels in the plasma and the tumor interstitial fluid of mice bearing PDAC tumors. (n = 7 samples/group). Statistical significance for indicated comparison quantified using a two-tailed unpaired t-test. Source numerical data are available in source data. Error bars in both graphs represent SEM.
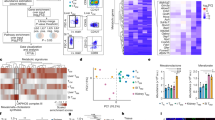
Extended Data Fig. 6 T cells exposed to pEtn have an altered intracellular lipid phenotype.
A) PCA analysis of lipidomics performed in 4I-O and S6B B) Heatmap showing Log2FC over mean for each lipid measured in lipidomics described in Fig. 4i-o). C-E) Lipidomics analysis of activated OT-I T cells cultured in either RPMI or RPMI supplemented with pEtn. C) Ratio of phosphatidylethanolamine (PE) to Phosphatidylcholine (PC) (p-value p = 0.0142). Boxes range from 25th to 75th percentiles, while the whiskers extend to the minimum and maximum value for each dataset. Fold change of D) PE species and E) PC species in pEtn treated OT-I T F-H) Cells treated with F) pEtn, G) Choline, or H) pCholine at TIFM-levels for 3 or 5 days and analyzed via lipidomics as described in Fig. 4i-o). Each volcano plot shows Log2 fold change of treated cells over RPMI-treated controls on the x axis, and Log10 p-value on the y axis. Red dots indicate DAG species, tan dots are PE species, blue dots are PC species, and purple dots are sphingomyelins. I) DAG levels in OT-I T cells that were cultured as described in 4I-O). Normalized DAG MFI is quantified on the left from six independent experiments (n = 18 mice/group, RPMI vs. pEtn p = 0.0037, RPMI vs. Chol p = 0.8314, RPMI vs. pChol p = 0.9923) and statistical analysis for all indicated comparisons in was determined using a one-way analysis of variance (ANOVA) with Tukey’s multiple comparisons test. J) RNA expression of choline and choline-like transporters in PD1+Tim3+ tumor-infiltrating CD8 + T cells. Data retrieved from previous report46. ND = transcripts not detected. K-M) Representative flow plots for quantification shown in Fig. 4 Panels Q-S). N) Western blot for Pcyt2 and β-Actin on Day 7 WT and Pcyt2 KO CD8 T cells from Fig. 4p-s. (n = 4 mice/group from 3 independent experiments) Statistical significance for indicated comparison in ED6C) quantified using a two-tailed paired t-test (p = 0.0098). Proteins were probed across multiple independent blots that were loaded with the same original proteins. Source numerical data are available in source data.
Extended Data Fig. 7 pEtn-treated T cells have changes in metabolic and transcriptional pathways, and functional changes driven by pEtn can be rescued with PMA/Ionomycin.
A) Quantification of metabolites in TCA cycle, oxidative phosphorylation, glycolysis, glutaminolysis and pentose phosphate pathway of SIINFEKL-activated OT-I T cells in RPMI+pEtn versus RPMI culture B) Quantification of lipid metabolites of SIINFEKL-activated OT-I T cells in RPMI+pEtn versus RPMI culture as shown in Fig. 3a. C) Volcano plot of RNA-Sequencing of activated OT-I T cells cultured in RPMI or RPMI +pEtn. Highlighted genes represent exhaustion associated genes. D) Gene set enrichment assay of RNA-Seq data shown in C) of activated OT-I cells cultured in either RPMI or RPMI+pEtn. (E-G) OT-I T cells activated by SIINFEKL O/N were cultured in RPMI for 2 days and then in either RPMI or RPMI+pEtn for 5 days as described in Fig. 3a). Then the cells were restimulated by either α-CD3 and α-CD28 or PMA/Ionomycin for 6 hours in RPMI. After restimulation, cells were analyzed for E) IFNγ and TNF co-expression F) Granzyme B expression, and G) IL-2 expression by flow cytometry. Normalized MFI ±SEM is quantified for Granzyme B and IL-2, while %IFNγ+TNF+ cells ±SEM are quantified on the left of each plot from three independent experiments (n = 6 mice/group, p-values left to right E) p = 0.0002, p = 0.6170, F) p = 0.0046, p = 0.0267, G) p = 0.0007, p = 0.2566). two-tailed paired t-test. ns, non-significant. Source numerical data are available in source data.
Extended Data Fig. 8 pEtn-treated T cells have altered lipid raft abundance, but no changes in cell cycle or steady state cytokine production.
A) OT-I CD8+ T cells were treated with RPMI + pEtn for 5 days as described in Fig. 3a then labeled with Cholera Toxin Subunit B-GFP. Sum fluorescent intensity of GFP is quantified on the left. (n = 70-80 cells/group) Statistical significance for indicated comparison was calculated using a two-tailed unpaired t-test (p < 0.0001). B) OT-I CD8+ T cells were treated with RPMI + pEtn for 5 days as described in Fig. 3a. On day 7, cell lysates were prepared, and western blot was performed with antibodies specific for Cdk6, Cyclin D3, phosphorylated and total Chk1 (pChk, and tChk, respectively). Representative blots are shown on the left. Quantification on the right is from 3 independent experiments (n = 6 mice/group, p values left to right, CDK6 p = 0.4213, Cyclin D3 p = 0.5531, p-Chk1 p = 0.5491) Proteins were probed across multiple, independent blots that were loaded with the same original proteins. C-E) OT-I T cells were treated as in A), then on Day 7, cells were treated with protein transport inhibitor cocktail for 6 hours without restimulation, and then C) Granzyme B MFI, p = 0.4806, D) IL-2 MFI, p = 0.9536, and E) %IFNγ+TNF+, p = 0.6104, was measured by flow cytometry. All data in C-E) are from 3 independent experiments (n = 6 mice/group). Statistical significance for indicated comparisons was calculated using a two-tailed paired t-test. Source numerical data and unprocessed blots are available in source data.
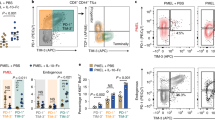
Extended Data Fig. 9 Pcyt2-overexpression in B16 melanoma does not alter PD1+Tim-3+ frequency among the endogenous CD8+ T cells.
A) DAG expression of the tumor infiltrating transferred Pmel T cells from the tumors in Fig. 6a. Bar graph on the left shows normalized DAG MFI ±SEM from three independent experiments (n = 7 mice/group, p = 0.0729) B) Western blot confirmation for Pcty2 and Actin in B16 EV and B16-Pcyt2 melanoma cell lines. (Data show n = 3 lysates from 1 independent experiment) Proteins were probed across multiple independent blots that were loaded with the same original proteins. C) PD1 and Tim3 co-expression of endogenous CD8+ T cells from tumors in Fig. 6i, Data quantified on the left show % PD1hiTim3hi from three independent experiments (n = 14-15 mice/group, p = 0.8162). Statistical significance for all indicated comparisons in this figure was calculated using a two-tailed unpaired t-test. D) Data from Fig. 6k were analyzed for Normalized TNF MFI of Live CD8+ TNF+ cells. Data are quantified from three independent experiments (n = 9-10 mice/group, p = 0.1188) E-F) pmel T cells from tumor-draining lymph nodes were restimulated with gp100 peptide for 1 hour and analyzed by flow cytometry for E) % p-S6+ (n = 8-10 mice/group from 3 independent experiments p = 0.8470) and F) % p-ERK+ (n = 8-10 mice/group from 3 independent experiments p = 0.7162) Statistical significance for all indicated comparisons in this figure was calculated using a two-tailed unpaired t-test. Source numerical data and unprocessed blots are available in source data.
Supplementary information
Supplementary Fig. 1
Gating strategy for CD8+ T cells isolated from cell culture: cells were collected on day 7 of In vitro RPMI culture. The flow cytograms show the representative gating strategy of CD8+ T cells used throughout all in vitro experiments.
Supplementary Table 1
TIFM formulation.
Source data
Source Data
Source data for all figures and extended data figures, with each figure as its own tab.
Unprocessed Blots
Unprocessed blot images for all figures (labelled on each page).
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, Y., Wilfahrt, D., Jonker, P. et al. Tumour interstitial fluid-enriched phosphoethanolamine suppresses T cell function. Nat Cell Biol 27, 835–846 (2025). https://doi.org/10.1038/s41556-025-01650-9
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41556-025-01650-9