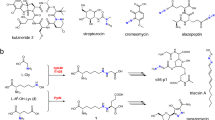
Abstract
Azides are energy-rich compounds with diverse representation in a broad range of scientific disciplines, including material science, synthetic chemistry, pharmaceutical science and chemical biology. Despite ubiquitous usage of the azido group, the underlying biosynthetic pathways for its formation remain largely unknown. Here we report the characterization of an enzymatic route for de novo azide construction. We demonstrate that Tri17, a promiscuous ATP- and nitrite-dependent enzyme, catalyses organic azide synthesis through sequential N-nitrosation and dehydration of aryl hydrazines. Through biochemical, structural and computational analyses, we further propose a plausible molecular mechanism for azide synthesis that sets the stage for future biocatalytic applications and biosynthetic pathway engineering.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All data supporting the findings of this study are available within the paper, the Supplementary Information, Supplementary data file, source data and extended data. The coordinates and structure factor amplitudes for the apo structure of Tri17 and the complex structure with ATP have been deposited to the Protein Data Bank (PDB) under accession codes 8TF7 and 9BQ0, respectively. The AlphaFold2 model is available in ModelArchive with accession code ma-zyg1d. Source data are provided with this paper.
References
Lin, T.-S. & Prusoff, W. H. Synthesis and biological activity of several amino analogs of thymidine. J. Med. Chem. 21, 109–112 (1978).
Liu, Y. et al. Anti-HIV agent azidothymidine decreases Tet(X)-mediated bacterial resistance to tigecycline in Escherichia coli. Commun. Biol. 3, 162 (2020).
Huynh, M. H. V., Hiskey, M. A., Chavez, D. E., Naud, D. L. & Gilardi, R. D. Synthesis, characterization and energetic properties of diazido heteroaromatic high-nitrogen C-N compound. J. Am. Chem. Soc. 127, 12537–12543 (2005).
Agrawal, J. P. & Hodgson, R. D. Organic Chemistry of Explosives (Wiley, 2007).
Bräse, S., Gil, C., Knepper, K. & Zimmermann, V. Organic azides: an exploding diversity of a unique class of compounds. Angew. Chem. Int. Ed. 44, 5188–5240 (2005).
Thirumurugan, P., Matosiuk, D. & Jozwiak, K. Click chemistry for drug development and diverse chemical-biology applications. Chem. Rev. 113, 4905–4979 (2013).
Zhu, X., Liu, J. & Zhang, W. De novo biosynthesis of terminal alkyne-labeled natural products. Nat. Chem. Biol. 11, 115–120 (2015).
Sletten, E. M. & Bertozzi, C. R. Bioorthogonal chemistry: fishing for selectivity in a sea of functionality. Angew. Chem. Int. Ed. 48, 6974–6998 (2009).
Grammel, M. & Hang, H. C. Chemical reporters for biological discovery. Nat. Chem. Biol. 9, 475–484 (2013).
Del Rio Flores, A. et al. Biosynthesis of isonitrile- and alkyne-containing natural products. Annu. Rev. Chem. Biomol. Eng. 13, 1–24 (2022).
Matthews, M. L. et al. Direct nitration and azidation of aliphatic carbons by an iron-dependent halogenase. Nat. Chem. Biol. 10, 209–215 (2014).
Kim, C. Y. et al. The chloroalkaloid (−)-acutumine is biosynthesized via a Fe(II)- and 2-oxoglutarate-dependent halogenase in Menispermaceae plants. Nat. Commun. 11, 1867 (2020).
Neugebauer, M. E. et al. A family of radical halogenases for the engineering of amino-acid-based products. Nat. Chem. Biol. 15, 1009–1016 (2019).
Voss, M., Honda Malca, S. & Buller, R. Exploring the biocatalytic potential of Fe/α-ketoglutarate-dependent halogenases. Chem. A Eur. J. 26, 7336–7345 (2020).
Gomez, C. A., Mondal, D., Du, Q., Chan, N. & Lewis, J. C. Directed evolution of an Iron(II)‐ and α‐ketoglutarate‐dependent dioxygenase for site‐selective azidation of unactivated aliphatic C−H bonds. Angew. Chem. 62, e202301370 (2023).
Rui, J. et al. Directed evolution of non-heme iron enzymes to access a non-natural radical-relay C(sp3)−H azidation. Science 376, 869–874 (2022).
Chan, N. H. et al. Non-native anionic ligand binding and reactivity in engineered variants of the Fe(II)- and α-ketoglutarate-dependent oxygenase, SadA. Inorg. Chem. 61, 14477–14485 (2022).
Del Rio Flores, A. et al. Biosynthesis of triacsin featuring an N-hydroxytriazene pharmacophore. Nat. Chem. Biol. 17, 1305–1313 (2021).
Matsuda, K. et al. Discovery of unprecedented hydrazine-forming machinery in bacteria. J. Am. Chem. Soc. 140, 9083–9086 (2018).
Zhao, G. et al. Molecular basis of enzymatic nitrogen–nitrogen formation by a family of zinc-binding cupin enzymes. Nat. Commun. 12, 7205 (2021).
He, H. Y., Niikura, H., Du, Y. L. & Ryan, K. S. Synthetic and biosynthetic routes to nitrogen–nitrogen bonds. Chem. Soc. Rev. 51, 2991–3046 (2022).
Matsuda, K. et al. A natural dihydropyridazinone scaffold generated from a unique substrate for a hydrazine-forming enzyme. J. Am. Chem. Soc. 144, 12954–12960 (2022).
Arima, K., Akiyama, S., Shin-ya, K., Matsuda, K. & Wakimoto, T. Carrier protein mediated formation of the dihydropyridazinone ring in actinopyridazinone biosynthesis. Angew. Chem. Int. Ed. https://doi.org/10.1002/anie.202305155 (2023).
Matsuda, K. & Wakimoto, T. Bacterial hydrazine biosynthetic pathways featuring cupin/methionyl tRNA synthetase-like enzymes. ChemBioChem https://doi.org/10.1002/cbic.202300874 (2024).
Waldman, A. J. & Balskus, E. P. Discovery of a diazo-forming enzyme in cremeomycin biosynthesis. J. Org. Chem. 83, 7539–7546 (2018).
Ma, G. L. et al. Biosynthesis of tasikamides via pathway coupling and diazonium-mediated hydrazone formation. J. Am. Chem. Soc. 144, 1622–1633 (2022).
Kawai, S., Hagihara, R., Shin-ya, K., Katsuyama, Y. & Ohnishi, Y. Bacterial avenalumic acid biosynthesis includes substitution of an aromatic amino group for hydride by nitrous acid dependent diazotization. Angew. Chem. 61, e202211728 (2022).
Kawai, S. et al. Identification and analysis of the biosynthetic gene cluster for the hydrazide-containing aryl polyene spinamycin. ACS Chem. Biol. 18, 1821–1828 (2023).
Yoshida, K. et al. Studies on new vasodilators, WS-1228 A and B I. Discovery, taxonomy, isolation and characterization. J. Antibiot. (Tokyo) 35, 151–156 (1981).
Reece, P. A. Hydralazine and related compounds: chemistry, metabolism and mode of action. Med. Res. Rev. 1, 73–96 (1981).
Arce, C. et al. Hydralazine target: from blood vessels to the epigenome. J. Transl. Med. 4, 31 (2006).
De Flora, S. et al. In vivo and in vitro genotoxicity of three antihypertensive hydrazine derivatives (hydralazine, dihydralazine and endralazine). Environ. Mutagen. 4, 605–619 (1982).
Thorson, M. K., Majtan, T., Kraus, J. P. & Barrios, A. M. Identification of cystathionine β-synthase inhibitors using a hydrogen sulfide selective probe. Angew. Chem. Int. Ed. 52, 4641–4644 (2013).
Chen, B. et al. Fluorescent probe for highly selective and sensitive detection of hydrogen sulfide in living cells and cardiac tissues. Analyst 138, 946–951 (2013).
Omura, S., Tomoda, H., Xu, M. Q., Takahashi, Y. & Iwai, Y. Triacsins, new inhibitors of acyl-CoA synthetase produced by Streptomyces sp. J. Antibiot. (Tokyo) 39, 1211–1218 (1986).
Twigg, F. F. et al. Identifying the biosynthetic gene cluster for triacsins with an N-hydroxytriazene moiety. ChemBioChem 20, 1145–1149 (2019).
Nunez Avila, A. G. et al. Surprising chemistry of 6-azidotetrazolo[5,1-a]phthalazine: what a purported natural product reveals about the polymorphism of explosives. J. Org. Chem. 87, 6680–6694 (2022).
Blair, L. M. & Sperry, J. Natural products containing a nitrogen–nitrogen bond. J. Nat. Prod. 76, 794–812 (2013).
Hossain, M. B., van der Helm, D., Sanduja, R. & Alam, M. Structure of 6-azidotetrazolo[5,1-a]phthalazine, C8H4N8, isolated from the toxic dinoflaggelate Gymnodinium breve. Acta Crystallogr. C Cryst. Struct. Commun. 41, 1199–1202 (1985).
Tišler, M. Some aspects of azido-tetrazolo isomerization. Synthesis 3, 123–136 (1973).
Krivopalov, V. P., Baram, S. G., Denisov, A. Y. & Mamatyuk, V. I. Azide–tetrazole tautomerism of diazidodiazines and their benzo analogs. Bull. Acad. Sci. USSR Div. Chem. Sci. 38, 1839–1844 (1989).
Hiroshi, T., Kazuaki, I. & Satoshi, O. Inhibition of acyl-CoA synthetase by triacsins. Biochim. Biophys. Acta 921, 595–598 (1987).
Li, Z. & Nair, S. K. Structural basis for specificity and flexibility in a plant 4-coumarate:CoA ligase. Structure 23, 2032–2042 (2015).
Gulick, A. M. Conformational dynamics in the acyl-CoA synthetases, adenylation domains of non-ribosomal peptide synthetases, and firefly luciferase. ACS Chem. Biol. 4, 811–827 (2009).
Holm, L. Dali server: structural unification of protein families. Nucleic Acids Res. 50, W210–W215 (2022).
Wang, Y., Yi, H., Wang, M., Yu, O. & Jez, J. M. Structural and kinetic analysis of the unnatural fusion protein 4-coumaroyl-CoA ligase::stilbene synthase. J. Am. Chem. Soc. 133, 20684–20687 (2011).
Yang, Z. et al. UCSF Chimera, MODELLER and IMP: an integrated modeling system. J. Struct. Biol. 179, 269–278 (2012).
Xu, T. et al. Induced-fit docking enables accurate free energy perturbation calculations in homology models. J. Chem. Theory Comput. 18, 5710–5724 (2022).
Tian, W., Chen, C., Lei, X., Zhao, J. & Liang, J. CASTp 3.0: computed atlas of surface topography of proteins. Nucleic Acids Res. 46, W363–W367 (2018).
Smith, R. H. B., Dar, A. C. & Schlessinger, A. PyVOL: a PyMOL plugin for visualization, comparison and volume calculation of drug-binding sites. Preprint at https://www.biorxiv.org/content/10.1101/816702v1 (2019).
Trott, O. & Olson, A. J. Autodock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization and multithreading. J. Comput. Chem. 31, 455–461 (2009).
Eberhardt, J., Santos-Martins, D., Tillack, A. F. & Forli, S. AutoDock Vina 1.2.0: new docking methods, expanded force field and Python bindings. J. Chem. Inf. Model. 61, 3891–3898 (2021).
Waldman, A. J., Ng, T. L., Wang, P. & Balskus, E. P. Heteroatom–heteroatom bond formation in natural product biosynthesis. Chem. Rev. 117, 5784–5863 (2017).
Myznikov, L. V., Vorona, S. V. & Zevatskii, Y. E. Biologically active compounds and drugs in the tetrazole series. Chem. Heterocycl. Compd. 57, 224–233 (2021).
Zou, Y., Liu, L., Liu, J. & Liu, G. Bioisosteres in drug discovery: focus on tetrazole. Future Med. Chem. 12, 91–93 (2020).
Ostrovskii, V. A., Trifonov, R. E. & Popova, E. A. Medicinal chemistry of tetrazoles. Russ. Chem. Bull. 61, 768–780 (2012).
Tsugawa, H. et al. MS-DIAL: data-independent MS/MS deconvolution for comprehensive metabolome analysis. Nat. Methods 12, 523–526 (2015).
Neese, F. Software update: the ORCA program system—Version 5.0. Wiley Interdiscip. Rev. Comput. Mol. Sci. 12, e1606 (2022).
Stephens, P. J., Devlin, F. J., Chabalowski, C. F. & Frisch, M. J. Ab initio calculation of vibrational absorption. J. Phys. Chem. 98, 11623–11627 (1994).
Lee, C., Yang, W. & Parr, R. G. Development of the Colle–Salvetti correlation-energy formula into a function of the electron density. Phys. Rev. B 37, 785–789 (1988).
Becke, A. D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 103, 5648–5652 (1993).
Grimme, S., Antony, J., Ehrlich, S. & Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 132, 154104 (2010).
Lange, A. W. & Herbert, J. M. A smooth, nonsingular and faithful discretization scheme for polarizable continuum models: the switching/Gaussian approach. J. Chem. Phys. 133, 244111 (2010).
York, D. M. A smooth solvation potential based on the conductor-like screening model. J. Phys. Chem. A 103, 11040–11044 (1999).
Henkelman, G. & Jónsson, H. Improved tangent estimate in the nudged elastic band method for finding minimum energy paths and saddle points. J. Chem. Phys. 113, 9978–9985 (2000).
Baker, J. An algorithm for the location of transition states. J. Comput. Chem. 7, 385–395 (1986).
Zhong, S., Barnes, E. C. & Petersson, G. A. Uniformly convergent n-tuple-ζ augmented polarized (nZaP) basis sets for complete basis set extrapolations. I. Self-consistent field energies. J. Chem. Phys. 129, 184116 (2008).
Helgaker, T., Klopper, W., Koch, H. & Noga, J. Basis-set convergence of correlated calculations on water. J. Chem. Phys. 106, 9639–9646 (1997).
Riplinger, C. & Neese, F. An efficient and near linear scaling pair natural orbital based local coupled cluster method. J. Chem. Phys. 138, 034106 (2013).
Møller, C. & Plesset, M. S. Note on an approximation treatment for many-electron systems. Phys. Rev. 46, 618–622 (1934).
Klamt, A. & Schürmann, G. COSMO: a new approach to dielectric screening in solvents with explicit expressions for the screening energy and its gradient. J. Chem. Soc. Perkin Trans. 2 1993, 799–805 (1993).
Kabsch, W. XDS. Acta Crystallogr. D Biol. Crystallogr. 66, 125–132 (2010).
Evans, P. R. & Murshudov, G. N. How good are my data and what is the resolution? Acta Crystallogr. D Biol. Crystallogr. 69, 1204–1214 (2013).
Jumper, J. et al. Highly accurate protein structure prediction with AlphaFold. Nature 596, 583–589 (2021).
Mirdita, M. et al. ColabFold: making protein folding accessible to all. Nat. Methods 19, 679–682 (2022).
McCoy, A. J. et al. Phaser crystallographic software. J. Appl. Crystallogr. 40, 658–674 (2007).
Adams, P. D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 66, 213–221 (2010).
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 60, 2126–2132 (2004).
Afonine, P. V. et al. Towards automated crystallographic structure refinement with phenix.refine. Acta Crystallogr. D Biol. Crystallogr. 68, 352–367 (2012).
Gulick, A. M., Starai, V. J., Horswill, A. R., Homick, K. M. & Escalante-Semerena, J. C. The 1.75 Å crystal structure of acetyl-CoA synthetase bound to adenosine-5′-propylphosphate and coenzyme A. Biochemistry 42, 2866–2873 (2003).
Hisanaga, Y. et al. Structural basis of the substrate-specific two-step catalysis of long chain fatty acyl-CoA synthetase dimer. J. Biol. Chem. 279, 31717–31726 (2004).
Reger, A. S., Carney, J. M. & Gulick, A. M. Biochemical and crystallographic analysis of substrate binding and conformational changes in acetyl-CoA synthetase. Biochemistry 46, 6536–6546 (2007).
Law, A. & Boulanger, M. J. Defining a structural and kinetic rationale for paralogous copies of phenylacetate-CoA ligases from the cystic fibrosis pathogen Burkholderia cenocepacia J2315. J. Biol. Chem. 286, 15577–15585 (2011).
Reger, A. S., Wu, R., Dunaway-Mariano, D. & Gulick, A. M. Structural characterization of a 140° domain movement in the two-step reaction catalyzed by 4-chlorobenzoate:CoA ligase. Biochemistry 47, 8016–8025 (2008).
Yonus, H. et al. Crystal structure of DltA: implications for the reaction mechanism of non-ribosomal peptide synthetase adenylation domains. J. Biol. Chem. 283, 32484–32491 (2008).
Kochan, G., Pilka, E. S., von Delft, F., Oppermann, U. & Yue, W. W. Structural snapshots for the conformation-dependent catalysis by human medium-chain acyl-coenzyme a synthetase ACSM2A. J. Mol. Biol. 388, 997–1008 (2009).
Shah, M. B. et al. The 2.1 Å crystal structure of an acyl-CoA synthetase from Methanosarcina acetivorans reveals an alternate acyl-binding pocket for small branched acyl substrates. Proteins Struct. Funct. Bioinform. 77, 685–698 (2009).
Hughes, A. J. & Keatinge-Clay, A. Enzymatic extender unit generation for in vitro polyketide synthase reactions: structural and functional showcasing of Streptomyces coelicolor MatB. Chem. Biol. 18, 165–176 (2011).
Sundlov, J. A., Shi, C., Wilson, D. J., Aldrich, C. C. & Gulick, A. M. Structural and functional investigation of the intermolecular interaction between NRPS adenylation and carrier protein domains. Chem. Biol. 19, 188–198 (2012).
Kaljunen, H. et al. Structural elucidation of the bispecificity of a domains as a basis for activating non-natural amino acids. Angew. Chem. Int. Ed. 54, 8833–8836 (2015).
Mitchell, C. A., Shi, C., Aldrich, C. C. & Gulick, A. M. Structure of PA1221, a nonribosomal peptide synthetase containing adenylation and peptidyl carrier protein domains. Biochemistry 51, 3252–3263 (2012).
Westfall, C. S. et al. Structural basis for prereceptor modulation of plant hormones by GH3 proteins. Science 336, 1708–1711 (2012).
Sundlov, J. A., Fontaine, D. M., Southworth, T. L., Branchini, B. R. & Gulick, A. M. Crystal structure of firefly luciferase in a second catalytic conformation supports a domain alternation mechanism. Biochemistry 51, 6493–6495 (2012).
Sundlov, J. A. & Gulick, A. M. Structure determination of the functional domain interaction of a chimeric nonribosomal peptide synthetase from a challenging crystal with noncrystallographic translational symmetry. Acta Crystallogr. D Biol. Crystallogr. 69, 1482–1492 (2013).
Henderson, J. C. et al. Antimicrobial peptide resistance of Vibrio cholerae results from an LPS modification pathway related to nonribosomal peptide synthetases. ACS Chem. Biol. 9, 2382–2392 (2014).
Thornburg, C. K., Wortas-Strom, S., Nosrati, M., Geiger, J. H. & Walker, K. D. Kinetically and crystallographically guided mutations of a Benzoate CoA Ligase (BadA) elucidate mechanism and expand substrate permissivity. Biochemistry 54, 6230–6242 (2015).
Huang, Y.-B., Luo, Y.-J., Del Rio Flores, A., Li, L.-C. & Wang, F. N-aryl pyrrole synthesis from biomass derived furans and arylamine over Lewis acidic Hf doped mesoporous SBA-15 catalyst. ACS Sustain. Chem. Eng. 8, 12161–12167 (2020).
Wang, N. et al. Natural separation of the acyl-CoA ligase reaction results in a non-adenylating enzyme article. Nat. Chem. Biol. 14, 730–737 (2018).
Cieślak, J. et al. Biochemical characterization and structural insight into aliphatic β-amino acid adenylation enzymes IdnL1 and CmiS6. Proteins Struct. Funct. Bioinform. 85, 1238–1247 (2017).
Han, X. et al. Cyclic AMP inhibits the activity and promotes the acetylation of acetyl-CoA synthetase through competitive binding to the ATP/AMP pocket. J. Biol. Chem. 292, 1374–1384 (2017).
Tripathi, A. et al. A defined and flexible pocket explains aryl substrate promiscuity of the Cahuitamycin starter unit–activating enzyme CahJ. ChemBioChem 19, 1595–1600 (2018).
Chen, Y. et al. Crystal structure of the thioesterification conformation of Bacillus subtilis o-succinylbenzoyl-CoA synthetase reveals a distinct substrate-binding mode. J. Biol. Chem. 292, 12296–12310 (2017).
Ishikawa, F. et al. An engineered aryl acid adenylation domain with an enlarged substrate binding pocket. Angew. Chem. 131, 6980–6984 (2019).
Miyanaga, A., Kurihara, S., Chisuga, T., Kudo, F. & Eguchi, T. Structural characterization of complex of adenylation domain and carrier protein by using pantetheine cross-linking probe. ACS Chem. Biol. 15, 1808–1812 (2020).
DeBouver, N. D. et al. Bacterial structural genomics target enabled by a recently discovered potent fungal acetyl-CoA synthetase inhibitor. Acta Crystallogr. F Struct. Biol. Commun. 79, 137–143 (2023).
Corpuz, J. C. et al. Essential role of loop dynamics in type II NRPS biomolecular recognition. ACS Chem. Biol. 17, 2890–2898 (2022).
Chen, I. H. et al. Characterization and structural determination of CmnG-A, the adenylation domain that activates the nonproteinogenic amino acid capreomycidine in capreomycin biosynthesis. ChemBioChem 23, e202200563 (2022).
Steinegger, M. & Söding, J. MMseqs2 enables sensitive protein sequence searching for the analysis of massive data sets. Nat. Biotechnol. 35, 1026–1028 (2017).
Heo, L. & Feig, M. Multi-state modeling of G-protein coupled receptors at experimental accuracy. Proteins Struct. Funct. Bioinform. 90, 1873–1885 (2022).
Del Alamo, D., Sala, D., McHaourab, H. S. & Meiler, J. Sampling alternative conformational states of transporters and receptors with AlphaFold2. eLife 11, e75751 (2022).
Zhang, Y. & Skolnick, J. TM-align: a protein structure alignment algorithm based on the TM-score. Nucleic Acids Res. 33, 2302–2309 (2005).
Xu, J. & Zhang, Y. How significant is a protein structure similarity with TM-score = 0.5? Bioinformatics 26, 889–895 (2010).
Williams, C. J. et al. MolProbity: more and better reference data for improved all-atom structure validation. Protein Sci. 27, 293–315 (2018).
Wiederstein, M. & Sippl, M. J. ProSA-web: interactive web service for the recognition of errors in three-dimensional structures of proteins. Nucleic Acids Res. 35, 407–410 (2007).
Jacobson, M. P. et al. A hierarchical approach to all-atom protein loop prediction. Proteins Struct. Funct. Genet. 55, 351–367 (2004).
Jacobson, M. P., Friesner, R. A., Xiang, Z. & Honig, B. On the role of the crystal environment in determining protein side-chain conformations. J. Mol. Biol. 320, 597–608 (2002).
Jorgensen, W. L., Maxwell, D. S. & Tirado-Rives, J. Development and testing of the OPLS all-atom force field on conformational energetics and properties of organic liquids. J. Am. Chem. Soc. 118, 11225–11236 (1996).
Sherman, W., Day, T., Jacobson, M. P., Friesner, R. A. & Farid, R. Novel procedure for modeling ligand/receptor induced fit effects. J. Med. Chem. 49, 534–553 (2006).
Miller, E. B. et al. Reliable and accurate solution to the induced fit docking problem for protein-ligand binding. J. Chem. Theory Comput. 17, 2630–2639 (2021).
Halgren, T. A. Identifying and characterizing binding sites and assessing druggability. J. Chem. Inf. Model. 49, 377–389 (2009).
Halgren, T. New method for fast and accurate binding-site identification and analysis. Chem. Biol. Drug Des. 69, 146–148 (2007).
Chovancova, E. et al. CAVER 3.0: a tool for the analysis of transport pathways in dynamic protein structures. PLoS Comput. Biol. 8, 23–30 (2012).
da Silva, T. U., de Pougy, K. C., Albuquerque, M. G., da Silva Lima, C. H. & de Machado, S. P. Development of parameters compatible with the CHARMM36 force field for [Fe4S4]2+ clusters and molecular dynamics simulations of adenosine-5′-phosphosulfate reductase in GROMACS 2019. J. Biomol. Struct. Dyn. 40, 3481–3491 (2022).
Schlick, T. et al. Algorithmic challenges in computational molecular biophysics. J. Comput. Phys. 151, 9–48 (1999).
Likić, V. A., Gooley, P. R., Speed, T. P. & Strehler, E. E. A statistical approach to the interpretation of molecular dynamics simulations of calmodulin equilibrium dynamics. Protein Sci. 14, 2955–2963 (2005).
Vanommeslaeghe, K. et al. CHARMM general force field: a force field for drug-like molecules compatible with the CHARMM all-atom additive biological force fields. J. Comput. Chem. 32, 174–182 (2012).
Helal, M. A. et al. Molecular basis of the potential interaction of SARS-CoV-2 spike protein to CD147 in COVID-19 associated-lymphopenia. J. Biomol. Struct. Dyn. 40, 1109–1119 (2022).
Golo, V. L. & Shaĭtan, K. V. Dynamic attractor for the Berendsen thermostat an the slow dynamics of biomacromolecules. Biofizika 47, 611–617 (2002).
Tuble, S. C., Anwar, J. & Gale, J. D. An approach to developing a force field for molecular simulation of Martensitic phase transitions between phases with subtle differences in energy and structure. J. Am. Chem. Soc. 126, 396–405 (2004).
Zallot, R., Oberg, N. & Gerlt, J. A. The EFI Web resource for genomic enzymology tools: leveraging protein, genome and metagenome databases to discover novel enzymes and metabolic pathways. Biochemistry 58, 4169–4182 (2019).
Oberg, N., Zallot, R. & Gerlt, J. A. EFI-EST, EFI-GNT and EFI-CGFP: Enzyme Function Initiative (EFI) Web resource for genomic enzymology tools. J. Mol. Biol. 435, 168018 (2023).
Copp, J. N., Akiva, E., Babbitt, P. C. & Tokuriki, N. Revealing unexplored sequence-function space using sequence similarity networks. Biochemistry 57, 4651–4662 (2018).
Shannon, P. et al. Cytoscape: a software environment for integrated models. Genome Res. 13, 2498–2504 (2003).
Smoot, M. E., Ono, K., Ruscheinski, J., Wang, P. L. & Ideker, T. Cytoscape 2.8: new features for data integration and network visualization. Bioinformatics 27, 431–432 (2011).
Altschul, S. F., Gish, W., Miller, W., Myers, E. W. & Lipman, D. J. Basic local alignment search tool. J. Mol. Biol. 215, 403–410 (1990).
Edgar, R. C. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 32, 1792–1797 (2004).
Tamura, K., Stecher, G. & Kumar, S. MEGA11: molecular evolutionary genetics analysis version 11. Mol. Biol. Evol. 38, 3022–3027 (2021).
Castro-Falcón, G. et al. Structure and candidate biosynthetic gene cluster of a manumycin-type metabolite from Salinispora pacifica. J. Nat. Prod. 85, 980–986 (2022).
Acknowledgements
This research was supported financially by grants from the National Institutes of Health (NIH; R01GM136758 and R35GM153289 for W.Z.). A.D.R.F. was supported financially by the Blavatnik Innovation Fellowship and UC Berkeley Chancellor’s Fellowship. X-ray data were collected at Beamline 8.3.1 of the Advanced Light Source, a DOE Office of Science User Facility under contract no. DE-AC02-05CH11231, which is supported in part by the ALS-ENABLE programme funded by the NIH, National Institute of General Medical Sciences (P30 GM124169-01). This work was supported in part by the National Science Foundation (CBET-1704266 and CBET-1846426 for H.J.K. and D.W.K.). H.J.K. holds a Career Award at the Scientific Interface from the Burroughs-Wellcome Fund (H.J.K. and D.W.K.). The funders had no role in study design, data collection and analysis, decision to publish or preparation of the paper. We thank J. Pelton and D. Millar for assistance with NMR and bioinformatic analyses, respectively. We also thank D. Hilvert and C. Khosla for helpful discussions on the proposed azido-forming mechanism.
Author information
Authors and Affiliations
Contributions
A.D.R.F. designed all the experiments, conceptualized and conceived the project, performed biochemical and bioinformatic analyses of Tri17 and variants, designed and conducted chemical synthesis schemes, analysed NMR data, aided with the structural analysis of Tri17, analysed the data and wrote the paper. R.Z. designed the experiments, conducted structural analysis for Tri17, performed docking and MD studies, analysed the data and wrote the paper. D.W.K. designed computational experiments, performed calculations, analysed the data and contributed to the writing about the computational work. K.S. helped conduct in vitro experiments, chemical synthesis, protein purification and kinetic characterization of substrates. W.C. analysed the NMR data and aided with chemical synthesis. S.Y., Y.S., K.D.M. and M.N. aided in protein purification, construction of plasmids and repeating biochemical assays for this study. N.B.D. aided in protein purification, biochemical assays, construction of plasmids and interpretation of LC-MS data. Z.X. aided R.Z. with the structural work of Tri17. D.A.M. helped collect and analyse the NMR data. H.J.K. designed computational experiments, analysed the data and wrote the paper. W.Z. designed the experiments, conceptualized and conceived the project, analysed the data and wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemistry thanks Satish Nair and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Biochemical analysis of Tri17 assays with 20.
a) EICs demonstrating production of 21 and 22 from assays containing Tri17, ATP, nitrite, and 20. Omission of any of these components led to the abolition of 21 and 22. Utilization of 15N-nitrite resulted in the expected mass spectral shifts for both 21 and 22. No new products were detected when Aha11 was used in place of Tri17. A 10-ppm error mass tolerance was used for each trace. At least three independent replicates were performed for each assay, and representative results are shown. b) Relative amounts of 21 and 22 quantified by LC-HRMS over a 6-hour time course of the Tri17 assay. Error bars correspond to standard deviation of the mean from three replicate experiments. c) Analysis of 22 production from Tri17 assays. A Tri17 biochemical assay with 20 was first incubated at room temperature for 30 minutes and the protein was removed immediately using an Amicon spin filter (2 kDa MWCO). The reaction flowthrough was extracted with ethyl acetate, dried, and served as substrates (containing a mixture of 20, 21, and 22) for new Tri17 reactions and the production of 22 was monitored in a time course. Tri17 wild-type and Tri17_H229F were used in new reactions together with no enzyme control. The data points and error bars represent the average and standard deviations from three independently performed experiments, respectively.
Extended Data Fig. 2 Biochemical analysis of Tri17-mediated dehydration of 18.
A Tri17 biochemical assay with 17 was incubated at room temperature for 30 minutes and the protein was removed immediately using an Amicon spin filter (2 kDa MWCO). The reaction flowthrough was extracted with ethyl acetate, dried, and served as substrates (containing a mixture of 17, 18, and 19) for new Tri17 reactions and the production of 19 was monitored. The EICs demonstrate increased production of 19 in a Tri17-dependent manner. A 10-ppm error mass tolerance was used for each trace. The data points and error bars present in the bar graph represent the average and standard deviations of 19 produced from three independently performed experiments.
Extended Data Fig. 3 Docking of selected nitrosylated species in the hydrophobic tunnel of Tri17 in the ConNuc model.
a–d, Docking poses were screened and clustered, and the stability was checked via MD simulation for 1P (a), 7P (b), 12P (c) and 18 (d). Three bulky aromatic residues (H275, F273, H230) were found to be located at the entrance, in the middle and in the deep end of the tunnel, respectively.
Extended Data Fig. 4 Biochemical analysis of Tri17_H229F with 17 and 20.
a) Relative amounts of 18 and 19 quantified by LC-HRMS over a 6-hour time course from assays containing Tri17_H229F, ATP, nitrite and 17. b) Relative amounts of 21 and 22 quantified by LC-HRMS over a 6-hour time course from assays containing Tri17_H229F, ATP, nitrite and 20. Error bars correspond to standard deviation of the mean from three replicate experiments.
Extended Data Fig. 5 Bioinformatic analysis of Tri17 and its homologs.
a) Sequence similarity network (constructed using the EFI-Enzyme Similarity Tool using default settings130,131) consisting of 1,471 Tri17 homologs represented as nodes. Each node represents proteins that are >45% identical. Tri17, AvaA6, and SpiA7 are highlighted in red, purple, and gray as part of Group 1 (1,159 members), respectively. CreM and Aha11 are highlighted in green and yellow, respectively, as part of Group 2 (191 members). Groups 3 and 4 are composed of 76 and 45 members, respectively. Two bacterial strains other than Streptomyces tsukubaensis have been reported to produce triacsins (Streptomyces aureofaciens29 and Salinispora cortesiana138), which possess Tri17 homologs with 89%/93% and 80%/86% identity/similarity, respectively. Tri17_Aur, reported in this work (Supplemental Fig. 14), is depicted by the same node as Tri17 due to their high sequence similarity. b) Phylogenetic tree of Tri17 suggests that Tri17 is located at a different clade than CreM and Aha11 (see Supplementary Fig. 57 for a larger representation of the phylogenetic tree). The Tri17 clade shown in yellow was putatively annotated to include homologs with >50% sequence identity. The CreM clade is shown in purple consisting of CreM and Aha11. Structural homologs of Tri17 from the Dali server are colored green. Other proteins in black correspond to BLAST results with less than 50% sequence identity with respect to Tri17. c) Sequence alignment between Tri17 and its homologs. The residues highlighted in yellow correspond to putative substrate binding residues.
Extended Data Fig. 6 Proposed reaction mechanism for azidation of 17 by Tri17.
Tri17 utilizes ATP to activate nitrite to generate a nitroso-AMP intermediate that is subjected to nucleophilic attack by 17. After tautomerization, compound 18 undergoes dehydration presumably through acid-base catalysis mediated by His229 to generate 19.
Supplementary information
Supplementary Data 1
Computational data from Supplementary Figs. 19–25.
Supplementary Data 2
Supplementary Fig. 10e source data.
Supplementary Data 3
Supplementary Fig. 11e source data.
Supplementary Data 4
Supplementary Fig. 27b source data.
Supplementary Data 5
Supplementary Fig. 52 source data.
Supplementary Data 6
Supplementary Fig. 56 source data.
Source data
Source Data Fig. 3
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 1/Table 1
Statistical source data.
Source Data Extended Data Fig. 2/Table 2
Statistical source data.
Source Data Extended Data Fig. 4/Table 4
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Del Rio Flores, A., Zhai, R., Kastner, D.W. et al. Enzymatic synthesis of azide by a promiscuous N-nitrosylase. Nat. Chem. 16, 2066–2075 (2024). https://doi.org/10.1038/s41557-024-01646-2
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41557-024-01646-2