Abstract
DNA-encoded libraries (DELs) are useful for hit discovery in the pharmaceutical industry. Although a large number of individually coded molecules are accessible through DELs, their structural diversity is limited because few transformations are benign and chemoselective enough to be applied in the presence of DNA in aqueous environments. In particular, C–H functionalization chemistry that could be ideally suited to increase structural diversity through late-stage functionalization is currently absent from DEL synthesis. Here we present a general C–H functionalization of electron-rich arenes on DNA. The development of a selenoxide reagent is key to achieving the regio- and chemoselective formation of arylselenonium salts in aqueous media. The introduction of arylselenonium salts offers a versatile linchpin on DNA conjugates, which gives access to a multitude of analogues through diverse subsequent reactions, including transition-metal-mediated and photochemical transformations for the formation of C–C, C–I and C–S bonds.

Similar content being viewed by others
Main
In DNA-encoded libraries (DELs), the DNA sequence acts as a covalently attached molecular barcode for each individual molecule in the library and allows its unique identification1. The library can be screened in a single bioassay against a target protein, and potential hits can be identified through polymerase chain reaction (PCR) and sequencing2. The key advantage of DELs is the ability to screen up to billions of compounds at once with only a fraction of the investment required for high-throughput screening approaches3. However, because the crucial information to identify the molecules is contained in the DNA sequence, the chemical toolbox suitable for the construction of the library is restricted. The reaction chemistry must be executed with the molecule on DNA, while maintaining the functionality of the DNA present during synthesis and DNA sequence growth4. Compatibility with aqueous environment, pH-buffered solutions, high selectivity and reaction temperature within the limits of DNA stability have constrained the synthesis of libraries to a few reactions5,6. Accessibility of a wide range of building blocks, although not a must, has also influenced the selection of reactions to produce successful DELs, favouring compounds that are widely available with diverse substituents. As a consequence, most libraries rely on robust transformations, such as amide couplings, SNAr reactions, reductive amination and metal-mediated cross-coupling reactions, that use broadly available building blocks—amines, carboxylic acids, aldehydes and aryl halides. Recently, efforts to explore a broader chemical space have spurred the development of new methodologies compatible with DNA-encoded conjugates, for example, Suzuki cross-coupling reactions7, Buchwald–Hartwig aminations8,9,10, a DNA-compatible synthesis of diazonium salts11, the formation of heterocycles directly on DNA12,13,14 and strain-promoted cycloadditions15. Other innovative methodologies, including photoredox16,17,18,19 and radical conjugated additions20, are also emerging in the field.
Direct functionalization of C–H bonds on DNA conjugates has been scarcely explored and is limited to a few isolated examples for the introduction of a single functionality. Early examples relied on ruthenium21- or palladium22-mediated C–H activation of DNA-conjugated olefins in a Heck-type reaction. The group of Yu developed a C–H activation with cyclic carboxylic acids as directing group, for the construction of new Csp2–Csp3 bonds with DNA-conjugated aryl iodides23. However, none of these examples shows the functionalization of an arene’s C–H bond. In 2020, Hou and Yang reported the first C–H functionalization of conjugated arenes for library synthesis24. They used a rhodium mediator and a pyrimidine scaffold as a directing group to introduce benzoselenazolone fragments to the C2 position of indole derivatives (Fig. 1a). Later, the same group leveraged the nucleophilicity of indoles to achieve its functionalization on the C3 position (Fig. 1b)25. Both of these methodologies, although first of their kind, are limited to the use of indoles and benzoselenazolone as coupling partners.
a, Rhodium-mediated C2 functionalization limited to indoles with pyrimidine as directing group. b, Lewis-acid-mediated C3 functionalization of free N–H indoles. c, Oxidative thioester synthesis between electron-rich arenes and arylthiols. d, A general C–H functionalization of arenes by means of a synthetic linchpin, and the subsequent transformations, as a strategy to expand the accessible chemical space for DEL synthesis. Depicted DNA fragment: HP–AOP–NH–. See the Methods for more details.
Another study by Li’s group expanded the C–H functionalization to other electron-rich arenes, such as anilines26. The oxidative synthesis of thioethers was possible through direct coupling between the arenes and thiophenol derivatives (Fig. 1c). Li’s study is the most general C–H functionalization of DNA-conjugated arenes so far, yet the transformation is still limited to the formation of C–S and C–Se bonds with aryl thiols and selenides. Traditionally, the functionalization of arene derivatives relies on halogenation procedures, which can then participate in various bond formations27. However, halogenation procedures often use highly oxidative or acidic conditions that are detrimental for DNA stability28. In addition, high selectivity is crucial to prevent the formation of complex mixtures of constitutional isomers and false-positive hits during library screening. Therefore, halogens must be present in the corresponding building blocks before their installation on DNA. The dependency of library synthesis on the access to the corresponding building block substantially reduces the available chemical space of the resulting library. The direct, regioselective introduction of a synthetic linchpin through C–H functionalization, which could be further transformed into different functional groups, remains an unsolved challenge in the field of DELs (Fig. 1d). Thianthrenation of arenes is often a more selective alternative to halogenation29. The resulting arylthiantrenium salts can be used in a variety of subsequent transformations, from metal-catalysed C–C cross couplings to radical arylation reactions30,31,32,33,34. Although selective, the activation of S-thianthreneoxide requires anhydrous conditions, as well as highly reactive activating reagents, such as trifluoroacetic anhydride, incompatible with DNA stability (Fig. 2b). Recent discoveries in our laboratory35 support that the use of a selenium analogue to thianthrene provides the opportunity to perform selective C–H functionalization in the presence of water36,37.
a, Different pKa values of protonated sulfoxide and selenoxides species highlight the structural effects on oxide basicity and their activation. b, Thianthrenium salt formation is incompatible with aqueous media. c, C–H functionalization in aqueous media by activated selenoxide reagent 2H. d, C–H functionalization in aqueous media by activated selenoxide reagent 3H. TT, thianthrene; TM, transition metal; FG, functional group. Depicted DNA fragment: HP–AOP–NH–. See the Methods for more details.
We envisioned the C–H modification of electron-rich arenes through sufficiently basic selenoxides, so that they can be activated by acid sufficiently weak to be endured by DNA. The pKa values of the corresponding protonated selenoxides, conjugated acids 1H and 2H, differ by as much as two orders of magnitude (Fig. 2a)38,39. Selenoxide-based reagent 2 not only exhibited compatibility with aqueous buffers but also demonstrated similar site selectivity to the thianthrenation methodology (Fig. 2c). Introduction of a pyridine moiety in 3 further increased the pKa of the conjugated acid 3H by another order of magnitude and allowed reactivity for C–H functionalization at pH 3.5. We first evaluated the reactivity of selenoxide 2 to functionalize a small set of DNA-conjugated arenes, and we observed full conversion for the most electron-rich heterocycles, such as indoles (Supplementary Information, pages 68–80). However, with less activated arenes, selenoxide 3 outperformed selenoxide 2, presumably due to its higher basicity, increasing the scope of C–H functionalization (Fig. 2d). The different mode of activation of the selenoxide reagents simplifies the functionalization protocol compared with thianthrenation; no anhydride is necessary, and an aqueous medium in the pH range 2–4 is acidic enough for the reaction to take place. Moreover, selenoxide 3 is a bench-stable solid, soluble in water.
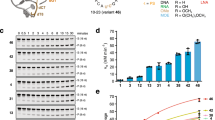
Next, we evaluated the scope of the linchpin installation with a wider range of DNA-conjugated arenes (Fig. 3). Electron-rich heteroarenes, such as indole (4–10 and 12) and pyrrole (11) derivatives, exhibited complete conversion in citrate-phosphate buffer as reaction medium within 1–16 h at 30 °C. Interestingly, unlike previously reported DEL reactions that often require a high excess of reagents, typically 40–100 equivalents5,6, our methodology achieved full conversion with only 2–10 molar equivalents of reagent in most cases. The functionalization of other electron-rich arenes, including primary (13–16 and 38), secondary (17–25) and tertiary anilines (26–28 and 36), proceeded smoothly, resulting in the formation of only one constitutional isomer detectable by high-performance liquid chromatography–mass spectrometry analysis. Benzylic alcohols (20) and anilines containing oxidation-sensitive groups are often found in medicinal chemistry, as are piperazines (26 and 28), morpholines (27) and benzylic amines (18 and 20–23). Our reaction conditions proved compatible with these scaffolds, with the exception of N-methylaniline (Supplementary Fig. 91), which underwent partial demethylation. Functional groups that can be further functionalized, such as bromide (18), chlorides (15, 24 and 25), esters (21) and primary amines (13–16 and 37), also remained untouched. When exploring less electron-rich diarylanilines (25) and phenols (29–31), we found that an excess of 10–50 equivalents of selenoxide was required. Nevertheless, these substrates still exhibited yields of over 70%. Heterocyclic aniline 36 was successfully functionalized, which showcases the application of the C–H functionalization to substrates resulting from SNAr, a reaction widely used in DEL synthesis. In addition, the fluorenylmethyloxycarbonyl (Fmoc) protecting group, commonly used in DEL libraries, proved to be compatible with our methodology (30). Electron-rich dimethoxyarenes (32 and 33) also underwent the desired transformation, whereas less activated anisole derivatives (Supplementary Fig. 91) remained unreactive. All tested benzene derivatives without at least one amino substituent or two alkoxides directly attached to the benzene ring failed to engage in the reaction, and no conversion was observed, with the only exception of dimethylanisole derivative 35. Finally, we selected a representative set of arenes to perform the selenation procedure ‘off DNA’, to determine and characterize the constitutional isomer obtained upon C–H functionalization (Supplementary Information, pages 169–186). To use the C–H functionalization protocol in the production of DEL, DNA integrity is a requirement. No acidic or oxidative DNA degradation, typically by loss of nucleobases40, was observed under any of the reaction conditions used for the scope shown here. When the DNA conjugates were exposed to longer reaction times: pH 3.0, 96 h, and pH 2.0, 24 h, partial degradation was detected (Supplementary Information, pages 148–163). Quantitative (q)PCR has become a key tool to assess potential DNA damage in DEL methodology development41. We synthesized a DNA conjugate containing 87 base pairs and exposed it to the C–H functionalization conditions (Supplementary Information, pages 163–169). After the reaction, we observed a good correlation of the qPCR efficacy between the sample exposed to acid, and the control, which indicates that the DNA conjugates maintain their structural integrity and replication capabilities.
aDNA conjugate (0.65 mM), selenoxide 3 (1.3 mM) in citrate-phosphate buffer (0.17 M, pH 3.5), 30 °C, 1 h. bDNA conjugate (0.1–0.65 mM), selenoxide 3 (1.3–4 mM) in citrate-phosphate buffer (0.17–0.2 M, pH 3), 30 °C, 1–24 h. cDNA conjugate (0.2–0.6 mM), selenoxide 3 (1.3–2 mM) in citrate-phosphate buffer (0.17–0.3 M, pH 2), 30 °C, 1 h. Depicted DNA fragment: HP–AOP–NH–. See the Supplementary Information for more details.
Subsequently, we assessed the versatility of the arylselenonium group as a linchpin for transition-metal-catalysed cross-coupling and radical reactions (Fig. 4). The robustness and wide availability of boronic acids makes the Suzuki cross-coupling one of the most widely used reactions in medicinal chemistry and hit identification42. Several metal-catalysed reactions such as Suzuki7 and Sonogashira43 couplings have been reported for DEL technology; however, these methods require a preinstalled halide or (pseudo)halide on the building block before DNA conjugation. When the arylselenonium salts were subjected to reaction conditions in the presence of a palladium catalyst and a boronic acid, we successfully achieved Suzuki cross-coupling to form C–C bonds with yields higher than 70% (40 and 41). Overall, the present study shows a general case of a full on-DNA two-step sequence for arene C–H bond functionalization leading to a cross-coupling product. Hydroxycarbonylation (42) via the use of trichlorophenyl formate as a CO surrogate44, and cyanation (43) via innocuous potassium ferrocyanide as a cyanide source, allows one to introduce a reactive functional group that could be further modified in the next step of a library synthesis45,46,47. Photoredox catalysis is a rapidly expanding field in organic and medicinal chemistry48,49,50 and often occurs at high dilution and mild temperature51, ideal for DEL reaction requisites. Indeed, selenonium salt 24 readily engaged in photoredox-mediated C–S bond formation (44) as well as Minisci-type reactions (45) under blue light irradiation in 15 min. Iodoarenes have been extensively exploited for the construction of DELs owing to their versatility in engaging in numerous transformations. However, to the best of our knowledge, there are no direct chemoselective monoiodination procedures for DNA-conjugated molecules. In fact, the only reported iodination of pyrroles produces mixtures of mono- and di-iodinated derivatives52. Conversion of the selenonium salt to the corresponding iodoarene 39 opens access to a variety of protocols already reported for DEL, such as C–N (refs. 8,9,10) or C–C (refs. 7,17,18,43,53) couplings without the need for further reaction development.
a, Photochemical iodination of selenonium salt: DNA conjugate (0.3 mM), NaI (1.67 M) in NaPi buffer (0.30 M, pH 6.0), Ar, 25 °C, 450-nm light-emitting diode (LED), 16 h. b, Suzuki cross-coupling: DNA conjugate (0.2 mM), APhos Pd G3 (4 mM), phenylboronic acid or methylboroxine (100 mM) in NaPi buffer (0.25 M, pH 8.0):DMA (3:2), 95 °C, 15 min. c, Pd-mediated hydroxycarbonylation: DNA conjugate (0.17 mM), XantPhos Pd G3 (1.7 mM), trichlorophenyl formate (170 mM) in NaPi buffer (0.17 M, pH 8.0):DMA (1:2), 80 °C, 30 min. d, Pd-mediated cyanation: DNA conjugate (0.2 mM), XantPhos Pd G3 (4 mM), potassium ferrocyanide (80 mM) in NaPi buffer (0.2 M, pH 8.0):NMP (2:1), 95 °C, 15 min. e, Photochemical C–S coupling: DNA conjugate (0.6 mM), 4-methylbenzenethiol (300 mM), K2CO3 (300 mM) in DMSO:water (2:1), Ar, 25 °C, 456-nm LED, 15 min. f, Minisci-type arylation: DNA conjugate (0.3 mM), 1,2,2,6,6-pentamethylpiperidine (16 mM), 3-methylisoquinoline (660 mM) in DMSO:water (9:1), Ar, 25 °C, 450-nm LED, 15 min. Ar, argon; APhos, (4-(N,N-dimethylamino)phenyl)di-tert-butyl phosphine; XantPhos, 4,5-bis(diphenylphosphino)-9,9-dimethylxanthene. Depicted DNA fragment: HP–AOP–NH–. See the Methods for more details.
To ensure the applicability of C–H functionalization in DEL synthesis, the following transformations need to be compatible with a range of different selenonium salts bearing different substitution patterns and electronic properties. Indole derivative (46), primary amine (47), tertiary aniline (48) and secondary anilines (49 and 50) all yielded the Suzuki cross-coupling products in more than 60% yield with boronic acids bearing different heterocycles (Fig. 5). We additionally tested the capability of the selenonium linchpin to be selectively activated by blue light irradiation in the presence of the labile group Br and observed C–Se bond scission exclusively (51). However, under palladium-mediated conditions, the same selenonium salt 18 undergoes double functionalization of both the bromide and selenonium groups.
DNA conjugate (0.2 mM), APhos Pd G3 (4 mM), boronic acid (100 mM) in NaPi buffer (0.25 M, pH 8.0):DMA (3:2), 95 °C, 15 min. a, 2-Methoxypyrimidine-5-boronic acid. b, 6-Chloro-3-pyridineboronic acid. c, 2-Methylpyridine-4-boronic acid, 80 °C, 10 min. d, DNA conjugate (0.3 mM), 1,2,2,6,6-pentamethylpiperidine (16 mM), 3-methylisoquinoline (660 mM) in DMSO:water (9:1), Ar, 25 °C, 450-nm LED, 15 min. Ar, argon; APhos, (4-(N,N-dimethylamino)phenyl)di-tert-butyl phosphine. Depicted DNA fragment: HP–AOP–NH–. See the Methods for more details.
Conclusions
In summary, C–H functionalization of DNA conjugates was made possible by the development of a family of selenoxide reagents. Selenoxides allowed us to synthesize a range of arylselenonium salts of different electron-rich arenes, while maintaining their structural integrity and replication capabilities as confirmed by qPCR. Furthermore, the versatility of the resultant selenonium linchpin is showcased by a variety of transformations with common building blocks for DEL synthesis. Our present study represents a general chemoselective on-DNA C–H functionalization that allows the introduction of more than one functionality, including C–C, C–S and C–I bond formation.
Methods
DNA sequence of DNA headpiece (HP–NH2)
DNA headpiece HP–NH2 (5′-/5Phos/GAGTCA/iSp9/iUniAmM/iSp9/TGACTCCC-3′) was purchased from LGC, Biosearch Technologies.
Reaction of DNA headpiece (HP–NH2) with linker Fmoc-AOP
At 20–25 °C, 100 µl of HP–NH2 (1.00 mM, 100 nmol, 1.00 equiv.) in borate buffer (pH 9.4, concentration (c) = 250 mM) was added to a 1.5-ml Eppendorf tube. Next, 10 µl of a Fmoc-15-amino-4,7,10,13-tetraoxapentadecanoic acid (Fmoc-AOP) stock solution (400 mM, 4.0 µmol, 40 equiv.) in dimethyl acetamide (DMA) was added. The mixture was vortexed for 5 s. Then, 10 µl of a 4-(4,6-dimethoxy-1,3,5-triazin-2-yl)-4-methyl-morpholinium chloride stock solution (400 mM, 4.0 µmol, 40 equiv.) in water was added. The mixture was vortexed for 5 s again, transferred into a Thermocycler at 25 °C and incubated at 25 °C for 16 h at 600 rpm. After 16 h, 12 µl of a 5 M solution of NaCl in water (10% volume of the reaction mixture) and 400 µl of ethanol at −20 °C were added to precipitate the DNA conjugate. The Eppendorf tube was placed in a freezer (−20 °C) for at least 1 h, and then it was centrifuged at 4 °C and 10,000g for at least 30 min. The supernatant was removed, and the pellet was redissolved in 50 µl of water to obtain an aqueous solution of HP–AOP–NHFmoc (2.00 mM, 100 nmol, 1.00 equiv.).
At 20–25 °C, to the 50 µl of HP–AOP–NHFmoc (2.00 mM, 100 nmol, 1.00 equiv.) in water was added 50 µl of a solution of piperidine in DMF (50% v/v). The mixture was vortexed for 5 s and left standing at 20–25 °C for 2 h. After 2 h, 10 µl of a 5 M solution of NaCl in water and 300 µl of ethanol at −20 °C were added to precipitate the DNA conjugate. The Eppendorf tube was placed in the freezer (−20 °C) for at least 1 h, and then it was centrifuged at 4 °C and 10,000g for at least 30 min. The supernatant was removed, and the pellet was redissolved in 20 µl of water. The remaining pellet was then dried under a flow of nitrogen, redissolved with 50 µl water and stored in the freezer at −20 °C to obtain HP–AOP–NH2 as a 2.00 mM aqueous solution.
General procedure for Se modification of DNA conjugates
At 20–25 °C, DNA-conjugated arene (0.40–1.0 mM, final concentration 0.20–0.65 mM) in citrate-phosphate buffer (pH 2.0–3.5, c = 0.3–1 M, final concentration 0.16–0.30 M) was added to a 1.5-ml Eppendorf tube. Then, a stock solution of selenoxide 3 (c = 4.0 mM, 2–10 equiv., final concentration 1.3–2.0 mM) in water was added (see the Supplementary Information for more details). The mixture was vortexed for 5 s, transferred into a Thermocycler preheated at 30 °C and incubated at 30 °C for 1–24 h at 600 rpm.
General procedure for palladium-mediated reactions of DNA conjugates
At 20–25 °C, DNA-conjugated selenonium salt (0.33 mM, final concentration 0.20 mM) in sodium-phosphate buffer (NaPi) (pH 8.0, c = 330 mM) was added to a 1.5-ml Eppendorf tube. Next, a stock solution of the Pd precatalyst (5–20 mM, final concentration 1.7–4 mM) in DMA or N-methyl-2-pyrrolidone (NMP) was added to the Eppendorf tube under air. The mixture was vortexed for 5 s. Lastly, a stock solution of the coupling partner (500 mM, final concentration 100–170 mM) in DMA or NMP was added. The mixture was vortexed for 5 s, transferred into a Thermocycler preheated at 80–95 °C and incubated at 80–95 °C for 15–30 min at 600 rpm.
General procedure for photochemical reactions of DNA conjugates
At 20–25 °C, DNA-conjugated selenonium salt (2.0 mM, final concentration 0.33–0.66 mM) in water was added to a 1.5-ml Eppendorf tube. Next, a stock solution of base (0.10–2.0 M, final concentration 16–300 mM) in dimethyl sulfoxide (DMSO) or water was added to the Eppendorf tube under air. The mixture was vortexed for 5 s. Lastly, a stock solution of the coupling partner (0.50–1.0 M, final concentration 0.33–0.66 M) in DMSO was added. Argon was fluxed over the reaction for 30 s. The mixture was vortexed for 5 s, transferred into a Penn PhD Photoreactor M2 and irradiated at 450 nm for 15 min at 25 °C.
Data availability
The data supporting the findings of this study are available within the Article and its Supplementary Information. Should any raw data files be needed in another format, they are available from the corresponding author upon reasonable request.
References
Brener, S. & Lerner, R. A. Encoded combinatorial chemistry. Proc. Natl Acad. Sci. USA 89, 5381–5383 (1992).
Nielsen, J., Brenner, S. & Janda, K. D. Synthetic methods for the implementation of encoded combinatorial chemistry. J. Am. Chem. Soc. 115, 9812–9813 (1993).
Hale, P. A Handbook for DNA‐Encoded Chemistry (John Wiley & Sons, 2014).
Shi, Y., Wu, Y.-R., Yu, J.-Q., Zhang, W.-N. & Zhuang, C.-L. DNA-encoded libraries (DELs): a review of on-DNA chemistries and their output. RSC Adv. 11, 2359–2376 (2021).
Fitzgerald, P. R. & Paegel, B. M. DNA-encoded chemistry: drug discovery from a few good reactions. Chem. Rev. 121, 7155–7177 (2021).
Fair, R. J., Walsh, R. T. & Hupp, C. D. The expanding reaction toolkit for DNA-encoded libraries. Bioorg. Med. Chem. Lett. 51, 128339 (2021).
Li, J.-Y. & Huang, H. Development of DNA-compatible Suzuki–Miyaura reaction in aqueous media. Bioconjug. Chem. 29, 3841–3846 (2018).
Lu, X., Roberts, S. E., Franklin, G. J. & Davie, C. P. On-DNA Pd and Cu promoted C–N cross-coupling reactions. Med. Chem. Commun. 8, 1614–1617 (2017).
de Pedro Beato, E. et al. Mild and efficient palladium-mediated C–N cross-coupling reaction between DNA-conjugated aryl bromides and aromatic amines. ACS Comb. Sci. 21, 69–74 (2019).
Chen, Y.-C. et al. C–N coupling of DNA-conjugated (hetero)aryl bromides and chlorides for DNA-encoded chemical library synthesis. Bioconjug. Chem. 31, 770–780 (2020).
Li, X. et al. Aryl diazonium intermediates enable mild DNA-compatible C–C bond formation for medicinally relevant combinatorial library synthesis. Chem. Sci. 13, 13100–13109 (2022).
Gerry, C. J., Yang, Z., Stasi, M. & Schreiber, S. L. DNA-compatible [3 + 2] nitrone-olefin cycloaddition suitable for DEL syntheses. Org. Lett. 21, 1325–1330 (2019).
Liu, S., Qi, J., Lu, W., Wang, X. & Lu, X. Synthetic studies toward DNA-encoded heterocycles based on the on-DNA formation of α,β-unsaturated ketones. Org. Lett. 23, 908–913 (2021).
Škopić, M. K. et al. Reagent-based scaffold diversity for DNA-encoded library design: solid phase synthesis of DNA-tagged sp3-rich heterocycles by SnAP chemistry. Org. Lett. 24, 1383–1387 (2022).
Westphal, M. V. et al. Water-compatible cycloadditions of oligonucleotide-conjugated strained allenes for DNA-encoded library synthesis. J. Am. Chem. Soc. 142, 7776–7782 (2020).
Phelan, J. P. et al. Open-air alkylation reactions in photoredox-catalyzed DNA-encoded library synthesis. J. Am. Chem. Soc. 141, 3723–3732 (2019).
Badir, S. O. et al. Multifunctional building blocks compatible with photoredox-mediated alkylation for DNA-encoded library synthesis. Org. Lett. 22, 1046–1051 (2020).
Kölmel, D. K., Ratnayake, A. S. & Flanagan, M. E. Photoredox cross-electrophile coupling in DNA-encoded chemistry. Biochem. Biophys. Res. Commun. 533, 201–208 (2020).
Kölmel, D. K. et al. On-DNA decarboxylative arylation: merging photoredox with nickel catalysis in water. ACS Comb. Sci. 21, 588–597 (2019).
Wang, J. et al. Kinetically guided radical-based synthesis of C(sp3)–C(sp3) linkages on DNA. Proc. Natl Acad. Sci. USA 115, E6404–E6410 (2018).
Wang, X. et al. Ruthenium-promoted C–H activation reactions between DNA-conjugated acrylamide and aromatic acids. Org. Lett. 20, 4764–4768 (2018).
Wang, X. et al. Palladium-promoted DNA-compatible Heck reaction. Org. Lett. 21, 719–723 (2019).
Fan, Z. et al. Merging C(sp3)–H activation with DNA-encoding. Chem. Sci. 11, 12282–12288 (2020).
Xu, H. et al. A chemistry for incorporation of selenium into DNA-encoded libraries. Angew. Chem. Int. Ed. 59, 13273–13280 (2020).
Xu, H. et al. Selenylation chemistry suitable for on-plate parallel and on-DNA library synthesis enabling high-throughput medicinal chemistry. Angew. Chem. Int. Ed. 61, e202206516 (2022).
Yang, S. et al. In-solution direct oxidative coupling for the integration of sulfur/selenium into DNA-encoded chemical libraries. Chem. Sci. 13, 2604–2613 (2022).
Biffis, A., Centomo, P., Del Zotto, A. & Zecca, M. Pd metal catalysts for cross-couplings and related reactions in the 21st century: a critical review. Chem. Rev. 118, 2249–2295 (2018).
Stanforth, S. P., Waldvogel, S. R. & Wehning, K. M. in Science of Synthesis Vol. 31 (ed. Ramsden, C. A.) 79–274 (Thieme, 2007).
Berger, F. et al. Site-selective and versatile aromatic C–H functionalization by thianthrenation. Nature 567, 223–228 (2019).
Lansbergen, B., Granatino, P. & Ritter, T. Site-selective C–H alkylation of complex arenes by a two-step aryl thianthrenation-reductive alkylation sequence. J. Am. Chem. Soc. 143, 7909–7914 (2021).
Alvarez, E. M., Karl, T., Berger, F., Torkowski, L. & Ritter, T. Late-stage heteroarylation of hetero(aryl)sulfonium salts activated by α-amino alkyl radicals. Angew. Chem. Int. Ed. 60, 13609–13613 (2021).
Ni, S. et al. Nickel meets aryl thianthrenium salts: Ni(I)-catalyzed halogenation of arenes. J. Am. Chem. Soc. 145, 9988–9993 (2023).
Ye, F. et al. Aryl sulfonium salts for site-selective late-stage trifluoromethylation. Angew. Chem. Int. Ed. 58, 14615–14619 (2019).
Zhao, D., Petzold, R., Yan, J., Muri, D. & Ritter, T. Tritiation of aryl thianthrenium salts with a molecular palladium catalyst. Nature 600, 444–449 (2021).
Lin, S. et al. A selenoxide for single-atom protein modification of tyrosine residues enabled by water-resistant chalcogen and hydrogen bonding. Nat. Chem. https://doi.org/10.1038/s41557-025-01842-8 (2025).
Iwaoka, M. & Kumakura, F. Applications of water-soluble selenides and selenoxides to protein chemistry. Phosph. Sulf. Silicon Relat. Elem. 183, 1009–1017 (2008).
Reich, H. J. & Hondal, R. J. Why nature chose selenium. ACS Chem. Biol. 11, 821–841 (2016).
Renault, E. & Le Questel, J.-Y. Selenoxides are better hydrogen-bond acceptors than sulfoxides: a crystallographic database and theoretical investigation. J. Phys. Chem. A 108, 7232–7240 (2004).
Laurence, C. et al. An enthalpic scale of hydrogen-bond basicity. 4. Carbon π bases, oxygen bases, and miscellaneous second-row, third-row, and fourth-row bases and a survey of the 4-fluorophenol affinity scale. J. Org. Chem. 75, 4105–4123 (2010).
Sunkari, Y. K., Nguyen, T.-L., Siripuram, V. K. & Flajolet, M. Impact of organic chemistry conditions on DNA durability in the context of DNA-encoded library technology. iScience 26, 107573 (2023).
Abel, G. R. Jr, Calabrese, Z. A., Ayco, J., Hein, J. E. & Ye, T. Measuring and suppressing the oxidative damage to DNA during Cu(I)-catalyzed azide–alkyne cycloaddition. Bioconjug. Chem. 27, 698–704 (2016).
Roughley, S. D. & Jordan, A. M. The medicinal chemist’s toolbox: an analysis of reactions used in the pursuit of drug candidates. J. Med. Chem. 54, 3451–3479 (2011).
Brown, D. G. & Boström, J. Analysis of past and present synthetic methodologies on medicinal chemistry: where have all the new reactions gone? J. Med. Chem. 59, 4443–4458 (2016).
Graham, J. S., Stanway-Gordon, H. A. & Waring, M. J. Micelle-mediated Sonogashira coupling for DNA-encoded library synthesis. Chem. Eur. J. 29, e202300603 (2023).
Ueda, T., Konishi, H. & Manabe, K. Trichlorophenyl formate: highly reactive and easily accessible crystalline CO surrogate for palladium-catalyzed carbonylation of aryl/alkenyl halides and triflates. Org. Lett. 14, 5370–5373 (2012).
Stanway-Gordon, H. A., Graham, J. S. & Waring, M. J. On-DNA transfer hydrogenolysis and hydrogenation for the synthesis of DNA-encoded chemical libraries. Angew. Chem. Int. Ed. 61, e202111927 (2022).
Du, H.-C., Matzuk, M. M. & Chen, Y.-C. Synthesis of 5-substituted tetrazoles via DNA-conjugated nitrile. Org. Biomol. Chem. 18, 9221–9226 (2020).
Chan, A. Y. et al. Metallaphotoredox: the merger of photoredox and transition metal catalysis. Chem. Rev. 122, 1485–1542 (2022).
Zhang, R. et al. Profiling and application of photoredox C(sp3)–C(sp2) cross-coupling in medicinal chemistry. ACS Med. Chem. Lett. 9, 773–777 (2018).
Fernández, D. F. et al. Redefining the synthetic logic of medicinal chemistry. Photoredox-catalyzed reactions as a general tool for aliphatic core functionalization. Org. Lett. 26, 2702–2707 (2023).
Callard-Langdon, E. E., Steven, A. & Kahan, R. J. Shining a light on the advances, challenges and realisation of utilising photoredox catalysis in pharmaceutical development. ChemCatChem 15, e202300537 (2023).
Qi, J., Liu, S., Seydimemet, M., Wang, X. & Lu, X. A general set of DNA-compatible reactions for preparing DNA-tagged multisubstituted pyrroles. Bioconjug. Chem. 32, 2290–2294 (2021).
Flood, D. T. et al. Expanding reactivity in DNA-encoded library synthesis via reversible binding of DNA to an inert quaternary ammonium support. J. Am. Chem. Soc. 141, 9998–10006 (2019).
Acknowledgements
We thank S. Lin, M. Hommrich, K. Bohdan and M. Hirao (MPI KOFO) for helpful discussions. E.d.P.B. acknowledges the Alexander von Humboldt Foundation for a Humboldt Research Fellowship. We thank the MPI für Kohlenforschung for funding. L.V. and K.-J.D. acknowledge funding by the DFG DI 346/23-1.
Funding
Open access funding provided by Max Planck Society.
Author information
Authors and Affiliations
Contributions
E.d.P.B. and T.R. conceived the project. E.d.P.B. developed the selenation reaction on DNA. E.d.P.B. and L.T. performed the reactions on small molecules and DNA. E.d.P.B. and P.H. carried out the DNA ligations. L.V. and K.-J.D. performed the qPCR experiments. E.d.P.B. and T.R. wrote the paper. T.R. directed the project. All authors read and finalized the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemistry thanks Yizhou Li, Xue-jing Zhang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–147, discussion, detailed methods, liquid chromatography–mass spectrometry traces and characterization data.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
de Pedro Beato, E., Torkowski, L., Hartmann, P. et al. On-DNA C–H functionalization of electron-rich arenes for DNA-encoded libraries. Nat. Chem. 17, 1340–1347 (2025). https://doi.org/10.1038/s41557-025-01844-6
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41557-025-01844-6
This article is cited by
-
Selenoxides unlock C–H bonds of biomacromolecules
Nature Chemistry (2025)