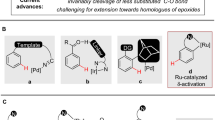
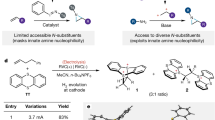
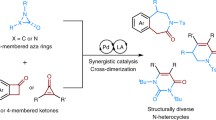
Abstract
Azoles are important synthetic targets due to their diverse applications in areas ranging from human health to food security. Accordingly, access to N-functionalized azoles is an essential goal in modern synthetic chemistry. Surprisingly, however, the relied-upon azole N-alkylation strategies fundamentally limit the structural diversity of these important compounds that can be synthesized and studied. Here we introduce an approach to prepare a broad array of important but difficult-to-access N-alkyl azole compounds. We accomplish this through the introduction of a base-catalysed hydroazolation of readily accessible alkenylthianthrenium electrophiles. This strategy circumvents the classical challenge of azole alkylation regiocontrol through an unusual reversible C–N-bond-forming step that exploits the thermodynamic differences between azole N-alkylation isomers. This reaction furnishes a class of versatile azolothianthrenium building blocks that provides a general platform to investigate diverse N-alkyl azole molecules. More broadly, the distinctive approach outlined through this project is poised to impact the design and development of diverse regioselective alkylation reactions.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All data supporting the findings of this paper are available within the article and its Supplementary Information files. Crystallographic data for the structures reported in this article have been deposited at the Cambridge Crystallographic Data Centre, under deposition numbers CCDC 2393749 (4), 2393750 (50), 2393751 (51) and 2393752 (3-N1). Copies of the data can be obtained free of charge at https://www.ccdc.cam.ac.uk/structures.
References
Vitaku, E., Smith, D. T. & Njardarson, J. T. Analysis of the structural diversity, substitution patterns, and frequency of nitrogen heterocycles among US FDA approved pharmaceuticals: miniperspective. J. Med. Chem. 57, 10257–10274 (2014).
Kerru, N., Gummidi, L., Maddila, S., Gangu, K. K. & Jonnalagadda, S. B. A review on recent advances in nitrogen-containing molecules and their biological applications. Molecules 25, 1909 (2020).
Bhutani, P. et al. US FDA approved drugs from 2015–June 2020: a perspective. J. Med. Chem. 64, 2339–2381 (2021).
Jørgensen, L. N. & Heick, T. M. Azole use in agriculture, horticulture, and wood preservation—is it indispensable? Front.Cell. Infect. Microbiol. 11, 730297 (2021).
Halder, P., Roy, T. & Das, P. Recent developments in selective N-arylation of azoles. Chem. Commun. 57, 5235–5249 (2021).
Blakemore, D. C. et al. Organic synthesis provides opportunities to transform drug discovery. Nat. Chem. 10, 383–394 (2018).
Roughley, S. D. & Jordan, A. M. The medicinal chemist’s toolbox: an analysis of reactions used in the pursuit of drug candidates. J. Med. Chem. 54, 3451–3479 (2011).
Brown, D. G. & Boström, J. Analysis of past and present synthetic methodologies on medicinal chemistry: where have all the new reactions gone? J. Med. Chem. 59, 4443–4458 (2016).
Karrouchi, K. et al. Synthesis and pharmacological activities of pyrazole derivatives: a review. Molecules 23, 134 (2018).
Alghamdi, S. S., Suliman, R. S., Almutairi, K., Kahtani, K. & Aljatli, D. Imidazole as a promising medicinal scaffold: current status and future direction. Drug Des. Devel. Ther. 15, 3289–3312 (2021).
Lovering, F., Bikker, J. & Humblet, C. Escape from Flatland: increasing saturation as an approach to improving clinical success. J. Med. Chem. 52, 6752–6756 (2009).
Lovering, F. Escape from Flatland 2: complexity and promiscuity. MedChemComm 4, 515–519 (2013).
Foley, D. J. et al. Synthesis and demonstration of the biological relevance of sp3-rich scaffolds distantly related to natural product frameworks. Chem. Eur. J. 23, 15227–15232 (2017).
McMurry, J. Organic Chemistry (Thomson Brooks/Cole, 2012).
Swamy, K. C. K., Kumar, N. N. B., Balaraman, E. & Kumar, K. V. P. P. Mitsunobu and related reactions: advances and applications. Chem. Rev. 109, 2551–2651 (2009).
Fletcher, S. The Mitsunobu reaction in the 21st century. Org. Chem. Front. 2, 739–752 (2015).
Bordwell, F. G. Equilibrium acidities in dimethyl sulfoxide solution. Acc. Chem. Res. 21, 456–463 (1988).
Huang, A. et al. Regioselective synthesis, NMR, and crystallographic analysis of N1-substituted pyrazoles. J. Org. Chem. 82, 8864–8872 (2017).
Iškauskienė, M., Ragaitė, G., Sløk, F. A. & Šačkus, A. Facile synthesis of novel amino acid-like building blocks by N-alkylationof heterocyclic carboxylates with N-Boc-3-iodoazetidine. Mol. Divers. 24, 1235–1251 (2020).
Ameziane El Hassani, I., Rouzi, K., Assila, H., Karrouchi, K. & Ansar, M. Recent advances in the synthesis of pyrazole derivatives: a review. Reactions 4, 478–504 (2023).
Tolomeu, H. V. & Fraga, C. A. M. Imidazole: synthesis, functionalization and physicochemical properties of a privileged structure in medicinal chemistry. Molecules 28, 838 (2023).
Krämer, K. The five reactions on every organic chemist’s wish list. Chemistry World https://www.chemistryworld.com/news/the-five-reactions-on-every-organic-chemists-wish-list/3010150.article (2019).
Constable, D. J. C. et al. Key green chemistry research areas—a perspective from pharmaceutical manufacturers. Green Chem. 9, 411–420 (2007).
Liang, Y., Zhang, X. & MacMillan, D. W. C. Decarboxylative sp3 C–N coupling via dual copper and photoredox catalysis. Nature 559, 83–88 (2018).
Sheng, T. et al. Electrochemical decarboxylative N-alkylation of heterocycles. Org. Lett. 22, 7594–7598 (2020).
Jiu, A. Y., Slocumb, H. S., Yeung, C. S., Yang, X.-H. & Dong, V. M. Enantioselective addition of pyrazoles to dienes. Angew. Chem. Int. Ed. 60, 19660–19664 (2021).
Dale, H. J. A., Hodges, G. R. & Lloyd-Jones, G. C. Taming ambident triazole anions: regioselective ion pairing catalyzes direct N-alkylation with atypical regioselectivity. J. Am. Chem. Soc. 141, 7181–7193 (2019).
Chen, S.-J., Golden, D. L., Krska, S. W. & Stahl, S. S. Copper-catalyzed cross-coupling of benzylic C–H bonds and azoles with controlled N-site selectivity. J. Am. Chem. Soc. 143, 14438–14444 (2021).
Guo, Q., Jiang, Y., Zhu, R., Yang, W. & Hu, P. Electrochemical azo-free Mitsunobu-type reaction. Angew. Chem. Int. Ed. 63, e202402878 (2024).
Bengel, L. L. et al. Engineered enzymes enable selective N-alkylation of pyrazoles with simple haloalkanes. Angew. Chem. Int. Ed. 60, 5554–5560 (2021).
Das, M., Zamani, L., Bratcher, C. & Musacchio, P. Z. Azolation of benzylic C–H bonds via photoredox-catalyzed carbocation generation. J. Am. Chem. Soc. 145, 3861–3868 (2023).
Górski, B., Barthelemy, A.-L., Douglas, J. J., Juliá, F. & Leonori, D. Copper-catalysed amination of alkyl iodides enabled by halogen-atom transfer. Nat. Catal. 4, 623–630 (2021).
Dow, N. W., Cabré, A. & MacMillan, D. W. C. A general N-alkylation platform via copper metallaphotoredox and silyl radicalactivation of alkyl halides. Chem 7, 1827–1842 (2021).
Chen, C., Wang, Z.-J., Lu, H., Zhao, Y. & Shi, Z. Generation of non-stabilized alkyl radicals from thianthrenium salts for C–B and C–C bond formation. Nat. Commun. 12, 4526 (2021).
Wagen, C. Organic chemistry’s wish list, four years later. Corin Wagen https://corinwagen.github.io/public/blog/20231020_wish_list.html (2023).
Meng, H., Liu, M.-S. & Shu, W. Organothianthrenium salts: synthesis and utilization. Chem. Sci. 13, 13690–13707 (2022).
Kim, M. J., Targos, K., Holst, D. E., Wang, D. J. & Wickens, Z. K. Alkene thianthrenation unlocks diverse cation synthons: recent progress and new opportunities. Angew. Chem. Int. Ed. 63, e202314904 (2024).
Chen, J., Li, J., Plutschack, M. B., Berger, F. & Ritter, T. Regio- and stereoselective thianthrenation of olefins to access versatile alkenyl electrophiles. Angew. Chem. Int. Ed. 59, 5616–5620 (2020).
Kaiser, D., Klose, I., Oost, R., Neuhaus, J. & Maulide, N. Bond-forming and -breaking reactions at sulfur(IV): sulfoxides, sulfonium salts, sulfur ylides, and sulfinate salts. Chem. Rev. 119, 8701–8780 (2019).
Holst, D. E., Wang, D. J., Kim, M. J., Guzei, I. A. & Wickens, Z. K. Aziridine synthesis by coupling amines and alkenes via an electrogenerated dication. Nature 596, 74–79 (2021).
Liu, M.-S., Du, H.-W., Cui, J.-F. & Shu, W. Intermolecular metal-free cyclopropanation and aziridination of alkenes with XH2 (X = N, C) by thianthrenation. Angew. Chem. Int. Ed. 61, e202209929 (2022).
Kim, M. J. et al. Diastereoselective synthesis of cyclopropanes from carbon pronucleophiles and alkenes. Angew. Chem. Int. Ed. 62, e202303032 (2023).
Moon, H., Jung, J., Choi, J.-H. & Chung, W. Stereospecific syn-dihalogenations and regiodivergent syn-interhalogenation of alkenes via vicinal double electrophilic activation strategy. Nat. Commun. 15, 3710 (2024).
Juliá, F., Yan, J., Paulus, F. & Ritter, T. Vinyl thianthrenium tetrafluoroborate: a practical and versatile vinylating reagent made from ethylene. J. Am. Chem. Soc. 143, 12992–12998 (2021).
Liu, M.-S., Du, H.-W., Meng, H., Xie, Y. & Shu, W. Unified metal-free intermolecular Heck-type sulfonylation, cyanation, amination, amidation of alkenes by thianthrenation. Nat. Commun. 15, 529 (2024).
Holst, D. E., Dorval, C., Winter, C. K., Guzei, I. A. & Wickens, Z. K. Regiospecific alkene aminofunctionalization via an electrogenerated dielectrophile. J. Am. Chem. Soc. 145, 8299–8307 (2023).
Breugst, M., Tokuyasu, T. & Mayr, H. Nucleophilic reactivities of imide and amide anions. J. Org. Chem. 75, 5250–5258 (2010).
Mahatthananchai, J., Dumas, A. M. & Bode, J. W. Catalytic selective synthesis. Angew. Chem. Int. Ed. 51, 10954–10990 (2012).
Larina, L. I. in Advances in Heterocyclic Chemistry Vol. 124 (eds. Scriven, E. F. V. & Ramsden, C. A.) 233–321 (Academic Press, 2018).
Horváth, A. Catalysis and regioselectivity in the Michael addition of azoles. Kinetic vs. thermodynamic control. Tetrahedron Lett. 37, 4423–4426 (1996).
Tomas, F. et al. Tautomerism and aromaticity in 1,2,3-triazoles: the case of benzotriazole. J. Am. Chem. Soc. 111, 7348–7353 (1989).
Ivanova, A. E. et al. Ambident polyfluoroalkyl-substituted pyrazoles in the methylation reactions. J. Fluor. Chem. 195, 47–56 (2017).
Norman, N. J. et al. Highly selective N-alkylation of pyrazoles: crystal structure evidence for attractive interactions. J. Org. Chem. 87, 10018–10025 (2022).
Bhanushali, M. J., Nandurkar, N. S., Bhor, M. D. & Bhanage, B. M. Y(NO3)3·6H2O catalyzed regioselective ring opening of epoxides with aliphatic, aromatic, and heteroaromatic amines. Tetrahedron Lett. 49, 3672–3676 (2008).
De, P. B., Pradhan, S. & Punniyamurthy, T. Stereoselective copper-catalyzed cross-coupling of aziridines with benzimidazoles via nucleophilic ring opening and C(sp2)–H functionalization. J. Org. Chem. 82, 3183–3191 (2017).
Czibula, L., Dobay, L., Papp, E. W., Bagdy, J. N. & Sebok, F. High purity butoconazole nitrate with specified particle size and a process for the preparation thereof. Patent US007625935B2 (2008).
Rotstein, D. M. & Walker, K. A. M. The synthesis and antifungal activity of the enantiomers of butoconazole nitrate. Tetrahedron Asymmetry 4, 1521–1526 (1993).
Varghese, S. et al. Discovery of potent N-ethylurea pyrazole derivatives as dual inhibitors of Trypanosoma brucei and Trypanosoma cruzi. ACS Med. Chem. Lett. 11, 278–285 (2020).
Acknowledgements
We thank A. Wendlandt and M. Levin for suggestions and paper proofreading. We thank the Weix, Stahl, Yoon and Schomaker groups for sharing their chemical inventory. B. J. Thompson is acknowledged for his assistance with power supply design and fabrication. T. Drier is acknowledged for electrochemical glassware fabrication. M. Horwitz is acknowledged for initiating the collaboration and suggestions at the beginning of the project. We also acknowledge support and suggestions from all Wickens group members throughout the investigation of this project. This work was financially supported by the Office of the Vice Chancellor for Research and Graduate Education at the University of Wisconsin–Madison with funding from the Wisconsin Alumni Research Foundation and from the NIH (R01 GM149674-01). This material is based upon work supported by the National Science Foundation Graduate Research Fellowship Program under grant number DGE-1747503 (A.D.M., K.T.). We acknowledge the UW-Madison Department of Chemistry SynCat Center for supporting this work. The Waters UPC2-MS instrument was supported by NIH 1S10OD036302-01, and we thank the UW-Madison Department of Chemistry SynCat Center for assisting with its operation. Any opinions, findings and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation. Spectroscopic instrumentation was supported by a generous gift from Paul J. and M. M. Bender, the NSF (CHE-1048642, CHE-1919350) and the NIH (1S10OD020022-1, S10 OD012245).
Author information
Authors and Affiliations
Contributions
Z.K.W. and C.D. designed the project. D.E.H. conducted preliminary studies. C.D., A.D.M., K.T., S.N.A., D.E.H. and Z.T. performed the experiments and collected the data. M.M. conducted all computational studies. J.B.D. and J.E.G. assisted with high-throughput experimentation. K.M.S. and I.A.G. collected and analysed X-ray diffraction data. All authors analysed the data and contributed to writing the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemistry thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
General methods and materials, supplementary data, computational studies, high-throughput experimentation set-up, characterization of N-regioselectivity, substrate preparation and characterization, general experimental procedures, and characterization data for all compounds synthesized in the preparation of this work.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dorval, C., Matthews, A.D., Targos, K. et al. Unlocking azole chemical space via modular and regioselective N-alkylation. Nat. Chem. 17, 1576–1585 (2025). https://doi.org/10.1038/s41557-025-01891-z
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41557-025-01891-z