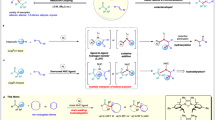
Abstract
Current methods of processing accumulated polyolefin waste typically require harsh conditions, precious metals or high metal loadings to achieve appreciable activities. Here we examined supported, single-site organonickel catalysts for polyolefin upcycling. Chemisorption of Ni(COD)2 (COD, 1,5-cyclooctadiene) onto Brønsted acidic sulfated alumina (AlS) yields a highly electrophilic Ni(I) precatalyst, AlS/Ni(COD)2, which is converted under H2 to the active AlS/NiIIH catalyst. This single-site system exhibits unique hydrogenolysis selectivity that favours cleaving branched polyolefin C–C linkages, enabling the hydrogenolytic separation of polyethylene and isotactic polypropylene (iPP) mixtures. Moreover, AlS/NiIIH remains highly selective and active for hydrogenolysis of iPP admixed with polyvinyl chloride, and the spent catalyst can be repeatedly regenerated by AlEt3 treatment. Experimental mechanistic analysis and density functional theory modelling reveal a turnover-limiting C–C scission pathway featuring β-alkyl transfer and strong olefin binding. These results highlight the potential of nickel-based systems for the selective upcycling of complex plastic waste streams.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All relevant data supporting the findings of this study are available in this paper and its Supplementary Information. Synthetic procedures and characterization for the catalysts, computational studies and all copies of NMR, XPS spectra and GPC traces are available in the Supplementary Information. Source data are provided with this paper.
References
Vollmer, I. et al. Beyond mechanical recycling: giving new life to plastic waste. Angew. Chem. Int. Ed. 59, 15402–15423 (2020).
Jehanno, C. et al. Critical advances and future opportunities in upcycling commodity polymers. Nature 603, 803–814 (2022).
Pires Costa, L., Vaz de Miranda, D. M. & Pinto, J. C. Critical evaluation of life cycle assessment analyses of plastic waste pyrolysis. ACS Sustain. Chem. Eng. 10, 3799–3807 (2022).
Dogu, O. et al. The chemistry of chemical recycling of solid plastic waste via pyrolysis and gasification: state-of-the-art, challenges, and future directions. Prog. Energy Combust. Sci. 84, 100901 (2021).
Rahimi, A. & García, J. M. Chemical recycling of waste plastics for new materials production. Nat. Rev. Chem. 1, 0046 (2017).
Solis, M. & Silveira, S. Technologies for chemical recycling of household plastics—a technical review and TRL assessment. Waste Manage. 105, 128–138 (2020).
Qureshi, M. S. et al. Pyrolysis of plastic waste: opportunities and challenges. J. Anal. Appl. Pyrolysis 152, 104804 (2020).
Miao, Y. et al. Photothermal recycling of waste polyolefin plastics into liquid fuels with high selectivity under solvent-free conditions. Nat. Commun. 14, 4242 (2023).
Wang, X.-Y., Gao, Y. & Tang, Y. Sustainable developments in polyolefin chemistry: progress, challenges, and outlook. Prog. Polym. Sci. 143, 101713 (2023).
Jubinville, D., Esmizadeh, E., Saikrishnan, S., Tzoganakis, C. & Mekonnen, T. A comprehensive review of global production and recycling methods of polyolefin (PO) based products and their post-recycling applications. Sustain. Mater. Technol. 25, e00188 (2020).
Zhang, F. et al. Polyethylene upcycling to long-chain alkylaromatics by tandem hydrogenolysis/aromatization. Science 370, 437–441 (2020).
Chen, L. et al. Disordered, sub-nanometer Ru structures on CeO2 are highly efficient and selective catalysts in polymer upcycling by hydrogenolysis. ACS Catal. 12, 4618–4627 (2022).
Rorrer, J. E., Troyano-Valls, C., Beckham, G. T. & Román-Leshkov, Y. Hydrogenolysis of polypropylene and mixed polyolefin plastic waste over Ru/C to produce liquid alkanes. ACS Sustain. Chem. Eng. 9, 11661–11666 (2021).
Celik, G. et al. Upcycling single-use polyethylene into high-quality liquid products. ACS Cent. Sci. 5, 1795–1803 (2019).
Tennakoon, A. et al. Catalytic upcycling of high-density polyethylene via a processive mechanism. Nat. Catal. 3, 893–901 (2020).
Rorrer, J. E., Beckham, G. T. & Román-Leshkov, Y. Conversion of polyolefin waste to liquid alkanes with Ru-based catalysts under mild conditions. JACS Au 1, 8–12 (2021).
Conk, R. J. et al. Catalytic deconstruction of waste polyethylene with ethylene to form propylene. Science 377, 1561–1566 (2022).
Goldman, A. S. et al. Catalytic alkane metathesis by tandem alkane dehydrogenation–olefin metathesis. Science 312, 257–261 (2006).
Rorrer, J. E. et al. Role of bifunctional Ru/acid catalysts in the selective hydrocracking of polyethylene and polypropylene waste to liquid hydrocarbons. ACS Catal. 12, 13969–13979 (2022).
Kots, P. A. et al. Polypropylene plastic waste conversion to lubricants over Ru/TiO2 catalysts. ACS Catal. 11, 8104–8115 (2021).
Musa, A., Jaseer, E. A., Barman, S. & Garcia, N. Review on catalytic depolymerization of polyolefin waste by hydrogenolysis: state-of-the-art and outlook. Energy Fuels 38, 1676–1691 (2024).
Jaydev, S. D. et al. Consumer grade polyethylene recycling via hydrogenolysis on ultrafine supported ruthenium nanoparticles. Angew. Chem. Int. Ed. 63, e202317526 (2024).
Hu, P. et al. Stable interfacial ruthenium species for highly efficient polyolefin upcycling. J. Am. Chem. Soc. 146, 7076–7087 (2024).
Sun, C. et al. Pt enhanced C–H bond activation for efficient and low-methane-selectivity hydrogenolysis of polyethylene over alloyed RuPt/ZrO2. Appl. Catal. B 353, 124046 (2024).
Dufaud, V. & Basset, J. M. Catalytic hydrogenolysis at low temperature and pressure of polyethylene and polypropylene to diesels or lower alkanes by a zirconium hydride supported on silica–alumina: a step toward polyolefin degradation by the microscopic reverse of Ziegler–Natta polymerization. Angew. Chem. Int. Ed. 37, 806–810 (1998).
Chen, S. et al. Ultrasmall amorphous zirconia nanoparticles catalyse polyolefin hydrogenolysis. Nat. Catal. 6, 161–173 (2023).
Mason, A. H. et al. Rapid atom-efficient polyolefin plastics hydrogenolysis mediated by a well-defined single-site electrophilic/cationic organo-zirconium catalyst. Nat. Commun. 13, 7187 (2022).
Edenfield, W. C. et al. Rapid polyolefin plastic hydrogenolysis mediated by single-site heterogeneous electrophilic/cationic organo-group IV catalysts. ACS Catal. 14, 554–565 (2024).
Lai, Q. et al. Rapid polyolefin hydrogenolysis by a single-site organo-tantalum catalyst on a super-acidic support: structure and mechanism. Angew. Chem. Int. Ed. 62, e202312546 (2023).
Gao, J., Zhu, L. & Conley, M. P. Cationic tantalum hydrides catalyze hydrogenolysis and alkane metathesis reactions of paraffins and polyethylene. J. Am. Chem. Soc. 145, 4964–4968 (2023).
Mason, A. H., Motta, A., Kratish, Y. & Marks, T. J. Demystifying group-4 polyolefin hydrogenolysis catalysis: gaseous propane hydrogenolysis mechanism over the same catalysts. Proc. Natl Acad. Sci. USA 121, e2406133121 (2024).
Vance, B. C., Kots, P. A., Wang, C., Granite, J. E. & Vlachos, D. G. Ni/SiO2 catalysts for polyolefin deconstruction via the divergent hydrogenolysis mechanism. Appl. Catal. B 322, 122138 (2023).
Zhao, Z. et al. Catalytic hydrogenolysis of plastic to liquid hydrocarbons over a nickel-based catalyst. Environ. Pollut. 313, 120154 (2022).
Chu, M. et al. Layered double hydroxide derivatives for polyolefin upcycling. J. Am. Chem. Soc. 146, 10655–10665 (2024).
Wang, M. et al. Complete hydrogenolysis of mixed plastic wastes. Nat. Chem. Eng. 1, 376–384 (2024).
Wilke, G. & Herrmann, G. Stabilized dialkylnickel compounds. Angew. Chem. Int. Ed. 5, 581–582 (1966).
Wilke G. et al. Cyclooligomerisation von Butadien und Übergangsmetall-π-Komplexe. Angew. Chem. 75, 10–20 (1963).
Wu, M. & Hercules, D. M. Studies of supported nickel catalysts by x-ray photoelectron and ion scattering spectroscopies. J. Phys. Chem. 83, 2003–2008 (1979).
Biesinger, M. C., Lau, L. W. M., Gerson, A. R. & Smart, R. S. C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Sc, Ti, V, Cu and Zn. Appl. Surf. Sci. 257, 887–898 (2010).
Grosvenor, A. P., Biesinger, M. C., Smart, R. S. C. & McIntyre, N. S. New interpretations of XPS spectra of nickel metal and oxides. Surf. Sci. 600, 1771–1779 (2006).
Klet, R. C. et al. Evidence for redox mechanisms in organometallic chemisorption and reactivity on sulfated metal oxides. J. Am. Chem. Soc. 140, 6308–6316 (2018).
Garribba, E. & Micera, G. The determination of the geometry of Cu(II) complexes: an EPR spectroscopy experiment. J. Chem. Educ. 83, 1229 (2006).
Bender, G. et al. Infrared and EPR spectroscopic characterization of a Ni(I) species formed by photolysis of a catalytically competent Ni(I)–CO intermediate in the acetyl-CoA synthase reaction. Biochem. 49, 7516–7523 (2010).
Schwab, M. M. et al. Ni(cod)2][Al(ORF)4], a source for naked nickel(I) chemistry. Angew. Chem. Int. Ed. 54, 14706–14709 (2015).
Eaton, G. R., Eaton, S., Barr, D. P. & Weber, R. T. Quantitative EPR (Springer, 2010).
Eberhardt, N. A. & Guan, H. Nickel hydride complexes. Chem. Rev. 116, 8373–8426 (2016).
Wang, Q., Duan, J. D., Goetjen, T., Hupp, J. & Notestein, J. Bimetallic NiCu catalysts supported on a metal–organic framework for non-oxidative ethanol dehydrogenation. J. Catal. 422, 86–98 (2023).
Morgan, D. J. XPS insights: sample degradation in X-ray photoelectron spectroscopy. Surf. Interface Anal. 55, 331–335 (2023).
Krzystek, J. et al. EPR spectra from ‘EPR-silent’ species: high-frequency and high-field EPR spectroscopy of pseudotetrahedral complexes of nickel(II). Inorg. Chem. 41, 4478–4487 (2002).
Albrahim, M. et al. Reduction and agglomeration of supported metal clusters induced by high-flux X-ray absorption spectroscopy measurements. J. Phys. Chem. C 125, 11048–11057 (2021).
Sehested, J., Gelten, J. A. P. & Helveg, S. Sintering of nickel catalysts: effects of time, atmosphere, temperature, nickel-carrier interactions, and dopants. Appl. Catal. A 309, 237–246 (2006).
Tomer, A. et al. Enhanced production and control of liquid alkanes in the hydrogenolysis of polypropylene over shaped Ru/CeO2 catalysts. Appl. Catal. A 666, 119431 (2023).
Garin, F., Hilaire, L. & Maire, G. in Studies in Surface Science and Catalysis Vol. 27 (ed. Cerveny, L.) 145–199 (Elsevier, 1986).
Lu, L., Li, W., Cheng, Y. & Liu, M. Chemical recycling technologies for PVC waste and PVC-containing plastic waste: a review. Waste Manage. 166, 245–258 (2023).
Chen, L. et al. Effect of reaction conditions on the hydrogenolysis of polypropylene and polyethylene into gas and liquid alkanes. React. Chem. Engin. 7, 844–854 (2022).
Yu, J., Sun, L., Ma, C., Qiao, Y. & Yao, H. Thermal degradation of PVC: a review. Waste Manage. 48, 300–314 (2016).
Drago, R. S., Petrosius, S. C. & Kaufman, P. B. Alkylation, isomerization and cracking activity of a novel solid acid, AlCl2(SG)n. J. Mol. Catal. 89, 317–327 (1994).
Luo, Y.-R. in Comprehensive Handbook of Chemical Bond Energies Chap. 4, 148–153 (CRC Press, 2007).
Acknowledgements
We thank the US Department of Energy (DOE), Office of Science, Office of Basic Energy Sciences under award number DOE DE-SC0024448, and The Dow Chemical Company for financial support of this work (T.J.M., Q.L., W.C.E., A.A., catalyst synthesis and characterization). X.Z. thanks the Center for Innovative and Strategic Transformation of Alkane Resources (CISTAR) for support under NSF award number EEC-1647722 (XPS). A part of this work (T.K., solid-state NMR) was supported by the US DOE, Office of Science, Basic Energy Sciences, Chemical Sciences, Geosciences, and Biosciences Division. The research was performed at the Ames National Laboratory, which is operated for the US DOE by Iowa State University under contract number DE-AC02-07CH11358. This work made use of IMSERC (RRID SCR_017874) facilities at Northwestern University, which received support from Soft and Hybrid Nanotechnology Experimental (SHyNE) Resource (NSFECCS-2025633), the International Institute of Nanotechnology and Northwestern University. This work made use of the Northwestern University QBIC supported by NASA Ames Research Center grant NNA04CC36G. This work made use of the REACT Facility of Northwestern University’s Center for Catalysis and Surface Science supported by grant DE-SC0001329 from the DOE. This work was supported by the US DOE, Office of Science, Office of Basic Energy Sciences, under award number DE-FG02-99ER14999 (M.R.W., EPR spectroscopy). This research used resources at the 8-ID beamline of the National Synchrotron Light Source II, a US DOE Office of Science User Facility operated for the DOE Office of Science by Brookhaven National Laboratory under contract number DE-SC0012704. This research was supported in part by computational resources and staff provided by the Quest High-Performance Computing Facility at NU, which is jointly supported by the Office of the Provost, the Office for Research and NU Information Technology. We also thank B. Tumanskii for informative and helpful discussions.
Author information
Authors and Affiliations
Contributions
Q.L., Y.K. and T.J.M. designed the study. Q.L., Y.K. and T.J.M. wrote the paper with contributions from all coauthors. Q.L. performed the catalyst preparation, characterization and catalytic tests. Y.K. carried out the theoretical calculations. X.Z. performed XPS measurements and analysis. S.J. and J.T.M. performed XAS measurement and analysis. M.D.K. performed EPR measurements and analysis. Y.L. performed the HAADF-STEM measurements. S.A. performed the DRIFTS measurements and analysis. T.K. performed the solid-state NMR experiments and analysis. X.Z. helped to set up the continuous-flow BenchCat reactor. Q.L., X.Z., S.J., M.D.K., S.A., A.A., M.R.W., V.D., Y.W., W.C.E., J.T.M., Y.K. and T.J.M. analysed the data and revised the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 potential cleaving point in PECO and analysis of DCM extract from hydrogenolysis of polyolefins or polyolefin mixtures.
a, GC–MS chromatogram of the DCM extract of PECO hydrogenolysis reactions run under identical conditions with the indicated catalysts; b, Potential C-C cleavage points in PECO hydrogenolysis. c, GPC analysis of the DCM extract of i-PP hydrogenolysis. d, GPC analysis of the DCM extract of a-PP hydrogenolysis. e, GPC analysis of DCM extract of hydrogenolysis of post-consumer i-PP and PE mixtures. f, GPC analysis of the solid fraction of hydrogenolysis of post-consumer i-PP and PE mixtures.
Extended Data Fig. 2 GPC analysis of DCM extract of i-PP hydrogenolysis in the presence of PVC.
a, GPC analysis of DCM extract of i-PP hydrogenolysis in the presence of PVC with constant total polymer mass (~500 mg). b, GPC analysis of DCM extract of i-PP hydrogenolysis in the presence of varied PVC content (~500 mg i-PP loading).
Extended Data Fig. 3 GPC analysis of DCM extract of i-PP hydrogenolysis under other conditions.
a, GPC analysis of DCM extract of i-PP hydrogenolysis in the presence of 10% PTFE. b, GPC analysis of DCM extract of i-PP hydrogenolysis with PVC pretreated AlS/Ni(COD)2.
Supplementary information
Supplementary Information
Supplementary procedures, physical methods, Figs. 1–36 and Tables 1–13.
Supplementary Data 1
DFT model construction and Cartesian coordinates.
Source data
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Computed energy values.
Source Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 3
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lai, Q., Zhang, X., Jiang, S. et al. Stable single-site organonickel catalyst preferentially hydrogenolyses branched polyolefin C–C bonds. Nat. Chem. 17, 1488–1496 (2025). https://doi.org/10.1038/s41557-025-01892-y
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41557-025-01892-y