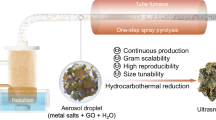
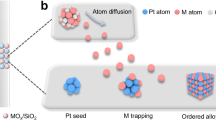
Abstract
High-entropy metal-containing nanomaterials have garnered interest in diverse fields such as electrocatalysis and energy conversion. Their synthesis typically requires high temperatures (>1,000 K) to facilitate homogeneous mixing and rapid transformation of metal precursors. However, current state-of-the-art approaches typically involve complex reaction environments and require specialized equipment and operations. Herein we demonstrate a versatile flame synthesis process to fabricate high-entropy metallic single atoms and/or nanoparticles supported on soot-like carbon via blending organometallic precursors into fuel (namely, paraffin wax) and subsequent burning. The high flame temperature (~1,800 K) enables strong metal–carbon association with tailorable chemistry and homogeneous bonding between dissimilar metallic elements (up to 25 metals studied), regardless of their thermodynamic compatibility. Additionally, we show high-performance electrosynthesis of hydrogen peroxide to highlight this approach as a promising method for electrocatalyst development.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Information. Source data are provided with this paper.
Code availability
Codes for MD simulations are provided as part of the replication package in the Supplementary Information.
References
Zhao, C., Ding, F., Lu, Y., Chen, L. & Hu, Y.-S. High-entropy layered oxide cathodes for sodium-ion batteries. Angew. Chem. Int. Ed. 59, 264–269 (2020).
Zeng, Y. et al. High-entropy mechanism to boost ionic conductivity. Science 378, 1320–1324 (2022).
Sun, Y. & Dai, S. High-entropy materials for catalysis: a new frontier. Sci. Adv. 7, eabg1600 (2021).
Miracle, D. B. & Senkov, O. N. A critical review of high entropy alloys and related concepts. Acta Mater. 122, 448–511 (2017).
Cui, M. et al. Multi-principal elemental intermetallic nanoparticles synthesized via a disorder-to-order transition. Sci. Adv. 8, eabm4322 (2022).
He, Q. F. et al. A highly distorted ultraelastic chemically complex Elinvar alloy. Nature 602, 251–257 (2022).
Lei, Z. et al. Enhanced strength and ductility in a high-entropy alloy via ordered oxygen complexes. Nature 563, 546–550 (2018).
Johnstone, G. H. J. et al. Entropy engineering and tunable magnetic order in the spinel high-entropy oxide. J. Am. Chem. Soc. 144, 20590–20600 (2022).
Loevlie, D. J., Ferreira, B. & Mpourmpakis, G. Demystifying the chemical ordering of multimetallic nanoparticles. Acc. Chem. Res. 56, 248–257 (2023).
Sun, Y. et al. A general approach to high-entropy metallic nanowire electrocatalysts. Matter 6, 193–205 (2023).
George, E. P., Raabe, D. & Ritchie, R. O. High-entropy alloys. Nat. Rev. Mater. 4, 515–534 (2019).
Li, T. et al. Denary oxide nanoparticles as highly stable catalysts for methane combustion. Nat. Catal. 4, 62–70 (2021).
Rost, C. M. et al. Entropy-stabilized oxides. Nat. Commun. 6, 8485 (2015).
Chen, H. et al. Entropy-stabilized metal oxide solid solutions as CO oxidation catalysts with high-temperature stability. J. Mater. Chem. A 6, 11129–11133 (2018).
Zheng, X. et al. Hydrogen-substituted graphdiyne-assisted ultrafast sparking synthesis of metastable nanomaterials. Nat. Nanotechnol. 18, 153–159 (2022).
Park, J. et al. Radially phase segregated PtCu@PtCuNi dendrite@frame nanocatalyst for the oxygen reduction reaction. ACS Nano 11, 10844–10851 (2017).
Yao, Y. et al. Extreme mixing in nanoscale transition metal alloys. Matter 4, 2340–2353 (2021).
Chen, P.-C. et al. Polyelemental nanoparticle libraries. Science 352, 1565–1569 (2016).
Yao, Y. et al. Carbothermal shock synthesis of high-entropy-alloy nanoparticles. Science 359, 1489–1494 (2018).
Qiao, Y. et al. Continuous fly-through high-temperature synthesis of nanocatalysts. Nano Lett. 21, 4517–4523 (2021).
Jones, J. et al. Thermally stable single-atom platinum-on-ceria catalysts via atom trapping. Science 353, 150–154 (2016).
Rao, P. et al. Movable type printing method to synthesize high-entropy single-atom catalysts. Nat. Commun. 13, 5071 (2022).
Wang, B. et al. General synthesis of high-entropy alloy and ceramic nanoparticles in nanoseconds. Nat. Synth. 1, 138–146 (2022).
Gu, K. et al. Defect-rich high-entropy oxide nanosheets for efficient 5-hydroxymethylfurfural electrooxidation. Angew. Chem. Int. Ed. 60, 20253–20258 (2021).
Minamihara, H. et al. Continuous-flow reactor synthesis for homogeneous 1 nm-sized extremely small high-entropy alloy nanoparticles. J. Am. Chem. Soc. 144, 11525–11529 (2022).
Liu, M. et al. Entropy-maximized synthesis of multimetallic nanoparticle catalysts via a ultrasonication-assisted wet chemistry method under ambient conditions. Adv. Mater. Interfaces 6, 1900015 (2019).
Cao, G. et al. Liquid metal for high-entropy alloy nanoparticles synthesis. Nature 619, 73–77 (2021).
Liao, Y. et al. High-entropy-alloy nanoparticles with 21 ultra-mixed elements for efficient photothermal conversion. Natl Sci. Rev. 9, nwac041 (2022).
Liu, S. et al. A general flame aerosol route to high-entropy nanoceramics. Matter 7, 3994–4013 (2024).
Pratsinis, S. E. Flame aerosol synthesis of ceramic powders. Prog. Energy Combust. Sci. 24, 197–219 (1998).
Laine, R. M., Bickmore, C. R., Treadwell, D. R. & Waldner, K. F. Ultrafine metal oxide powders by flame spray pyrolysis. US patent 5,958,361 (1999).
Koirala, R., Pratsinis, S. E. & Baiker, A. Synthesis of catalytic materials in flames: opportunities and challenges. Chem. Soc. Rev. 45, 3053–3068 (2016).
Johansson, K. O., Head-Gordon, M. P., Schrader, P. E., Wilson, K. R. & Michelsen, H. A. Resonance-stabilized hydrocarbon-radical chain reactions may explain soot inception and growth. Science 361, 997–1000 (2018).
Chen, W. et al. A map of single-phase high-entropy alloys. Nat. Commun. 14, 2856 (2023).
Han, Y.-C. et al. A general method for rapid synthesis of refractory carbides by low-pressure carbothermal shock reduction. Proc. Natl Acad. Sci. USA 119, e2121848119 (2022).
He, T. et al. Mastering the surface strain of platinum catalysts for efficient electrocatalysis. Nature 598, 76–81 (2021).
Qin, Y. et al. Fine-tuning intrinsic strain in penta-twinned Pt–Cu–Mn nanoframes boosts oxygen reduction catalysis. Adv. Funct. Mater. 30, 1910107 (2020).
Yang, C. et al. Continuous roll-to-roll production of carbon nanoparticles from candle soot. Nano Lett. 21, 3198–3204 (2021).
Huang, Y. et al. Metal nanoparticle harvesting by continuous rotating electrodeposition and separation. Matter 3, 1294–1307 (2020).
Dong, Q. et al. Depolymerization of plastics by means of electrified spatiotemporal heating. Nature 616, 488–494 (2023).
Thomson, M. & Mitra, T. A radical approach to soot formation. Science 361, 978–979 (2018).
Homann, K.-H. Fullerenes and soot formation—new pathways to large particles in flames. Angew. Chem. Int. Ed. 37, 2434–2451 (1998).
Plimpton, S. Fast parallel algorithms for short-range molecular dynamics. J. Comput. Phys. 117, 1–19 (1995).
Totton, T. S., Misquitta, A. J. & Kraft, M. A quantitative study of the clustering of polycyclic aromatic hydrocarbons at high temperatures. Phys. Chem. Chem. Phys. 14, 4081–4094 (2012).
Bowal, K., Martin, J. W., Misquitta, A. J. & Kraft, M. Ion-induced soot nucleation using a new potential for curved aromatics. Combust. Sci. Technol. 191, 747–765 (2019).
Sharma, A., Mukut, K. M., Roy, S. P. & Goudeli, E. The coalescence of incipient soot clusters. Carbon 180, 215–225 (2021).
Heine, M. C., Mädler, L., Jossen, R. & Pratsinis, S. E. Direct measurement of entrainment during nanoparticle synthesis in spray flames. Combust. Flame 144, 809–820 (2006).
Nielson, K. D., Van Duin, A. C. T., Oxgaard, J., Deng, W.-Q. & Goddard, W. A. Development of the ReaxFF reactive force field for describing transition metal catalyzed reactions, with application to the initial stages of the catalytic formation of carbon nanotubes. J. Phys. Chem. A 109, 493–499 (2005).
Islam, M. M., Zou, C., Van Duin, A. C. T. & Raman, S. Interactions of hydrogen with the iron and iron carbide interfaces: a ReaxFF molecular dynamics study. Phys. Chem. Chem. Phys. 18, 761–771 (2016).
Yeh, J.-W. Alloy design strategies and future trends in high-entropy alloys. JOM 65, 1759–1771 (2013).
Acknowledgements
We thank C. Ma from the Analytical Instrumentation Center of Hunan University for his assistance with the spherical aberration-corrected transmission electron microscopy analysis. The electron spin resonance spectroscopy experiments were performed at the State Key Laboratory of Chemo and Biosensing. E.G. acknowledges the Materials and Process Simulation (MAPS) Scienomics platform. In terms of funding, this research was supported by the following grants, schemes and fellowships: National Natural Science Foundation of China (S.P., grant nos 22378105 and 23FAA02526), Department of Science and Technology of Hunan Province (S.P., project no. 2022TP2032), National Supercomputing Center in Changsha (S.P., grant no. G2023016), Australian Research Council Future Fellowship (J.J.R., grant no. FT210100669) and the XAS and the SAXS/WAXS beamlines at the Australian Synchrotron, part of the Australian Nuclear Science and Technology Organisation (S.P., project nos 18766, 20570 and 21771).
Author information
Authors and Affiliations
Contributions
S.P. conceived the idea (with the assistance of Z.L. and R.G.) and designed and led the project. All authors performed research and/or analysed data with intellectual contributions. S.P., F.C. and Z.L. drafted the manuscript with input from all authors. All authors approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemistry thanks Feng Jiao, Mark Swihart and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–98, Tables 1–15, Materials and Methods, captions for Supplementary Videos 1–9, captions for Supplementary Data 1 and 2, captions for Supplementary Codes 1–5 and refs. 1–58.
Supplementary Data 1
Literature review on metal–carbon nanomaterials including HMNs. These reports mentioned in our work are a random selection of the literature published in the past decade. These reports are all based on carbon-supported metal-containing nanomaterials in the field including catalysis and energy storage. Specifically, the number of metals was collated in our work (Fig. 1e). This file is provided separately.
Supplementary Data 2
Flow information from Aspen simulations. The data include the flow details occurring at the reaction, the separation and the concentration sections, which were extracted from the Aspen simulation module.
Supplementary Code 1
The file describes the initial configuration of 200 coronene molecules and 400 Co atoms.
Supplementary Code 2
The file describes the ReaxFF force-field parameters.
Supplementary Code 3
The file describes the main input script.
Supplementary Code 4
The file is the ReaxFF control file.
Supplementary Code 5
The file is the charge equilibration parameters file.
Supplementary Video 1
Scaled-up production of candle soot (CS)-based nanomaterials. Four candles (each with a diameter of 2.5 cm) were ignited simultaneously for scaled-up production. A stainless steel collector (50 cm × 50 cm) was used for collection. A quantity of 4.56 g CS NPs per hour was achieved. In principle, the production of CS-based nanomaterials can be readily scaled up. This file is provided separately.
Supplementary Video 2
Collection of CS-based nanomaterials using a rotating collector. The collector was driven by a motor, and the rotating speed could be adjusted. This file is provided separately.
Supplementary Video 3
CS production using solid (powder) fuel. By burning target (metal) precursors with solid fuels, such as coal dust and graphite powder, using a solid burner, the production of CS-based nanomaterials (including HMNs) can be further increased. This file is provided separately.
Supplementary Video 4
Soot clustering simulations at 700 K in the absence of metals. Coronene molecules (200) were used as the PAH intermediates for soot formation. The simulation was carried out in a constant NST (number, size and temperature) ensemble using LAMMPS. Coalescence-induced clustering occurred relatively faster than that at 1,000 K (Supplementary Video 5). This file is provided separately.
Supplementary Video 5
Soot clustering simulations at 1,000 K in the absence of metals. This file is provided separately.
Supplementary Video 6
MD simulations in the presence of 28.2 wt% Co. In this experiment, 200 coronene molecules (plate-like objects) were simulated together with 400 Co atoms (red spheres; that is, low metal concentration). Metal aggregation was observed, suggesting the possible formation of Co NPs in the CS matrix. This file is provided separately.
Supplementary Video 7
MD simulations in the presence of 2.4 wt% Co. In this experiment, 200 coronene molecules (plate-like objects) were simulated together with 25 Co atoms (red spheres; that is, low metal concentration). No apparent metal aggregation was observed at the end of the simulation studies, suggesting the possible formation of Co SAs within the CS matrix. This file is provided separately.
Supplementary Video 8
Water droplets sticking on the CS-based coating (N doping). The coating was prepared by burning candles mixed with a N source (as detailed in the Materials and Methods section). The volume of each water droplet was about 10 μL. The tilt angle of the surface was ~15°. After releasing, water droplets rebounded at the surface and sat on the surface owing to the high hysteresis (that is, high interactions between the coating and water droplet). This file is provided separately.
Supplementary Video 9
Water droplets rolling off the CS-based coating (F doping). The coating was prepared by burning candles mixed with an F source (as detailed in the Materials and Methods section). The volume of each water droplet was about 10 μL. The tilt angle of the surface was ~15°. After releasing, water droplets rebounded freely at the surface and rolled off the surface owing to the low hysteresis (that is, low interactions between the coating and water droplet). This file is provided separately.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, Z., Goudeli, E., Guo, R. et al. Flame synthesis achieves compositionally tailorable high-entropy metal-containing nanomaterials. Nat. Chem. 17, 1497–1504 (2025). https://doi.org/10.1038/s41557-025-01894-w
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41557-025-01894-w
This article is cited by
-
High-entropy nanomaterials by candlelight
Nature Chemistry (2025)