Abstract
Plasmids are extrachromosomal genetic elements commonly found in bacteria. They are known to fuel bacterial evolution through horizontal gene transfer, and recent analyses indicate that they can also promote intragenomic adaptations. However, the role of plasmids as catalysts of bacterial evolution beyond horizontal gene transfer is poorly explored. In this study, we investigated the impact of a widespread conjugative plasmid, pOXA-48, on the evolution of several multidrug-resistant clinical enterobacteria. Combining experimental and within-patient evolution analyses, we unveiled that plasmid pOXA-48 promotes bacterial evolution through the transposition of plasmid-encoded insertion sequence 1 (IS1) elements. Specifically, IS1-mediated gene inactivation expedites the adaptation rate of clinical strains in vitro and fosters within-patient adaptation in the gut microbiota. We deciphered the mechanism underlying the plasmid-mediated surge in IS1 transposition, revealing a negative feedback loop regulated by the genomic copy number of IS1. Given the overrepresentation of IS elements in bacterial plasmids, our findings suggest that plasmid-mediated IS1 transposition represents a crucial mechanism for swift bacterial adaptation.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The sequences generated for the EE assay, analysed during this work, can be found at the NCBI under BioProject PRJNA1076826. The genomic sequences used to study within-patient evolution were previously generated and uploaded to BioProject PRJNA626430. The reference genomes previously generated are available under BioProjects PRJNA626430 and PRJNA838107. Illumina reads from BioProject PRJNA641166 were used to close some of the reference genomes (Supplementary Table 1). The RNA-seq data are available under BioProject PRJNA1071971 and the Gene Expression Omnibus under accession no. GSE255663.
Code availability
All code developed for the analyses included in this work can be found at GitHub (https://github.com/jorgEVOplasmids/rapid_adaptation_pOXA48). The extended code for the RNA-seq analysis can be found at https://github.com/LaboraTORIbio/RNA-Seq_enterobacteria_pOXA-48.
References
Wiedenbeck, J. & Cohan, F. M. Origins of bacterial diversity through horizontal genetic transfer and adaptation to new ecological niches. FEMS Microbiol. Rev. 35, 957–976 (2011).
Ochman, H., Lawrence, J. G. & Groisman, E. A. Lateral gene transfer and the nature of bacterial innovation. Nature 405, 299–304 (2000).
Harrison, E. & Brockhurst, M. A. Plasmid-mediated horizontal gene transfer is a coevolutionary process. Trends Microbiol. 20, 262–267 (2012).
Botelho, J. & Schulenburg, H. The role of integrative and conjugative elements in antibiotic resistance evolution. Trends Microbiol. 29, 8–18 (2021).
San Millan, A. Evolution of plasmid-mediated antibiotic resistance in the clinical context. Trends Microbiol. 26, 978–985 (2018).
MacLean, R. C. & San Millan, A. The evolution of antibiotic resistance. Science 365, 1082–1083 (2019).
Castañeda-Barba, S., Top, E. M. & Stalder, T. Plasmids, a molecular cornerstone of antimicrobial resistance in the One Health era. Nat. Rev. Microbiol. 22, 18–32 (2024).
Friedman, N. D., Temkin, E. & Carmeli, Y. The negative impact of antibiotic resistance. Clin. Microbiol. Infect. 22, 416–422 (2016).
San Millan, A., Escudero, J. A., Gifford, D. R., Mazel, D. & MacLean, R. C. Multicopy plasmids potentiate the evolution of antibiotic resistance in bacteria. Nat. Ecol. Evol. 1, 10 (2016).
Rodriguez-Beltran, J. et al. Multicopy plasmids allow bacteria to escape from fitness trade-offs during evolutionary innovation. Nat. Ecol. Evol. 2, 873–881 (2018).
Rodríguez-Beltrán, J., DelaFuente, J., León-Sampedro, R., MacLean, R. C. & San Millán, Á. Beyond horizontal gene transfer: the role of plasmids in bacterial evolution. Nat. Rev. Microbiol. 19, 347–359 (2021).
Alonso-Del Valle, A. et al. Antimicrobial resistance level and conjugation permissiveness shape plasmid distribution in clinical enterobacteria. Proc. Natl Acad. Sci. USA 120, e2314135120 (2023).
Hernandez-Beltran, J. C. R. et al. Plasmid-mediated phenotypic noise leads to transient antibiotic resistance in bacteria. Nat. Commun. 15, 2610 (2024).
Mazzamurro, F. et al. Intragenomic conflicts with plasmids and chromosomal mobile genetic elements drive the evolution of natural transformation within species. Preprint at bioRxiv https://doi.org/10.1101/2023.11.06.565790 (2023).
Bottery, M. J., Wood, A. J. & Brockhurst, M. A. Adaptive modulation of antibiotic resistance through intragenomic coevolution. Nat. Ecol. Evol. 1, 1364–1369 (2017).
San Millan, A., Heilbron, K. & MacLean, R. C. Positive epistasis between co-infecting plasmids promotes plasmid survival in bacterial populations. ISME J. 8, 601–612 (2014).
Dunn, S., Carrilero, L., Brockhurst, M. & McNally, A. Limited and strain-specific transcriptional and growth responses to acquisition of a multidrug resistance plasmid in genetically diverse Escherichia coli lineages. mSystems 6, e00083-21 (2021).
Thompson, C. M. A. et al. Plasmids manipulate bacterial behaviour through translational regulatory crosstalk. PLoS Biol. 21, e3001988 (2023).
Remigi, P. et al. Transient hypermutagenesis accelerates the evolution of legume endosymbionts following horizontal gene transfer. PLoS Biol. 12, e1001942 (2014).
Horne, T., Orr, V. T. & Hall, J. P. How do interactions between mobile genetic elements affect horizontal gene transfer? Curr. Opin. Microbiol. 73, 102282 (2023).
Benz, F. & Hall, A. R. Host-specific plasmid evolution explains the variable spread of clinical antibiotic-resistance plasmids. Proc. Natl Acad. Sci. USA 120, e2212147120 (2023).
Carrilero, L., Dunn, S. J., Moran, R. A., McNally, A. & Brockhurst, M. A. Evolutionary responses to acquiring a multidrug resistance plasmid are dominated by metabolic functions across diverse Escherichia coli lineages. mSystems 8, e0071322 (2023).
Siguier, P., Gourbeyre, E., Varani, A., Ton-Hoang, B. & Chandler, M. in Mobile DNA III (eds Craig, N. L. et al.) 555–590 (ASM, 2015).
Casacuberta, E. & González, J. The impact of transposable elements in environmental adaptation. Mol. Ecol. 22, 1503–1517 (2013).
Gusa, A. et al. Genome-wide analysis of heat stress-stimulated transposon mobility in the human fungal pathogen Cryptococcus deneoformans. Proc. Natl Acad. Sci. USA 120, e2209831120 (2023).
Senft, A. D. & Macfarlan, T. S. Transposable elements shape the evolution of mammalian development. Nat. Rev. Genet. 22, 691–711 (2021).
McClintock, B. The origin and behavior of mutable loci in maize. Proc. Natl Acad. Sci. USA 36, 344–355 (1950).
Modzelewski, A. J., Gan Chong, J., Wang, T. & He, L. Mammalian genome innovation through transposon domestication. Nat. Cell Biol. 24, 1332–1340 (2022).
Parkhill, J. et al. Genome sequence of Yersinia pestis, the causative agent of plague. Nature 413, 523–527 (2001).
Héritier, C., Poirel, L. & Nordmann, P. Cephalosporinase over-expression resulting from insertion of ISAba1 in Acinetobacter baumannii. Clin. Microbiol. Infect. 12, 123–130 (2006).
Schneider, D. & Lenski, R. E. Dynamics of insertion sequence elements during experimental evolution of bacteria. Res. Microbiol. 155, 319–327 (2004).
Good, B. H., McDonald, M. J., Barrick, J. E., Lenski, R. E. & Desai, M. M. The dynamics of molecular evolution over 60,000 generations. Nature 551, 45–50 (2017).
Wedel, E. et al. Insertion sequences determine plasmid adaptation to new bacterial hosts. mBio 14, e0315822 (2023).
Consuegra, J. et al. Insertion-sequence-mediated mutations both promote and constrain evolvability during a long-term experiment with bacteria. Nat. Commun. 12, 980 (2021).
Goswami, C. et al. Origin, maintenance and spread of antibiotic resistance genes within plasmids and chromosomes of bloodstream isolates of Escherichia coli. Microb. Genom. 6, e000353 (2020).
Harrison, E., Guymer, D., Spiers, A. J., Paterson, S. & Brockhurst, M. A. Parallel compensatory evolution stabilizes plasmids across the parasitism–mutualism continuum. Curr. Biol. 25, 2034–2039 (2015).
Wang, Y., Batra, A., Schulenburg, H. & Dagan, T. Gene sharing among plasmids and chromosomes reveals barriers for antibiotic resistance gene transfer. Philos. Trans. R. Soc. Lond. B Biol. Sci. 377, 20200467 (2022).
Yao, Y. et al. Intra- and interpopulation transposition of mobile genetic elements driven by antibiotic selection. Nat. Ecol. Evol. 6, 555–564 (2022).
Che, Y. et al. Conjugative plasmids interact with insertion sequences to shape the horizontal transfer of antimicrobial resistance genes. Proc. Natl Acad. Sci. USA 118, e2008731118 (2021).
Alonso-Del Valle, A. et al. Variability of plasmid fitness effects contributes to plasmid persistence in bacterial communities. Nat. Commun. 12, 2653 (2021).
Fernández-Calvet, A. et al. The distribution of fitness effects of plasmid pOXA-48 in clinical enterobacteria. Microbiology 169, 001369 (2023).
Pitout, J. D. D., Peirano, G., Kock, M. M., Strydom, K.-A. & Matsumura, Y. The global ascendency of OXA-48-type carbapenemases. Clin. Microbiol. Rev. 33, e00102–e00119 (2019).
David, S. et al. Genomic surveillance of multidrug-resistant Klebsiella in Wales reveals persistent spread of Klebsiella pneumoniae ST307 and adaptive evolution of pOXA-48-like plasmids. Microb. Genom. 9, mgen001016 (2023).
Hernández-García, M. et al. Characterization of carbapenemase-producing Enterobacteriaceae from colonized patients in a university hospital in Madrid, Spain, during the R-GNOSIS project depicts increased clonal diversity over time with maintenance of high-risk clones. J. Antimicrob. Chemother. 73, 3039–3043 (2018).
Deatherage, D. E. & Barrick, J. E. Identification of mutations in laboratory evolved microbes from next-generation sequencing data using breseq. Methods Mol. Biol. 1151, 165–188 (2014).
Card, K. J., Thomas, M. D., Graves, J. L.Jr, Barrick, J. E. & Lenski, R. E. Genomic evolution of antibiotic resistance is contingent on genetic background following a long-term experiment with Escherichia coli. Proc. Natl Acad. Sci. USA 118, e2016886118 (2021).
Lieberman, T. D. et al. Parallel bacterial evolution within multiple patients identifies candidate pathogenicity genes. Nat. Genet. 43, 1275–1280 (2011).
Cooper, V. S. Experimental evolution as a high-throughput screen for genetic adaptations. mSphere 3, e00121-18 (2018).
Lange, R. & Hengge-Aronis, R. The nlpD gene is located in an operon with rpoS on the Escherichia coli chromosome and encodes a novel lipoprotein with a potential function in cell wall formation. Mol. Microbiol. 13, 733–743 (1994).
Nucci, A., Rocha, E. P. C. & Rendueles, O. Adaptation to novel spatially-structured environments is driven by the capsule and alters virulence-associated traits. Nat. Commun. 13, 4751 (2022).
Ton-Hoang, B., Turlan, C. & Chandler, M. Functional domains of the IS1 transposase: analysis in vivo and in vitro. Mol. Microbiol. 53, 1529–1543 (2004).
Zerbib, D., Polard, P., Escoubas, J. M., Galas, D. & Chandler, M. The regulatory role of the IS1-encoded InsA protein in transposition. Mol. Microbiol. 4, 471–477 (1990).
Luria, S. E. & Delbrück, M. Mutations of bacteria from virus sensitivity to virus resistance. Genetics 28, 491–511 (1943).
Kawecki, T. J. et al. Experimental evolution. Trends Ecol. Evol. 27, 547–560 (2012).
Brockhurst, M. A. Experimental evolution can unravel the complex causes of natural selection in clinical infections. Microbiology 161, 1175–1179 (2015).
DelaFuente, J. et al. Within-patient evolution of plasmid-mediated antimicrobial resistance. Nat. Ecol. Evol. 6, 1980–1991 (2022).
León-Sampedro, R. et al. Pervasive transmission of a carbapenem resistance plasmid in the gut microbiota of hospitalized patients. Nat. Microbiol. 6, 606–616 (2021).
Coll, F. et al. Definition of a genetic relatedness cutoff to exclude recent transmission of meticillin-resistant Staphylococcus aureus: a genomic epidemiology analysis. Lancet Microbe 1, e328–e335 (2020).
Maldonado, R. F., Sá-Correia, I. & Valvano, M. A. Lipopolysaccharide modification in Gram-negative bacteria during chronic infection. FEMS Microbiol. Rev. 40, 480–493 (2016).
Meng, J., Young, G. & Chen, J. The Rcs system in Enterobacteriaceae: envelope stress responses and virulence regulation. Front. Microbiol. 12, 627104 (2021).
Artyszuk, D. et al. The impact of insertion sequences on O-serotype phenotype and its O-locus-based prediction in Klebsiella pneumoniae O2 and O1. Int. J. Mol. Sci. 21, 6572 (2020).
Szijártó, V. et al. Both clades of the epidemic KPC-producing Klebsiella pneumoniae clone ST258 share a modified galactan O-antigen type. Int. J. Med. Microbiol. 306, 89–98 (2016).
Aubry, C. et al. OatA, a peptidoglycan O-acetyltransferase involved in Listeria monocytogenes immune escape, is critical for virulence. J. Infect. Dis. 204, 731–740 (2011).
Chen, G. Y., Thorup, N. R., Miller, A. J., Li, Y.-C. & Ayres, J. S. Cooperation between physiological defenses and immune resistance produces asymptomatic carriage of a lethal bacterial pathogen. Sci. Adv. 9, eadg8719 (2023).
Scanlan, P. D. Microbial evolution and ecological opportunity in the gut environment. Proc. Biol. Sci. 286, 20191964 (2019).
Fishbein, S. R. S., Mahmud, B. & Dantas, G. Antibiotic perturbations to the gut microbiome. Nat. Rev. Microbiol. 21, 772–788 (2023).
Xie, Z. & Tang, H. ISEScan: automated identification of insertion sequence elements in prokaryotic genomes. Bioinformatics 33, 3340–3347 (2017).
Wu, Y., Aandahl, R. Z. & Tanaka, M. M. Dynamics of bacterial insertion sequences: can transposition bursts help the elements persist? BMC Evol. Biol. 15, 288 (2015).
Iranzo, J., Gómez, M. J., López de Saro, F. J. & Manrubia, S. Large-scale genomic analysis suggests a neutral punctuated dynamics of transposable elements in bacterial genomes. PLoS Comput. Biol. 10, e1003680 (2014).
Giordano, C. et al. Expansion of KPC-producing Klebsiella pneumoniae with various mgrB mutations giving rise to colistin resistance: the role of ISL3 on plasmids. Int. J. Antimicrob. Agents 51, 260–265 (2018).
Fordham, S. M. E., Mantzouratou, A. & Sheridan, E. Prevalence of insertion sequence elements in plasmids relating to mgrB gene disruption causing colistin resistance in Klebsiella pneumoniae. Microbiologyopen 11, e1262 (2022).
Zheng, Q. rSalvador: an R package for the fluctuation experiment. G3 7, 3849–3856 (2017).
Acknowledgements
We thank J. A. Escudero for constructive comments on the manuscript, O. Rendueles for helping the discussion regarding K. pneumoniae capsules, C. W. Marshall for bioinformatics support, F. Trigo da Roza for helping with long-read sequencing, and L. Jaraba and A. Alonso-Del Valle for technical support. This work was supported by the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme (ERC grant no. 757440-PLASREVOLUTION to A.S.M.), the Instituto de Salud Carlos III (grant no. PI19/00749 to A.S.M.) co-funded by the European Development Regional Fund ‘A way to achieve Europe’, the ‘La Caixa’ Foundation (ID 100010434) under project LCF/BQ/PR22/11920001 (to A.S.-L.), the European Commission (nos. H2020-MSCA-IF-2019 and 895671-REPLAY to A.S.-L.) and the European Society of Clinical Microbiology and Infectious Diseases (research grant 2022 to A.S.-L.). J.R.-B. acknowledges support from a Miguel Servet contract from the Carlos III Health Institute (ISCIII) (grant no. CP20/00154), co-founded by the European Social Fund, ‘Investing in your future’, PI21/01363, funded by the Carlos III Health Institute (ISCIII) and co-funded by the European Union and the ERC HorizonGT (no. 101077809). The research by R.C. is supported by CIBER de Enfermedades Infecciosas (CIBERINFEC) (CB21/13/00081 and CB21/13/00084), Instituto de Salud Carlos III. M.H.-G. is supported by a postdoctoral contract from CIBERINFEC (CB21/13/00084). C.H. is supported by a Sara Borrel contract from the Instituto de Salud Carlos III (ISCIII) (grant no. CD21/00115), the Convocatoria Intramural Emergentes 2021 FIBioHRC-IRYCIS (cod. IMP-21 no. C13) and project PI23/01945 funded by the Carlos III Health Institute (ISCIII).
Author information
Authors and Affiliations
Contributions
J.S.-D., A.S.-L. and A.S.M. conceptualized the study. J.S.-D., A.S.-L. and A.S.M. designed the methodology. J.S.-D. analysed the genomic data. A.S.-L., J.D.F., C.H., C.C., P.H.-G. and J.R.-B. performed the experiments. J.D.F. and L.T.-C. contributed to the data analysis. M.H.-G. and R.C. designed and supervised the sampling and collection of bacterial isolates. J.S.-D., A.S.-L. and A.S.M. analysed the data, prepared the original draft of the manuscript and reviewed and edited the manuscript. A.S.-L. and A.S.M. acquired the funding and supervised the study.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Ecology & Evolution thanks the anonymous reviewers for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Main parameters of bacterial growth curves.
a) Schematic representation of the different parameters that can be extracted from growth curves as proxies for fitness. Growth curves of plasmid-carrying and plasmid-free isogenic strains. The comparison of the maximum growth rate (μmax), maximum OD (ODmax), duration of the lag phase (lag), and the area under the curve (AUC) of plasmid-carrying and plasmid-free clones gives an idea of the fitness effect of the plasmid40. The AUC integrates information about μmax, ODmax and lag in a single metric, and thus, is the one we used for our analysis (see Fig. 1 in the main text). b) Fitness effects of pOXA-48 in the strains under study using ODmax as metric (relative ODmax of plasmid-carrying vs plasmid-free clones). We did not detect any significant cost in any of the strains (two-sided paired t-tests after Bonferroni correction; p > 0.05; n = 13 strains, 6 biological replicates per strain). Black error bars represent the standard deviation (SD) of the relative ODmax for each strain. c) Fitness effects of pOXA-48 in the strains under study using μmax as metric (relative μmax of plasmid-carrying vs plasmid-free clones). We did not detect any significant cost in any of the strains (two-sided paired t-tests after Bonferroni correction; p > 0.05; n = 13 strains, 6 biological replicates per strain). Black error bars represent the standard deviation (SD) of the relative μmax for each strain.
Extended Data Fig. 2 Growth curves during EE.
Optical Density at 600 nm wavelength (OD600) of the strains included in this study measured during 24 hours in alternative days during the EE assay. The day of the EE assay in which we measured bacterial growth is indicated on the right, as well as the experimental conditions (presence/absence of pOXA-48 and presence/absence of AMC). Each strain name is indicated on the top. Each color of the curves represents a different replicate for each strain and condition.
Extended Data Fig. 3 Frequency of New Junction events (NJ) and Single Nucleotide Polymorphisms (SNPs) or small insertions or deletions (INDELs) during the EE in different conditions.
NJ events are mutations predicted from split-read sequences which match distant locations in the reference sequence, such as large (>150 bp) deletions/insertions45. The frequency of each of the mutational events is shown for each strain and experimental condition at the end of the EE. In general, NJs showed high frequencies for all the strains and conditions (n = 13 strains, 3 biological replicates per strain and condition), suggesting that these potentially produced more notable effects on bacterial fitness during the experiment. From all the mutations captured, very few were fixed (100% freq.; n = 26/256 (10.2%) of the total SNPs; n = 27/376 (7.1%) of the total NJs) in the populations at the end of the experiment.
Extended Data Fig. 4 Overview of the mutational profile in each evolved replicate population.
Summary of the genetic changes in the evolved replicate populations in the different conditions (grouped in y axis) and species (grouped in x axis). We show the number of events in each replicate population (n = 3) grouped by its strain. The label of each strain (E. coli n = 4; K. pneumoniae n = 7 and C. freundii n = 2) is indicated in the lower part of the barplot. The colors of the bars depict the different types of mutations occurring during the EE classified in NJs (IS1-mediated, Non-IS-mediated or mediated by other ISs) and SNPs (intergenic, synonymous or non-synonymous). A general increase in the number of IS1-mediated NJ events can be observed for each replicate population propagated during the EE in presence of pOXA-48 both for K. pneumoniae and C. freundii.
Extended Data Fig. 5 IS families transposition during EE.
Number of transposition events associated with each of the IS families during the evolution for each species and experimental condition. Barplots represent the number of transposition events per IS family summarized for all the strains of each species studied. We identified those NJ events mediated by ISs in each population, and classified them depending on the family. We noticed that IS1 elements showed specially high transposition levels in most of the species and conditions. However, both in C. freundii and K. pneumoniae we observed that the presence of pOXA-48 significantly triggered the mobilization of IS1 during evolution (stats in Fig. 2b).
Extended Data Fig. 6 Analysis of the specificity of the types of mutations for each experimental condition during evolution.
The average similarity (in percentage) among all replicate population pairs both within groups (circular arrows) and between groups (straight arrows) for each experimental condition and each species is shown. Briefly, we analyzed the specificity of the different types of mutations (classified in NJs: Non-IS mediated, Other IS or IS1 mediated; and in SNPs: intergenic, synonymous or non-synonymous) for each condition separately for each species as described in ref. 46. Significant differences between groups are indicated (***: p < 0.05). Based on these values, we performed two-sided permutation tests to study the significance of the specificity of mutations associated with both pOXA-48 presence (No pOXA-48 vs pOXA-48) and AMC presence (pOXA-48 vs pOXA-48 + AMC) considering all replicate populations of each species. Our results show that in none of the species the presence of AMC affected the types of mutations reported during the EE (E. coli, p = 0.124; K. pneumoniae, p = 0.231; C. freundii, p = 0.914), whereas the presence of the plasmid pOXA-48 produced significant differences in mutation types both in K. pneumoniae (p = 0.003) and C. freundii (p = 0.008), but not in E. coli (p = 0.952). The average similarity in the types of mutations was significantly lower in K. pneumoniae and C.freundii when comparing pOXA-48 carrying populations vs pOXA-48 free populations. Overall, these results support the idea that the mutation profiles were dependent on pOXA-48 presence, but not on AMC presence, in K. pneumoniae and C. freundii.
Extended Data Fig. 7 Capsule loss in the different conditions tested during the fluctuation assay of IS1 transposition and its results including all the controls.
a) Scheme showing capsule loss in the different conditions both with and without pIS1. Our EE observations indicated that non-capsulated K. pneumoniae mutants appeared mainly due to IS1 insertions in the capsule operon in presence of pOXA-48. Hence, the induction of pIS1 in presence of arabinose should repress the transposition of pOXA-48 IS1 elements into the capsule operon due to increased levels of repressor (InsA). b) Phenotypic mutation rate of non-capsulated mutants in logarithmic scale. Left side of the plot shows all the results for the samples tested in LB, including the different phenotypes with 1, 2 or none of the plasmids (pOXA-48; pIS1), while the right plot shows the results of the fluctuation assay in presence of arabinose (LB + ARA). We could detect a clear repression of non-capsulated phenotype in pOXA-48 + pIS1 samples in presence of arabinose, reaching levels very similar to those of pOXA-48-free samples (n = 80 independent cultures for No pOXA-48 and pOXA-48 genotypes; n = 20 independent cultures for pOXA-48 + pIS1 and pIS1 genotypes). Error bars indicate the likelihood ratio-based 95% confidence interval centered around the maximum likelihood estimate of each genotype. c) Relative fitness levels of non-capsulated vs. capsulated bacteria without and with pOXA-48 resulting from competition assays (n = 3 biological replicates for clones 81 and 92; n = 2 biological replicates for clones 82, 105, 107 and 110). We selected clones with mutations in different genes along the capsule operon: pe (81 [stop codon], 105 [IS1 inactivation]), pb (82 [IS1 inactivation]), and wcaJ (92 [IS1 inactivation], 107 [IS1 inactivation] and 110 [stop codon]). Increase in relative fitness ranged from 14% to 53% in non-capsulated mutants, both in the presence and absence of pOXA-48.
Extended Data Fig. 8 GAM model results.
a) Predicted partial effects of genomic IS1 copies in the transposition rate of in vivo lineages analyzed through a Generalized Additive Model (GAM). The smooth function (number of basis functions: k = 6) shows a significant decrease in transposition rate (p = 0.032; F = 3.28; R-squared = 0.203; Deviance explained = 29.7%) as genomic IS1 copies increase in the strains, supporting our previous observations. Grey shade indicates the 95% confidence interval area centered around the fitted values predicted by the model. b) Predicted partial effects for the different species (parametric coefficients) included in the GAM. No significant differences were reported between species (two-sided; Intercept t = −0.704, p = 0.4864; E. coli t = 1.73, p = 0.0929; K. pneumoniae t = 0.1654, p = 0.4366). Solid lines indicate the mean partial effect and dashed lines indicate the 95% confidence interval for each species.
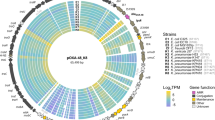
Extended Data Fig. 9 pOXA-48 variants detected in the selected strains.
Scheme of pOXA-48 variants in the selected strains for this work as compared against the most common variant of pOXA-48 (K8). Each concentric circle represents one plasmid. From the inner to the outer, the strains are: C021, C286, C309, C324, CF12, CF13, H53, K091, K147, K153, K163, K209, K25. Most mutations found were accumulated in ltrA, a mobile element of the plasmid, in both C. freundii strains, as well as a non-synonymous SNP in a hypothetical protein of unknown function. For E. coli, only one strain showed a synonymous mutation (C286), whereas for K. pneumoniae strains, three showed differences compared with the K8 variant. Interestingly, K153 showed a deletion in the IS1 element around the 10 kbp, which could potentially affect transposition activity. This strain did not show plasmid-mediated IS1 transposition. K209 showed an intergenic SNP in the region between repC and repA, potentially increasing pOXA-48 PCN. K25 showed two SNPs in conjugation related genes.
Extended Data Fig. 10 Schematic representation of EE + WGS workflow.
This scheme summarizes the main technologies used to sequence each of the samples analyzed during the experimental evolution (Illumina = short-read; Oxford Nanopore Technologies (ONT) = long-read). We combined both short and long read sequencing for getting hybrid closed assemblies of both the ancestors of each strain and of the evolved clones isolated from the populations at day 15. We mainly used short read WGS to filter out possible mutations at day 1 (as a control for the EE), as well as for analyzing the mutations present in the evolved populations and isolated clones at day 15. We used the hybrid assemblies of the ancestors as the reference for the variant calling both populations and clones. From the 117 evolved populations, we discarded 4: a contaminated replicate of C. freundii without pOXA-48, a contaminated replicate of E. coli due to low mapping % when performing the variant calling, and the two hypermutator strains indicated in the main text. We used breseq to perform the variant calling with short-read data. To confirm the results reported by breseq we complemented the variant calling of the clonal samples using long-read data and the Sniffles software (specifically developed to detect Structural Variants). Finally, we also supported the variant calling results with the closed genomes of the evolved clones. We indicate the sequencing technology or the software used for the hybrid assembly/variant calling next to each arrow. We show input samples and analysis results inside rectangles.
Supplementary information
Supplementary Information
Supplementary Figs. 1–13.
Supplementary Data 1
Information and sequencing details of the bacterial strains used in the experimental evolution assay, within the patient evolution and RNA-seq analyses presented in this work.
Supplementary Data 2
Summary of the mutational events identified by breseq at the end of the experimental evolution in all the evolved populations sequenced. The table shows SNPs/indels, NJ events, IS1 transposition events per replicate, as well as the breseq mapping statistics.
Supplementary Data 3
Mutation spectrum for each of the species studied during the experimental evolution.
Supplementary Data 4
Summary of the mutational events identified by breseq (SNPs/indels and NJs) and by sniffles2 (structural variants, such as IS transpositions) at the end of the experimental evolution in the clones selected to be sequenced per population.
Supplementary Data 5
Summary of the results for the fluctuation tests of K25 (capsulated versus non-capsulated mutants). The table includes the results of the variant calling of the non-capsulated mutants using breseq against the K25 ancestral reference, as well as the competition results for non-capsulated versus capsulated K25 clones.
Supplementary Data 6
Summary of the mutational events identified by breseq in the within-patient evolution analysis.
Supplementary Data 7
Primers used for the construction of the plasmid pIS1.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sastre-Dominguez, J., DelaFuente, J., Toribio-Celestino, L. et al. Plasmid-encoded insertion sequences promote rapid adaptation in clinical enterobacteria. Nat Ecol Evol 8, 2097–2112 (2024). https://doi.org/10.1038/s41559-024-02523-4
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41559-024-02523-4
This article is cited by
-
A plasmid-chromosome crosstalk in multidrug resistant enterobacteria
Nature Communications (2024)