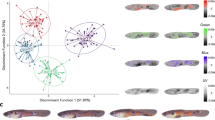
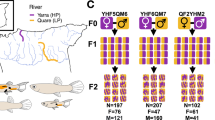
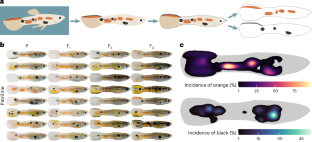
Abstract
The extraordinary variation in male guppy (Poecilia reticulata) colouration is a powerful model for studying the interplay of natural and sexual selection. However, the complexity of this variation has hampered the high-resolution characterization and determination of the genetic architecture underlying male guppy colour and clouded our understanding of how this exceptional level of diversity is maintained. Here we identify the heritability and genetic basis of male colour variation using convolutional neural networks for high-resolution phenotyping coupled with selection experiments, controlled pedigrees and whole-genome resequencing for a genome-wide association study of colour traits. Our phenotypic and genomic results converge to show that colour patterning in guppies is a combination of many heritable features, each with a largely independent genetic architecture spanning the entire genome. Autosomally inherited ornaments are polygenic, with significant contributions from loci involved in neural crest cell migration. Unusually, the results of our genome-wide association study suggest that gene duplicates from the autosomes to the Y chromosome are responsible for much of the sex-linked variation in colour in guppies, providing a potential mechanism for the maintenance of variation of this classic model trait.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Sequence data are available via the SRA under accession PRJNA1262490 (http://www.ncbi.nlm.nih.gov/bioproject/1262490), image data are available via the Federated Research Data Repository94 and tabular data are available via Zenodo at https://doi.org/10.5281/zenodo.15499676 (ref. 19). Source data are provided with this paper.
Code availability
Code is available via GitHub at https://github.com/Ax3man/vdBijl_etal_2025_GuppyColorPatterns and via Zenodo at https://doi.org/10.5281/zenodo.15499676 (ref. 19).
References
Stephenson, J. F., Stevens, M., Troscianko, J. & Jokela, J. The size, symmetry, and color saturation of a male guppy’s ornaments forecast his resistance to parasites. Am. Nat. 196, 597–608 (2020).
Karino, K., Shinjo, S. & Sato, A. Algal-searching ability in laboratory experiments reflects orange spot coloration of the male guppy in the wild. Behaviour 144, 101–113 (2007).
Evans, J. P., Kelley, J. L., Bisazza, A., Finazzo, E. & Pilastro, A. Sire attractiveness influences offspring performance in guppies. Proc. R. Soc. Lond. B 271, 2035–2042 (2004).
Endler, J. A. Natural selection on color patterns in Poecilia reticulata. Evolution 34, 76–91 (1980).
Potter, T. et al. Female preference for rare males is maintained by indirect selection in Trinidadian guppies. Science 380, 309–312 (2023).
Houde, A. E. Mate choice based upon naturally occurring color-pattern variation in a guppy population. Evolution 41, 1–10 (1987).
Houde, A. E. & Endler, J. A. Correlated evolution of female mating preferences and male color patterns in the guppy Poecilia reticulata. Science 248, 1405–1408 (1990).
Haskins, C. P., Haskins, E. F., McLaughlin, J. J. A. & Hewitt, R. E. in Vertebrate Speciation (ed Blair, W. F.) 320–395 (Univ. of Texas at Austin Press, 1961).
Kemp, D. J., Reznick, D. N., Grether, G. F. & Endler, J. A. Predicting the direction of ornament evolution in Trinidadian guppies (Poecilia reticulata). Proc. R. Soc. B 276, 4335–4343 (2009).
Herdegen-Radwan, M., Cattelan, S., Buda, J., Raubic, J. & Radwan, J. What do orange spots reveal about male (and female) guppies? A test using correlated responses to selection. Evolution 75, 3037–3055 (2021).
Brooks, R. & Endler, J. A. Direct and indirect sexual selection and quantitative genetics of male traits in guppies (Poecilia reticulata). Evolution 55, 1002–1015 (2001).
Morris, J., Darolti, I., van der Bijl, W. & Mank, J. E. High-resolution characterization of male ornamentation and re-evaluation of sex linkage in guppies. Proc. R. Soc. B 287, 20201677 (2020).
Hughes, K. A., Houde, A. E., Price, A. C. & Rodd, F. H. Mating advantage for rare males in wild guppy populations. Nature 503, 108–110 (2013).
Farr, J. A. Male rarity or novelty, female choice behavior, and sexual selection in the guppy, Poecilia reticulata Peters (Pisces: Poeciliidae). Evolution 31, 162–168 (1977).
Lindholm, A. K. & Breden, F. Sex chromosomes and sexual selection in poeciliid fishes. Am. Nat. 160, S214–S224 (2002).
Tripathi, N. et al. Genetic linkage map of the guppy, Poecilia reticulata, and quantitative trait loci analysis of male size and colour variation. Proc. R. Soc. B 276, 2195–2208 (2009).
Nanda, I. et al. Sex chromosome polymorphism in guppies. Chromosoma 123, 373–383 (2014).
Kirpichnikov, V. S. Genetic Bases of Fish Selection (Springer, 1981).
van der Bijl, W. et al. Ax3man/vdBijl_etal_2025_GuppyColorPatterns. Zenodo https://doi.org/10.5281/zenodo.15499676 (2025).
Kruuk, L. E. B. Estimating genetic parameters in natural populations using the ‘animal model. Philos. Trans. R. Soc. Lond. B 359, 873–890 (2004).
Winge, Ø. One-sided masculine and sex-linked inheritance in Lebistes reticulatus. J. Genet. 12, 145–162 (1922).
Winge, Ø. & Ditlevsen, E. Colour inheritance and sex determination in Lebistes. Heredity 1, 65–83 (1947).
Haskins, C. P., Young, P., Hewitt, R. E. & Haskins, E. F. Stabilised heterozygosis of supergenes mediating certain Y-linked colour patterns in populations of Lebistes reticulatus. Heredity 25, 575–589 (1970).
Parichy, D. M., Ransom, D. G., Paw, B., Zon, L. I. & Johnson, S. L. An orthologue of the kit-related gene fms is required for development of neural crest-derived xanthophores and a subpopulation of adult melanocytes in the zebrafish, Danio rerio. Development 127, 3031–3044 (2000).
Wilkins, A. S., Wrangham, R. W. & Fitch, W. T. The “domestication syndrome” in mammals: a unified explanation based on neural crest cell behavior and genetics. Genetics 197, 795–808 (2014).
Liley, N. R. Ethological isolating mechanisms in four sympatric species of poeciliid fishes. Behav. Suppl. 1, 1–197 (1966).
Houde, A. E. Sex, Color, and Mate Choice in Guppies (Princeton Univ. Press, 1997).
Brooks, R. & Endler, J. A. Female guppies agree to differ: phenotypic and genetic variation in mate-choice behavior and the consequences for sexual selection. Evolution 55, 1644–1655 (2001).
Schroff, F., Kalenichenko, D. & Philbin, J. FaceNet: A unified embedding for face recognition and clustering. In 2015 IEEE Conference on Computer Vision and Pattern Recognition 815–823 (IEEE, 2015); https://doi.org/10.1109/CVPR.2015.7298682
Hoyal Cuthill, J. F., Guttenberg, N., Ledger, S., Crowther, R. & Huertas, B. Deep learning on butterfly phenotypes tests evolution’s oldest mathematical model. Sci. Adv. 5, eaaw4967 (2019).
Almeida, P. et al. Divergence and remarkable diversity of the Y chromosome in guppies. Mol. Biol. Evol. 38, 619–633 (2021).
Du, K. et al. Identification of the male-specific region on the guppy Y chromosome from a haplotype-resolved assembly. Genome Res. https://doi.org/10.1101/gr.279582.124 (2025).
Wright, A. E. et al. Convergent recombination suppression suggests role of sexual selection in guppy sex chromosome formation. Nat. Commun. 8, 14251 (2017).
Darolti, I. et al. Extreme heterogeneity in sex chromosome differentiation and dosage compensation in livebearers. Proc. Natl Acad. Sci. USA 116, 19031–19036 (2019).
Künstner, A. et al. The genome of the Trinidadian guppy, Poecilia reticulata, and variation in the Guanapo population. PLoS ONE 11, e0169087 (2016).
Fraser, B. A. et al. Improved reference genome uncovers novel sex-linked regions in the guppy (Poecilia reticulata). Genome Biol. Evol. 12, 1789–1805 (2020).
Barriga, E. H. & Mayor, R. in Current Topics in Developmental Biology (ed. Yap, A. S.) vol. 112, 301–323, Ch. 9 (Academic Press, 2015).
Parichy, D. M. & Spiewak, J. E. Origins of adult pigmentation: diversity in pigment stem cell lineages and implications for pattern evolution. Pigment Cell Melanoma Res. 28, 31–50 (2015).
Yin, L. et al. Epidermal gene expression and ethnic pigmentation variations among individuals of Asian, European and African ancestry. Exp. Dermatol. 23, 731–735 (2014).
Yong, L., Croft, D. P., Troscianko, J., Ramnarine, I. W. & Wilson, A. J. Sensory-based quantification of male colour patterns in Trinidadian guppies reveals no support for parallel phenotypic evolution in multivariate trait space. Mol. Ecol. 31, 1337–1357 (2022).
Hoek, K. S. et al. Novel MITF targets identified using a two-step DNA microarray strategy. Pigment Cell Melanoma Res. 21, 665–676 (2008).
Zhang, Y. et al. Transcriptome analyses of betta fish (Betta splendens) provide novel insights into fin regeneration and color-related genes. Gene 876, 147508 (2023).
Yu, H.-H. & Moens, C. B. Semaphorin signaling guides cranial neural crest cell migration in zebrafish. Dev. Biol. 280, 373–385 (2005).
Koludrovic, D. et al. Chromatin-remodelling complex NURF is essential for differentiation of adult melanocyte stem cells. PLoS Genet. 11, e1005555 (2015).
Miyadai, M. et al. Pax3 and Pax7 function in combination with Mitf to generate melanophores and xanthophores in medaka and zebrafish. Preprint at bioRxiv https://doi.org/10.1101/2023.06.22.546052 (2023).
Korzeniwsky, K. G. et al. Dominant negative Mitf allele impacts melanophore and xanthophore development and reveals collaborative interactions with Tfec in zebrafish chromatophore lineages. Pigment Cell Melanoma Res. 38, e70009 (2025).
Bhattacharya, D., Rothstein, M., Azambuja, A. P. & Simoes-Costa, M. Control of neural crest multipotency by Wnt signaling and the Lin28/let-7 axis. eLife 7, e40556 (2018).
Takahashi, K. et al. A globin-family protein, cytoglobin 1, is involved in the development of neural crest-derived tissues and organs in zebrafish. Dev. Biol. 472, 1–17 (2021).
Boer, E. F., Howell, E. D., Schilling, T. F., Jette, C. A. & Stewart, R. A. Fascin1-dependent filopodia are required for directional migration of a subset of neural crest cells. PLoS Genet. 11, e1004946 (2015).
Lin, Y. et al. Gene duplication to the Y chromosome in Trinidadian guppies. Mol. Ecol. 31, 1853–1863 (2022).
Bergero, R., Gardner, J., Bader, B., Yong, L. & Charlesworth, D. Exaggerated heterochiasmy in a fish with sex-linked male coloration polymorphisms. Proc. Natl Acad. Sci. USA 116, 6924–6931 (2019).
Tomaszkiewicz, M., Chalopin, D., Schartl, M., Galiana, D. & Volff, J.-N. A multicopy Y-chromosomal SGNH hydrolase gene expressed in the testis of the platyfish has been captured and mobilized by a Helitron transposon. BMC Genet. 15, 44 (2014).
Sandkam, B. A. et al. Extreme Y chromosome polymorphism corresponds to five male reproductive morphs of a freshwater fish. Nat. Ecol. Evol. 5, 939–948 (2021).
Lindsay, C. R. et al. P-Rex1 is required for efficient melanoblast migration and melanoma metastasis. Nat. Commun. 2, 555 (2011).
Endler, J. A. in Evolutionary Ecology of Neotropical Freswater Fishes (ed Zaret, T. M.) 95–111 (Springer, 1984).
Kotrschal, A. et al. Artificial selection on relative brain size in the guppy reveals costs and benefits of evolving a larger brain. Curr. Biol. 23, 168–171 (2013).
Fong, S. et al. Rapid mosaic brain evolution under artificial selection for relative telencephalon size in the guppy (Poecilia reticulata). Sci. Adv. 7, eabj4314 (2021).
Kotrschal, A. et al. Rapid evolution of coordinated and collective movement in response to artificial selection. Sci. Adv. 6, eaba3148 (2020).
Houde, A. E. Effect of artificial selection on male colour patterns on mating preference of female guppies. Proc. R. Soc. Lond. B 256, 125–130 (1994).
Cole, G. L. & Endler, J. A. Change in male coloration associated with artificial selection on foraging colour preference. J. Evol. Biol. 31, 1227–1238 (2018).
Yamamoto, T. in Handbook of Genetics: Vertebrates of Genetic Interest vol. 4 (ed. King, R. C.) 133–149 (Springer, 1975); https://doi.org/10.1007/978-1-4613-4470-4_7
Houde, A. E. Sex-linked heritability of a sexually selected character in a natural population of Poecilia reticulata (Pisces: Poeciliidae) (guppies). Heredity 69, 229–235 (1992).
Gordon, S. P., López-Sepulcre, A. & Reznick, D. N. Predation-associated differences in sex linkage of wild guppy coloration. Evolution 66, 912–918 (2012).
Kotrschal, A., Corral-Lopez, A. & Kolm, N. Large brains, short life: selection on brain size impacts intrinsic lifespan. Biol. Lett. 15, 20190137 (2019).
Lin, Y., Darolti, I., Bijl, W., van der, Morris, J. & Mank, J. E. Extensive variation in germline de novo mutations in Poecilia reticulata. Genome Res. 33, 1317–1324 (2023).
Kottler, V. A., Fadeev, A., Weigel, D. & Dreyer, C. Pigment pattern formation in the guppy, Poecilia reticulata, involves the Kita and Csf1ra receptor tyrosine kinases. Genetics 194, 631–646 (2013).
Kottler, V. A. et al. Adenylate cyclase 5 is required for melanophore and male pattern development in the guppy (Poecilia reticulata). Pigment Cell Melanoma Res. 28, 545–558 (2015).
Paris, J. R. et al. A large and diverse autosomal haplotype is associated with sex-linked colour polymorphism in the guppy. Nat. Commun. 13, 1233 (2022).
Pérez-Gálvez, A. & Mínguez-Mosquera, M. I. Esterification of xanthophylls and its effect on chemical behavior and bioavailability of carotenoids in the human. Nutr. Res. 25, 631–640 (2005).
Breithaupt, D. E., Bamedi, A. & Wirt, U. Carotenol fatty acid esters: easy substrates for digestive enzymes? Comp. Biochem. Physiol. B 132, 721–728 (2002).
Bruders, R. et al. A copy number variant is associated with a spectrum of pigmentation patterns in the rock pigeon (Columba livia). PLoS Genet. 16, e1008274 (2020).
Weich, K. et al. Pigment intensity in dogs is associated with a copy number variant upstream of KITLG. Genes 11, 75 (2020).
Kokko, H., Jennions, M. D. & Houde, A. Evolution of frequency-dependent mate choice: keeping up with fashion trends. Proc. R. Soc. B 274, 1317–1324 (2007).
Gordon, S. P., López-Sepulcre, A., Rumbo, D. & Reznick, D. N. Rapid changes in the sex linkage of male coloration in introduced guppy populations. Am. Nat. 189, 196–200 (2017).
Bürkner, P.-C. Advanced Bayesian multilevel modeling with the R package brms. R J. 10, 395–411 (2018).
Bürkner, P.-C. brms: an R package for Bayesian multilevel models using Stan. J. Stat. Softw. 80, 1–28 (2017).
Lenth, R. V. emmeans: Estimated Marginal Means, Aka Least-Squares Means (CRAN, 2023); https://CRAN.R-project.org/package=emmeans
Bates, D., Mächler, M., Bolker, B. & Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48 (2015).
Pérez-Escudero, A., Vicente-Page, J., Hinz, R. C., Arganda, S. & de Polavieja, G. G. idTracker: tracking individuals in a group by automatic identification of unmarked animals. Nat. Methods 11, 743–748 (2014).
Brooks, M. E. et al. glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. R J. 9, 378–400 (2017).
Carpenter, B. et al. Stan: a probabilistic programming language. J. Stat. Softw. https://doi.org/10.18637/jss.v076.i01 (2017).
Wolak, M. E. nadiv: an R package to create relatedness matrices for estimating non-additive genetic variances in animal models. Methods Ecol. Evol. 3, 792–796 (2012).
Giovanny, C.-P. Genome assisted prediction of quantitative traits using the R package sommer. PLoS ONE 11, 1–15 (2016).
Zhang, Z. et al. Mixed linear model approach adapted for genome-wide association studies. Nat. Genet. 42, 355–360 (2010).
Zhou, X. & Stephens, M. Genome-wide efficient mixed-model analysis for association studies. Nat. Genet. 44, 821–824 (2012).
Legarra, A., Aguilar, I. & Misztal, I. A relationship matrix including full pedigree and genomic information. J. Dairy Sci. 92, 4656–4663 (2009).
Li, X. & Zhu, X. in Statistical Human Genetics: Methods and Protocols (ed. Elston, R. C.) 455–467 (Springer, 2017); https://doi.org/10.1007/978-1-4939-7274-6_22
Benjamini, Y. & Hochberg, Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B 57, 289–300 (1995).
Krassowski, M. ComplexUpset. Zenodo https://doi.org/10.5281/zenodo.3700590 (2020).
Baglama, J., Reichel, L. & Lewis, B. W. Irlba: Fast Truncated Singular Value Decomposition and Principal Components Analysis for Large Dense and Sparse Matrices (2022).
Li, H. Minimap2: pairwise alignment for nucleotide sequences. Bioinformatics 34, 3094–3100 (2018).
Tang, A. D. et al. Full-length transcript characterization of SF3B1 mutation in chronic lymphocytic leukemia reveals downregulation of retained introns. Nat. Commun. 11, 1438 (2020).
R Core Team. R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, 2023).
van der Bijl, W. et al. Male guppy ornamentation: a multi-generational dataset of aligned images of guppies and their orange and black coloration. Federated Research Data Repository https://doi.org/10.20383/103.01281 (2025).
Acknowledgements
We thank L. Yong for sharing insights on guppy photography. We thank B. Sandkam, B. Furman, D. Metzger, L. Fong, Y. Lin, C. Hodson and J. Lewis for help with animal husbandry and valuable discussions and T. Booker for advice and comments on an earlier draft. Y. Lin contributed the female coverage data. Funding was provided by a Canada 150 Research Chair, NSERC and an ERC grant (680951) to J.E.M.
Author information
Authors and Affiliations
Contributions
Conceptualization: W.v.d.B., A.C.-L. and J.E.M.; methodology: W.v.d.B.; formal analysis: W.v.d.B. and A.C.-L.; investigation: W.v.d.B., J.J.S., V.S.G., L.M.S., C.J., M.C.-C., A.C.-L. and J.E.M.; writing—original draft: W.v.d.B. and J.E.M.; writing—review and editing: W.v.d.B., J.J.S., V.S.G., L.M.S., C.J., M.C.-C., A.C.-L. and J.E.M.; visualization: W.v.d.B.; supervision: J.E.M.; project administration: J.J.S.; funding acquisition: J.E.M.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Ecology & Evolution thanks Nidal Karagic, Claudius Kratochwil and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Variance components and heritability of total color, as estimated by a Bayesian animal model.
a) Cumulative posterior density bar graphs, for each component of total orange (left) and black (right) color. Bars represent in order: autosomal, X-linked and Y-linked genetic variance, maternal effects variance, tank environment variance, other environmental variance, variance due to asymmetry, and measurement error. b) Posterior distributions of the total, X-linked and Y-linked heritability of total color. c) Posterior distributions of the correlation between orange and black variance components. Posterior summaries are shown as posterior means (point) and 66% (thick lines) and 95% (thin lines) credible intervals in all panels.
Extended Data Fig. 2 Correlated effects of selection on orange area on the incidence of black coloration.
a) Density plots show the distribution of the percent of black coloration per generation and selection regime. Thin lines between generations connect fathers and sons. Thick lines inside the densities show the median value of color area. b) Heatmaps illustrating the effect of selection across the body. Each heatmap cell is colored by the log odds ratio (as estimated by a generalized linear mixed model), illustrating the relative odds that a male has black color at that location. Body positions where the incidence of black color is less than 1% are colored grey. c) Effect of selection on orange on location in black patternspace. Axes show black patternspace (after UMAP dimension reduction), with points indicating individuals, colored by selection direction, and facets showing the four generations.
Extended Data Fig. 3 Effects of artificial selection for orange coloration on sexual behavior, gross morphology and life history.
a to e) Comparison of display and sexually coercive behavior between down-selected (n = 46) and up-selected (n = 49) males from the F3 generation. f to h) Comparison of gross morphology between down-selected (n = 63) and up-selected (n = 62) males from the F3 generation. i & j) Comparison for life history parameters between down-selected (nF1 = 106, nF2 = 106) and up-selected (nF1 = 104, nF2 = 105) females from the F1 and F2 generations. All panels: Violin plots show the kernel densities of observations, and point estimates and error bars show the point estimate and 95% confidence intervals for the groups means estimated by a linear mixed model.
Extended Data Fig. 4 Black ornaments are heritable but responded only weakly to selection on orange color.
a) Pictograms of eight black ornaments. b) Heritabilities of the incidence and size (when present) of each ornament. Dots and lines reflect point estimates and 95% credible intervals, and the gradient bars show the cumulative posterior density. c&d) Effect of selection on the incidence and size of black ornaments. Error bars show 95% bootstrapped confidence intervals, and points represent means. X-axes show consecutive generations. Sample size (number of fish); P generation: 300; down-selected F1, F2, F3: 453, 570, 441; up-selected F1, F2, F3: 508, 513, 444.
Extended Data Fig. 5 Top 30 most common ornament combinations.
The y-axis shows the median color pattern for each combination of ornaments, while x-axis shows its count (top) and frequency in the population (bottom). The bars indicate the observed frequency, while the orange lines show the frequency predicted from the incidence of each of the pattern’s ornaments. Inset: The number of unique ornament combinations in the full dataset of all males (black line), compared the expectation derived from ornament incidence (orange). The expected distribution was calculated by randomly generating 3,229 males and their ornaments with P(incidence), repeated 1,000 times. The point and errorbar denote the mean and 95% confidence interval.
Extended Data Fig. 6 Pattern space encodes variation in the incidence and size of ornaments.
a&c) Pictograms of seven orange and eight black ornaments, matching with the associated panels in B&D respectively. b&d) Axes are the UMAP reduced representation of five-dimensional pattern space. Individuals are colored by the size of their relevant ornament, expressed as a fraction of the largest observed size, or colored grey if they lack the ornament. Note the use of a logarithmic scale for ornament size.
Extended Data Fig. 7 GWAS results for the presence/absence of six orange ornaments, as in Fig. 5.
Points represent SNPs and small indels, with their genomic location on the x-axis, and the p-value of the association on the y-axis. Numbers along the x axis denote linkage groups. Sex-linked loci are placed to the side, with both the sex-chromosome (LG12) and sex-linked loci cross-mapping to the autosomes shown, colored by their putative origin (red = X, blue = Y). Peaks are labelled with gene names, but unnamed and uncharacterized genes are not shown. Names with asterisk (*) indicate that more significant cross-mapping SNPs were present in the same peak.
Extended Data Fig. 8 GWAS results for the presence/absence of six black ornaments, as in Fig. 5.
Points represent SNPs and small indels, with their genomic location on the x-axis, and the p-value of the association on the y-axis. Numbers along the x axis denote linkage groups. Sex-linked loci are placed to the side, with both the sex-chromosome (LG12) and sex-linked loci cross-mapping to the autosomes shown, colored by their putative origin (red = X, blue = Y). Peaks are labelled with gene names, but unnamed and uncharacterized genes are not shown. Names with asterisk (*) indicate that more significant cross-mapping SNPs were present in the same peak.
Extended Data Fig. 9 Autosomally inherited ornaments are complex traits.
a) Pictograms of eight ornaments with (partial) autosomal inheritance. b) Illustrative GWAS peaks for the association with the ornaments depicted in A. Each point is a genetic variant, with black points surpassing the FDR-threshold, and the red point is the top variant. Horizontal lines show the location of all annotated genes, with labels only shown for characterized genes. c) Bars indicate the proportion of individuals with the ornament (depicted in A) depending on their genotype at the top variant. The proportion of variation in the phenotype that is explained by the top variant is displayed as Nagelkerke’s R2 calculated from a logistic regression. d) PheWAS heatmaps displaying Z-scores for the effect of the top variant on orange or black color across the body, controlling for autosomal and sex-linked relatedness.
Extended Data Fig. 10 Expression at texim and relation to ornament O6.
a) Expression of texim copy 6531 in three male and three female pools, as counts per million (CPM). b) The proportion of males with ornament O6, as a function of the presence of texim copy 6531, as determined by reads with diagnostic SNPs for that copy. The proportion of phenotypic variance is shown as Nagelkerke’s R2.
Supplementary information
Supplementary Information
Supplementary Notes, Figs. 1–14 and Table 1.
Source data
Source Data Figs. 1–6
Statistical source data.
Source Data Extended Data Figs. 1–6 and 10
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
van der Bijl, W., Shu, J.J., Goberdhan, V.S. et al. Deep learning reveals the complex genetic architecture of male guppy colouration. Nat Ecol Evol 9, 1614–1625 (2025). https://doi.org/10.1038/s41559-025-02781-w
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41559-025-02781-w
This article is cited by
-
The next generation of colour pattern genomics
Nature Ecology & Evolution (2025)