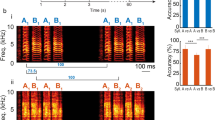
Abstract
Signals in vocal communication systems range from innate to learned. Although innate and learned signals are often assumed to be independent, Darwin speculated that they could be evolutionarily related, with the former being the foundation of the latter even in our own communication system, language. Here we test this hypothesis by studying the vocal communication systems of avian hosts of brood parasites. First, we show that 21 bird species separated by approximately 53 million years of evolution produce structurally similar ‘whining’ vocalizations towards their respective brood parasites. Exploring the social correlates of whining vocalization production, we find that species that produce this vocalization often exist in areas with dense parasite–host networks, suggesting that its production facilitates interactions among host species. Experiments across three continents show that this vocalization is referential towards brood parasites in multiple host species, that hearing them elicits an innate rapid recruiting response, and that host species from different continents respond equally to the whining vocalizations of each other, indicating that convergent use facilitates cooperative defences across species. Our results provide an example of a referential animal vocalization for which sound production in the correct context is learned but for which hearing it elicits an innate response, representing an intermediate between innate and learned signals.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
Datasets used in this study are available in the Supplementary Data.
Code availability
An R script containing all the codes used for statistical analyses is available in the Supplementary Code.
References
Darwin, C. The Descent of Man, and Selection in Relation to Sex Vol. 1 (John Murray, 1871).
Számadó, S. & Szathmáry, E. Selective scenarios for the emergence of natural language. Trends Ecol. Evol. 21, 555–561 (2006).
Wheeler, B. C. & Fischer, J. Functionally referential signals: a promising paradigm whose time has passed. Evol. Anthropol. 21, 195–205 (2012).
Jurisevic, M. A. & Sanderson, K. J. A comparative analysis of distress call structure in Australian passerine and non-passerine species: influence of size and phylogeny. J. Avian Biol. 29, 61–71 (1998).
Aubin, T. Why do distress calls evoke interspecific responses? An experimental study applied to some species of birds. Behav. Process. 23, 103–111 (1991).
Lingle, S. & Riede, T. Deer mothers are sensitive to infant distress vocalizations of diverse mammalian species. Am. Nat. 184, 510–522 (2014).
Pinker, S. Words and Rules: The Ingredients of Language (Basic Books, 1999).
Blasi, D. E., Wichmann, S., Hammarström, H., Stadler, P. F. & Christiansen, M. H. Sound–meaning association biases evidenced across thousands of languages. Proc. Natl Acad. Sci. USA 113, 10818–10823 (2016).
Johansson, N., Anikin, A. & Aseyev, N. Color sound symbolism in natural languages. Lang. Cogn. 12, 56–83 (2020).
Joo, I. Phonosemantic biases found in Leipzig–Jakarta lists of 66 languages. Linguist. Typol. 24, 1–12 (2020).
Winter, B., Sóskuthy, M., Perlman, M. & Dingemanse, M. Trilled /r/ is associated with roughness, linking sound and touch across spoken languages. Sci. Rep. 12, 1035 (2022).
Ćwiek, A. et al. Novel vocalizations are understood across cultures. Sci. Rep. 11, 10108 (2021).
Ludwig, V. U., Adachi, I. & Matsuzawa, T. Visuoauditory mappings between high luminance and high pitch are shared by chimpanzees (Pan troglodytes) and humans. Proc. Natl Acad. Sci. USA 108, 20661–20665 (2011).
Seyfarth, R. M. & Cheney, D. L. The origin of meaning in animal signals. Anim. Behav. 124, 339–346 (2017).
Marler, P. The logical analysis of animal communication. J. Theor. Biol. 1, 295–317 (1961).
Cheney, D. L. & Seyfarth, R. M. Flexible usage and social function in primate vocalizations. Proc. Natl Acad. Sci. USA 115, 1974–1979 (2018).
Davies, N. B. Cuckoos, Cowbirds and Other Cheats (T & A. D. Poyser, 2000).
Pollock, H. S., Hoover, J. P., Uy, F. M. K. & Hauber, M. E. Brood parasites are a heterogeneous and functionally distinct class of natural enemies. Trends Parasitol. 37, 588–596 (2021).
Langmore, N. E. et al. Coevolution with hosts underpins speciation in brood-parasitic cuckoos. Science 384, 1030–1036 (2024).
Feeney, W. E., Welbergen, J. A. & Langmore, N. E. Advances in the study of coevolution between avian brood parasites and their hosts. Annu. Rev. Ecol. Evol. Syst. 45, 227–246 (2014).
Soler, M. Long-term coevolution between avian brood parasites and their hosts. Biol. Rev. 89, 688–704 (2014).
Kennerley, J. A. et al. The overlooked complexity of avian brood parasite–host relationships. Ecol. Lett. 25, 1889–1904 (2022).
Feeney, W. E., Welbergen, J. A. & Langmore, N. E. The frontline of avian brood parasite–host coevolution. Anim. Behav. 84, 3–12 (2012).
Macedonia, J. M. & Evans, C. S. Essay on contemporary issues in ethology: variation among mammalian alarm call systems and the problem of meaning in animal signals. Ethology 93, 177–197 (1993).
Gill, S. A. & Sealy, S. G. Functional reference in an alarm signal given during nest defence: seet calls of yellow warblers denote brood-parasitic brown-headed cowbirds. Behav. Ecol. Sociobiol. 56, 71–80 (2004).
Feeney, W. E. et al. Brood parasitism and the evolution of cooperative breeding in birds. Science 342, 1506–1508 (2013).
Feeney, W. E. & Langmore, N. E. Social learning of a brood parasite by its host. Biol. Lett. 9, 20130443 (2013).
Langmore, N. E. et al. Learned recognition of brood parasitic cuckoos in the superb fairy-wren Malurus cyaneus. Behav. Ecol. 23, 798–805 (2012).
Lawson, S. L. et al. Absence of referential alarm calls in long-term allopatry from the referent: a case study with Galapagos yellow warblers. Behav. Ecol. Sociobiol. 77, 99 (2023).
Feeney, W. E. & Langmore, N. E. Superb fairy-wrens (Malurus cyaneus) increase vigilance near their nest with the perceived risk of brood parasitism. Auk 132, 359–364 (2015).
Payne, R. B., Rowley, I. & Payne, L. L. Splendid wren Malurus splendens response to cuckoos: an experimental test of social organization in a communal bird. Behaviour 94, 108–126 (1985).
Noh, H.-J., Jacomb, F., Gloag, R. & Langmore, N. E. Frontline defences against cuckoo parasitism in the large-billed gerygones. Anim. Behav. 174, 51–61 (2021).
Wheatcroft, D. & Price, T. D. Rates of signal evolution are associated with the nature of interspecific communication. Behav. Ecol. 26, 83–90 (2015).
Jetz, W., Thomas, G. H., Joy, J. B., Hartmann, K. & Mooers, A. O. The global diversity of birds in space and time. Nature 491, 444–448 (2012).
Aubin, T. & Bremond, J. C. Perception of distress call harmonic structure by the starling (Sturnus vulgaris). Behaviour https://doi.org/10.1163/156853992X00570 (1992).
Rowley, I. The life history of the superb blue wren Malurus cyaneus. Emu 64, 251–297 (1965).
Sorenson, M. D. & Payne, R. B. Molecular genetic perspectives on avian brood parasitism. Integr. Comp. Biol. 42, 388–400 (2002).
Gloag, R., Fiorini, V. D., Reboreda, J. C. & Kacelnik, A. The wages of violence: mobbing by mockingbirds as a frontline defence against brood-parasitic cowbirds. Anim. Behav. 86, 1023–1029 (2013).
Davies, N. B. & Brooke, M. de L. Cuckoos versus reed warblers: adaptations and counteradaptations. Anim. Behav. 36, 262–284 (1988).
Langmore, N. E., Cockburn, A., Russell, A. F. & Kilner, R. M. Flexible cuckoo chick-rejection rules in the superb fairy-wren. Behav. Ecol. 20, 978–984 (2009).
Hoover, J. P. & Robinson, S. K. Retaliatory mafia behavior by a parasitic cowbird favors host acceptance of parasitic eggs. Proc. Natl Acad. Sci. USA 104, 4479–4483 (2007).
Ficken, M. S. & Popp, J. A comparative analysis of passerine mobbing calls. Auk 113, 370–380 (1996).
Davies, N. B. & Welbergen, J. A. Social transmission of a host defense against cuckoo parasitism. Science 324, 1318–1320 (2009).
Campobello, D., Sealy, S. G. & Welbergen, J. A. in Avian Brood Parasitism (ed. Soler, M.) 421–436 (Springer, 2017).
Lawson, S. L., Enos, J. K., Gill, S. A. & Hauber, M. E. Red-winged blackbirds nesting nearer to yellow warbler and conspecific nests experience less brood parasitism. Ecol. Evol. 13, e9818 (2023).
Canestrari, D., Marcos, J. M. & Baglione, V. Cooperative breeding in carrion crows reduces the rate of brood parasitism by great spotted cuckoos. Anim. Behav. 77, 1337–1344 (2009).
Trnka, A. & Prokop, P. Polygynous great reed warblers Acrocephalus arundinaceus suffer more cuckoo Cuculus canorus parasitism than monogamous pairs. J. Avian Biol. 42, 192–195 (2011).
Brown, M. & Lawes, M. J. Colony size and nest density predict the likelihood of parasitism in the colonial Southern red bishop Euplectes orix–Diderick cuckoo Chrysococcyx caprius system. Ibis 149, 321–327 (2007).
Wells, M. T. & Barker, F. K. Big groups attract bad eggs: brood parasitism correlates with but does not cause cooperative breeding. Anim. Behav. 133, 47–56 (2017).
Brooker, M. G. & Brooker, L. C. Cuckoo hosts in Australia. Aust. Zoo. Rev. 2, 1–67 (1989).
Curio, E., Ernst, U. & Vieth, W. Cultural transmission of enemy recognition: one function of mobbing. Science 202, 899–901 (1978).
Marler, P. Characteristics of some animal calls. Nature 176, 6–8 (1955).
Morton, E. S. On the occurrence and significance of motivation-structural rules in some bird and mammal sounds. Am. Nat. 111, 855–869 (1977).
King, S. L. & Janik, V. M. Bottlenose dolphins can use learned vocal labels to address each other. Proc. Natl Acad. Sci. USA 110, 13216–13221 (2013).
Pardo, M. A. et al. African elephants address one another with individually specific name-like calls. Nat. Ecol. Evol. 8, 1353–1364 (2024).
Heine, B. The Grammar of Interactives (Oxford Univ. Press, 2023).
Ameka, F. Interjections: the universal yet neglected part of speech. J. Pragmat. 18, 101–118 (1992).
Watson, S. K. et al. Optionality in animal communication: a novel framework for examining the evolution of arbitrariness. Biol. Rev. 97, 2057–2075 (2022).
Flower, T. P., Gribble, M. & Ridley, A. R. Deception by flexible alarm mimicry in an African bird. Science 344, 513–516 (2014).
R Core Team R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, 2022).
Ripley, B. et al. MASS: support functions and datasets for Venables and Ripley’s MASS. R package version 7.3-61 (2023).
Brooks, M. et al. glmmTMB: Generalized linear mixed models using template model builder. R package version 1.1.10 (2023).
Lenth, R. V. emmeans: Estimated marginal means, aka Least-Squares Means. R package version 1.8.7 (2023).
Revell, L. J. phytools: Phylogenetic tools for comparative biology (and other things). R package version 0.7.47 (2023).
Adams, D. C. Comparing evolutionary rates for different phenotypic traits on a phylogeny using likelihood. Syst. Biol. 62, 181–192 (2013).
Paradis, E. et al. ape: Analyses of phylogenetics and evolution. R package version 5.7 (2023).
Lawson, S. L. et al. Do hosts of avian brood parasites discriminate parasitic vs. predatory threats? A meta-analysis. in Advances in the Study of Behaviour Vol. 53 (eds Naguib, M. et al.) 63–95 (Academic Press, 2021).
Cockburn, A. Prevalence of different modes of parental care in birds. Proc. R. Soc. B Biol. Sci. 273, 1375–1383 (2006).
Sahr, K., White, D. & Kimerling, A. J. Geodesic discrete global grid systems. Cartogr. Geogr. Inf. Sci. 30, 121–134 (2003).
BirdLife International and Handbook of the Birds of the World: Bird Species Distribution Maps of the World (BirdLife International, 2017; accessed 9 June 2020); http://datazone.birdlife.org/species/requestdis
Dormann, C. F., Gruber, B. & Fruend, J. Introducing the bipartite package: analysing ecological networks. R. J. 8, 8–11 (2008).
Lawson, S. L., Enos, J. K., Mendes, N. C., Gill, S. A. & Hauber, M. E. Heterospecific eavesdropping on an anti-parasitic referential alarm call. Commun. Biol. 3, 143 (2020).
Yasukawa, K. & Cockburn, A. Antipredator vigilance in cooperatively breeding superb fairy-wrens (Malurus cyaneus). Auk 126, 147–154 (2009).
Hartig, F. & Lohse, L. DHARMa: residual diagnostics for hierarchical (multi-level/mixed) regression models. R package version 0.4.6 (2022).
Thorsten, P. PMCMRplus: calculate pairwise multiple comparisons of mean rank sums extended. R package version 1.9.6 (2022).
Raven Pro: interactive sound analysis software (K. Lisa Yang Center for Conservation Bioacoustics at the Cornell Lab of Ornithology, 2023).
Tobias, J. A. et al. AVONET: morphological, ecological and geographical data for all birds. Ecol. Lett. 25, 581–597 (2022).
Lawson, S. L. et al. Referential alarm calling elicits future vigilance in a host of an avian brood parasite. Biol. Lett. 17, 20210377 (2021).
Acknowledgements
This work was supported by the Alexander von Humboldt Foundation (to W.E.F. and M.E.H.), Birds Queensland (to W.E.F. and M.S.W.), the British Ornithologists’ Union (to W.E.F.), the Hermon-Slade Foundation (no. HS15/1 to W.E.F.), Griffith University and the University of Queensland (to W.E.F.), a Whitten PhD Studentship in the Department of Zoology, University of Cambridge (to J.A.K.), a Clare Hall Research Award, University of Cambridge (to J.A.K.), an Edward W. Rose Postdoctoral Fellowship in the Cornell Lab of Ornithology for part of this work (to J.A.K.), the US National Science Foundation (no. 1353681 to M.S.W., no. 1953226 to M.E.H. and no. 1952726 to S.A.G.), the National Key R & D Program of China (2023YFF1304600 to W.L.), the National Natural Science Foundation of China (32270526 and 32470513 to W.L.), the National Geographic Society (NGS-60453R-19 to M.E.H.), the Australian Research Council (FT110100505 and DP150103595 to A.P.), the Australian Wildlife Conservancy (to N.T. and A.P.), a Royal Society Dorothy Hodgkin Fellowship (to C.N.S.), a Biotechnology and Biological Sciences Research Council David Phillips Research Fellowship (BB/J014109/1) (to C.N.S.), the National Agency for the Promotion of Science and Technology (ANPCyT), National Science Centre, Poland (grants 2012/05/E/NZ8/02694, 2016/23/B/NZ8/03082 and 2022/45/B/NZ8/03740 to A.A., R. Gula and J.T.), and the National Scientific and Technical Research Council (CONICET) (to V.D.F.). We also thank E. Miller for sharing pipelines, which helped with constructing phylogenetically corrected linear models, as well as B. Kempenaers, C. Riehl and J. Fischer for comments on the manuscript. Finally, we thank all our field assistants (E. Aarsvold, A. Branney, R. Bracken, C. Brock, J. D. Brooks, M. Chan, L. Clarke, J. Cosentino, Z. Davis, W. Deptula, B. Donnelly, V. Drolet-Gratton, S. Dougill, R. Green, D. Erickson, M. Freeby, D. Ferraro, L. Fried, J. Grayum, K. Gielow, M. Grundler, J. Grudens, C. Hawey, N. Hunt, L. Huntsmith, O. Kashembe, S. LeQuier, L. Lichtenauer, M. Marsh, A. Miller, C. Moya, S. Mwanza, J. Platzer, R. Neil, A. Sargent, M. Scheuering, D. Thrasher, D. Tolman, R. Weisbeck, J. Welklin, A. Werrell, J. Upton and E. Zarri) for their time and expertise, without which this research would not have been possible.
Author information
Authors and Affiliations
Contributions
W.E.F. conceived the study with input from J.A.K., D.W., D.E.B., A.M., W.L. and M.S.W. W.E.F., J.A.K., B.Z., S.L.L., J.K.E., N.M.R., N.T., M.A., S.A.G., V.D.F., J.B., M.Z., A.A., R. Gula, J.T. and M.E.H. collected the data. W.E.F., J.A.K., A.M. and D.W. implemented the analyses. W.E.F., J.A.K., D.W. and D.E.B. wrote the manuscript with input from all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Ecology & Evolution thanks Sonia Kleindorfer, Jingyi Yang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Vocalization spectrograms.
Representative spectrograms of vocalizations of 26 species, grouped by family, included in the study produced in response to either predators or brood parasites. Top row, from left: brown thornbill (Acanthiza chrysorrhoa), fan-tailed gerygone (Gerygone flavolateralis), white-browed scrubwren (Sericornis frontalis), great reed warbler (Acrocephalus arundinaceus), oriental reed warbler (Acrocephalus orientalis), common tailorbird (Orthotomus sutorius), rufescent prinia (Prinia rufescens), tawny-flankd prinia (Prinia subflava), purple-crowned fairy-wren (Malurus coronatus), superb fairy-wren (Malurus cyaneus), variegated fairy-wren (Malurus lamberti), yellow-faced honeyeater (Caligavis chrysops), brown honeyeater (Lichmera indistincta), noisy friarbird (Philemon corniculatus). Bottom row, from left: chalk-browed mockingbird (Mimus saturninus), yellow warbler (Setophaga petechia), Hume’s leaf warbler (Phylloscopus humei), Ijima’s leaf warbler (Phylloscopus ijimae), western crowned warbler (Phylloscopus occipitalis), buff-barred warbler (Phylloscopus pulcher), mountain chiffchaff (Phylloscopus sindianus), greenish warbler (Phylloscopus trochiloides), willow warbler (Phylloscopus trochilus), Japanese leaf warbler (Phylloscopus xanthodryas), grey-hooded warbler (Phylloscopus xanthoschistos), grey fantail (Rhipidura albiscapa). Color above spectrograms denote vocalization type as in Fig. 1 in the main text. Green: alarm; blue: ‘whining’; yellow: ‘seet’; ‘red’: no unique vocalization.
Extended Data Fig. 2 Estimating the correlation between whining vocalization behaviour and linkage density.
Distribution of posterior means (N = 4,000 posterior samples per tree) obtained following MCMC sampling in a Bayesian threshold model to estimate the evolutionary correlation between the presence of the whining vocalization behaviour in a species and the median linkage density across the species’ range. MCMC sampling was conducted over 5,000,000 iterations with a thinning rate of 1000. The first 20% of iterations (1,000,000 iterations) was discarded as burn-in. The analysis was conducted using 1,000 randomly sampled trees based on the Hackett backbone, and 1,000 randomly sampled trees based on the Ericson backbone. Of these trees, 970 and 985 out of 1000 trees, respectively, had posterior distributions excluding zero.
Extended Data Fig. 3 Phylogeny of species that attended cuckoo model presentation trials or were detected in the vicinity of nests during point counts but did not attend the trial.
For each species it is denoted whether it is known to be a host species of a brood parasite, and whether the species belongs to the order Passeriformes (passerines) which includes the species most frequently parasitized by brood parasitic cuckoos and species known to produce whining vocalizations, or not (non-passerines).
Extended Data Fig. 4 Yellow warbler responses to playbacks.
Responses of yellow warblers (Setophaga petechia) towards 30 s playbacks of brood parasite-context whining vocalizations by superb fairy-wrens (Malurus cyaneus), conspecific predator-context chip, conspecific brood parasite-context seet vocalizations and wood thrush (Hylocichla mustelina) song, a non-threatening control (N = 14 for all treatments). Generalized linear mixed models were used (see Methods for more detail on statistical procedures). Boxes denote median and interquartile ranges, whiskers denote 1.5x interquartile range, and dots indicate data that lies outside of 1.5x interquartile range.
Supplementary information
Supplementary Information
Supplementary Methods and Results, Supplementary Tables 1–4 and Supplementary References.
Supplementary Data
Zip file containing all data.
Supplementary Code
Feeneyetal_Code.Rmd.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Feeney, W.E., Kennerley, J.A., Wheatcroft, D. et al. Learned use of an innate sound-meaning association in birds. Nat Ecol Evol 9, 2103–2115 (2025). https://doi.org/10.1038/s41559-025-02855-9
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41559-025-02855-9