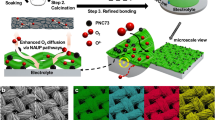
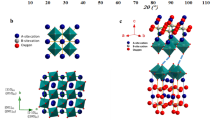
Abstract
Protonic ceramic electrochemical cells (PCECs) have potential as long-duration energy storage systems. However, their operational stability is limited under industrially relevant conditions due to the intrinsic chemical instability of doped barium cerate-based electrolytes and oxygen electrodes against H2O, as well as the poor electrode–electrolyte interfacial contact. Here we present a conformally coated scaffold (CCS) design to comprehensively address these issues. A porous proton-conducting scaffold is constructed and conformally coated with Pr1.8Ba0.2NiO4.1 electrocatalyst, which has high chemical stability against H2O, triple conductivity and hydration capability, and protects vulnerable electrolytes from H2O. The CCS structure consolidates the electrode–electrolyte interfacial bonding to enable fast proton transfer in the percolated network. This design enables PCECs to reach electrolysis stability for 5,000 h at −1.5 A cm−2 and 600 °C in 40% H2O. This work provides a general strategy to stabilize PCECs and offers guidance for designing resilient and stable solid-state energy storage systems.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All of the data supporting the findings of this study are available within this Article and its Supplementary Information. Source data are provided with this paper.
References
Gür, T. M. Review of electrical energy storage technologies, materials and systems: challenges and prospects for large-scale grid storage. Energy Environ. Sci. 11, 2696–2767 (2018).
Zhang, W. et al. Water electrolysis toward elevated temperature: advances, challenges and frontiers. Chem. Rev. 123, 7119–7192 (2023).
Duan, C. C. et al. Highly efficient reversible protonic ceramic electrochemical cells for power generation and fuel production. Nat. Energy 4, 230–240 (2019).
Tian, H. et al. Protonic ceramic materials for clean and sustainable energy: advantages and challenges. Int. Mater. Rev. 68, 272–300 (2023).
Zvonareva, I., Fu, X.-Z., Medvedev, D. & Shao, Z. Electrochemistry and energy conversion features of protonic ceramic cells with mixed ionic-electronic electrolytes. Energy Environ. Sci. 15, 439–465 (2022).
He, F. et al. Catalytic self-assembled air electrode for highly active and durable reversible protonic ceramic electrochemical cells. Adv. Funct. Mater. 32, 2206756 (2022).
Pellow, M. A., Emmott, C. J. M., Barnhart, C. J. & Benson, S. M. Hydrogen or batteries for grid storage? A net energy analysis. Energy Environ. Sci. 8, 1938–1952 (2015).
Peng, X. et al. Hierarchical electrode design of highly efficient and stable unitized regenerative fuel cells (URFCs) for long-term energy storage. Energy Environ. Sci. 13, 4872–4881 (2020).
Wang, Y., Leung, D. Y. C., Xuan, J. & Wang, H. A review on unitized regenerative fuel cell technologies, part-A: unitized regenerative proton exchange membrane fuel cells. Renew. Sustain. Energy Rev. 65, 961–977 (2016).
Wang, Y., Leung, D. Y. C., Xuan, J. & Wang, H. A review on unitized regenerative fuel cell technologies, part B: unitized regenerative alkaline fuel cell, solid oxide fuel cell, and microfluidic fuel cell. Renew. Sustain. Energy Rev. 75, 775–795 (2017).
Duan, C., Huang, J., Sullivan, N. & O’Hayre, R. Proton-conducting oxides for energy conversion and storage. Appl. Phys. Rev. 7, 011314 (2020).
Lei, L. et al. Progress report on proton conducting solid oxide electrolysis cells. Adv. Funct. Mater. 29, 1903805 (2019).
Vollestad, E. et al. Mixed proton and electron conducting double perovskite anodes for stable and efficient tubular proton ceramic electrolysers. Nat. Mater. 18, 752–759 (2019).
Murphy, R. et al. A new family of proton-conducting electrolytes for reversible solid oxide cells: BaHfxCe0.8−xY0.1Yb0.1O3−δ. Adv. Funct. Mater. 30, 2002265 (2020).
Xu, K. et al. An efficient steam-induced heterostructured air electrode for protonic ceramic electrochemical cells. Adv. Funct. Mater. 32, 2110998 (2022).
Luo, Z. Y. et al. Critical role of acceptor dopants in designing highly stable and compatible proton-conducting electrolytes for reversible solid oxide cells. Energy Environ. Sci. 15, 2992–3003 (2022).
Kim, D. et al. High-performance protonic ceramic electrochemical cells. ACS Energy Lett. 7, 2393–2400 (2022).
Choi, S., Davenport, T. C. & Haile, S. M. Protonic ceramic electrochemical cells for hydrogen production and electricity generation: exceptional reversibility, stability, and demonstrated Faradaic efficiency. Energy Environ. Sci. 12, 206–215 (2019).
Pei, K. et al. Surface restructuring of a perovskite-type air electrode for reversible protonic ceramic electrochemical cells. Nat. Commun. 13, 2207 (2022).
Wu, W. et al. 3D self-architectured steam electrode enabled efficient and durable hydrogen production in a proton-conducting solid oxide electrolysis cell at temperatures lower than 600 °C. Adv. Sci. 5, 1800360 (2018).
He, F. et al. An efficient high-entropy perovskite-type air electrode for reversible oxygen reduction and water splitting in protonic ceramic cells. Adv. Mater. 35, 2209469 (2023).
Zhou, Y. C. et al. An active and robust air electrode for reversible protonic ceramic electrochemical cells. ACS Energy Lett. 6, 1511–1520 (2021).
Luo, Z. Y. et al. Highly conductive and durable Nb(Ta)-doped proton conductors for reversible solid oxide cells. ACS Energy Lett. 7, 2970–2978 (2022).
Song, Y. et al. Nanocomposites: a new opportunity for developing highly active and durable bifunctional air electrodes for reversible protonic ceramic cells. Adv. Energy Mater. 11, 2101899 (2021).
Saqib, M. et al. Transition from perovskite to misfit-layered structure materials: a highly oxygen deficient and stable oxygen electrode catalyst. Energy Environ. Sci. 14, 2472–2484 (2021).
Niu, Y. H. et al. Highly active and durable air electrodes for reversible protonic ceramic electrochemical cells enabled by an efficient bifunctional catalyst. Adv. Energy Mater. 12, 2103783 (2022).
Su, H. & Hu, Y. H. Degradation issues and stabilization strategies of protonic ceramic electrolysis cells for steam electrolysis. Energy Sci. Eng. 10, 1706–1725 (2022).
Bian, W. et al. Revitalizing interface in protonic ceramic cells by acid etch. Nature 604, 479–485 (2022).
Tong, H., Fu, M., Yang, Y., Chen, F. & Tao, Z. A novel self-assembled cobalt-free perovskite composite cathode with triple-conduction for intermediate proton-conducting solid oxide fuel cells. Adv. Funct. Mater. 32, 2209695 (2022).
Ding, D., Li, X., Lai, S. Y., Gerdes, K. & Liu, M. Enhancing SOFC cathode performance by surface modification through infiltration. Energy Environ. Sci. 7, 552–575 (2014).
Pei, K. et al. Constructing an active and stable oxygen electrode surface for reversible protonic ceramic electrochemical cells. Appl. Catal. B 330, 122601 (2023).
Duan, C. et al. Readily processed protonic ceramic fuel cells with high performance at low temperatures. Science 349, 1321–1326 (2015).
Tarutina, L. R., Kasyanova, A. V., Starostin, G. N., Vdovin, G. K. & Medvedev, D. A. Electrochemical activity of original and infiltrated Fe-doped Ba(Ce,Zr,Y)O3-based electrodes to be used for protonic ceramic fuel cells. Catalysts 12, 1421 (2022).
Sun, C. et al. Tailoring a micro-nanostructured electrolyte–oxygen electrode interface for proton-conducting reversible solid oxide cells. J. Power Sources 449, 227498 (2020).
Saqib, M. et al. Modification of oxygen-ionic transport barrier of BaCo0.4Zr0.1Fe0.4Y0.1O3 steam (air) electrode by impregnating samarium-doped ceria nanoparticles for proton-conducting reversible solid oxide cells. J. Electrochem. Soc. 166, F746–F754 (2019).
Zhou, Y. C. et al. An efficient bifunctional air electrode for reversible protonic ceramic electrochemical cells. Adv. Funct. Mater. 31, 2105386 (2021).
Kim, H. et al. Unveiling the key factor for the phase reconstruction and exsolved metallic particle distribution in perovskites. Nat. Commun. 12, 6814 (2021).
He, F. et al. A surface reconfiguration of a perovskite air electrode enables an active and durable reversible protonic ceramic electrochemical cell. Energy Storage Mater. 53, 754–762 (2022).
Tian, H. et al. Deconvolution of water-splitting on the triple-conducting Ruddlesden–Popper-phase anode for protonic ceramic electrolysis cells. ACS Appl. Mater. Interfaces 12, 49574–49585 (2020).
Ding, H. et al. Self-sustainable protonic ceramic electrochemical cells using a triple conducting electrode for hydrogen and power production. Nat. Commun. 11, 1907 (2020).
Wang, N. et al. Machine-learning-accelerated development of efficient mixed protonic–electronic conducting oxides as the air electrodes for protonic ceramic cells. Adv. Mater. 34, 2203446 (2022).
Kim, J. et al. Triple-conducting layered perovskites as cathode materials for proton-conducting solid oxide fuel cells. ChemSusChem 7, 2811–2815 (2014).
Liang, M. et al. A new durable surface nanoparticles-modified perovskite cathode for protonic ceramic fuel cells from selective cation exsolution under oxidizing atmosphere. Adv. Mater. 34, 2106379 (2022).
Choi, M. et al. Exceptionally high performance of protonic ceramic fuel cells with stoichiometric electrolytes. Energy Environ. Sci. 14, 6476–6483 (2021).
Duan, C. et al. Highly durable, coking and sulfur tolerant, fuel-flexible protonic ceramic fuel cells. Nature 557, 217–222 (2018).
Song, Y. et al. Self-assembled triple-conducting nanocomposite as a superior protonic ceramic fuel cell cathode. Joule 3, 2842–2853 (2019).
Shi, H. et al. Building Ruddlesden–Popper and single perovskite nanocomposites: a new strategy to develop high-performance cathode for protonic ceramic fuel cells. Small 17, 2101872 (2021).
Regmi, Y. N. et al. A low temperature unitized regenerative fuel cell realizing 60% round trip efficiency and 10 000 cycles of durability for energy storage applications. Energy Environ. Sci. 13, 2096–2105 (2020).
Luo, Z. et al. A new class of proton conductors with dramatically enhanced stability and high conductivity for reversible solid oxide cells. Small 19, 2208064 (2023).
Liang, M. et al. High-temperature water oxidation activity of a perovskite-based nanocomposite towards application as air electrode in reversible protonic ceramic cells. Appl. Catal. B 331, 122682 (2023).
Park, K. et al. Understanding the highly electrocatalytic active mixed triple conducting NaxCa3−xCo4O9−δ oxygen electrode materials. Adv. Energy Mater. 13, 2202999 (2023).
Naeini, M., Cotton, J. S. & Adams, T. A. An eco-technoeconomic analysis of hydrogen production using solid oxide electrolysis cells that accounts for long-term degradation. Front. Energy Res. 10, 1015465 (2022).
Mebane, D. S., Liu, Y. & Liu, M. A two-dimensional model and numerical treatment for mixed conducting thin films: the effect of sheet resistance. J. Electrochem. Soc. 154, A421–A426 (2007).
Lee, Y.-L. et al. Defect thermodynamics and transport properties of proton conducting oxide BaZr1−xYxO3−δ (x ≤ 0.1) guided by density functional theory modeling. JOM 74, 4506–4526 (2022).
Lee, Y.-L. et al. Kinetics of oxygen surface exchange on epitaxial Ruddlesden–Popper phases and correlations to first-principles descriptors. J. Phys. Chem. Lett. 7, 244–249 (2016).
Tarutin, A. P., Danilov, N. A., Kalinin, A. A., Murashkina, A. A. & Medvedev, D. A. Ba-doped Pr2NiO4+δ electrodes for proton-conducting electrochemical cells. Part 1: structure, mechanical, and chemical properties. Int. J. Hydrogen Energy 48, 22531–22544 (2023).
Zou, J. et al. Electrochemical compression technologies for high-pressure hydrogen: current status, challenges and perspective. Electrochem. Energy Rev. 3, 690–729 (2020).
Li, W. et al. Low-temperature water electrolysis: fundamentals, progress, and new strategies. Mater. Adv. 3, 5598–5644 (2022).
Tong, W. et al. Electrolysis of low-grade and saline surface water. Nat. Energy 5, 367–377 (2020).
Acknowledgements
X. Liu acknowledges funding from the US Department of Energy, Office of Energy Efficiency and Renewable Energy (EERE) under contract no. DE-E0008378. We thank the technology managers N. Stetson, D. Peterson and W. Gibbons for technical guidance and financial support. J.L. and K.H. jointly acknowledge partial support by the National Science Foundation Materials Research Science and Engineering Center programme through the University of California Irvine Center for Complex and Active Materials (DMR-2011967). K.H. and H.Z. acknowledge support by the National Science Foundation through the CAREER programme (DMR-2239598) and the use of facilities and instrumentation at the University of California Irvine Materials Research Institute (IMRI), supported in part by the National Science Foundation Materials Research Science and Engineering Center programme through the UC Irvine Center for Complex and Active Materials (DMR-2011967). G.L. and Xiaolin Li acknowledge the US Department of Energy, Office of Electricity (Pacific Northwest National Laboratory project number 70247) for supporting 3D XRM instrumentation and characterization. M.R.R. and F.X. acknowledge the Australian Research Council for funding the in situ XRD instrument through project LE170100199. M.K. acknowledges Murdoch University for providing a Murdoch International Postgraduate Scholarship (MIPS). The use of the WVU Shared Research Facilities is acknowledged. H.T. thanks C. Li at Xi’an Jiaotong University for technical suggestions on cell fabrication and testing. We thank L. Hu at the University of Maryland, College Park and Yale University for technical discussions.
Author information
Authors and Affiliations
Contributions
X. Liu led the project, directed the formulation of overarching project goals, acquired the financial support and supervised the project. H.T., Wenyuan Li and X. Liu conceived the original concept of conformally coated scaffold design. Wei Li conceived the concept of unitized regenerative protonic ceramic electrochemical cell applications for long-duration energy storage cycling and seawater electrolysis. H.T. and Wei Li directed the evolution of the research planning and execution designed the screening criteria for materials, device fabrication, experiments and methodology, fabricated the cells, conducted most of the electrochemical, chemical, material and post-mortem characterizations, performed the CFD modelling, analysed the data and prepared most of the figures. Y.-L.L. conducted the DFT and AIMD calculations and prepared related figures. H.Z. and K.H. performed most of the TEM characterizations and analysis. Q.L. contributed to the electrochemical characterization of symmetric cells and part of the materials characterization. L.M. performed part of the electrochemical characterizations and assisted in the fabrication of the button and large cells and process development and optimization. D.B. conducted the technoeconomic analysis and wrote the related discussion. X.C. performed part of the materials characterization. D.Z. and J.L. contributed to part of the TEM characterizations. G.L. and Xiaolin Li contributed to the XRM characterizations. Y.W. assisted in part of the electrochemical characterizations and button cell fabrication. L.L. and Q.W. assisted in investigations and discussions on the Pr2−xBaxNiO4+δ defects and valence state change mechanism. F.X., M.K. and M.R.R. contributed to the in situ XRD characterizations. Z.S. performed part of the materials characterization and cell fabrication. Wenyuan Li provided suggestions on cell fabrication and electrochemical characterization and assisted in the acquisition of project funding. W.A.S. provided technical suggestions on the DFT and AIMD calculations. C.L. conducted part of the Raman spectroscopy characterizations. Xuemei Li assisted in the fabrication and electrochemical characterization of the nickel-based symmetric cells. Wei Li and H.T. wrote the original draft of the manuscript, response letter, revised manuscript and supplementary information. Y.-L.L. wrote the DFT and AIMD discussion in the original manuscript, response letter and revised manuscript. X. Liu reviewed and revised the paper. All authors discussed, commented and reviewed the paper.
Corresponding authors
Ethics declarations
Competing interests
H.T., Wenyuan Li and X. Liu have filed a patent application (US patent application no. 18/586661) on ‘Conformal coating scaffold electrodes for reversible solid oxide cells’. The other authors declare no competing interests.
Peer review
Peer review information
Nature Energy thanks Andrea Lanzini, Ryan O’Hayre, Emilia Olsson and Jose M. Serra for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Comparison of the fuel cell stability of the CCS-based PCECs in terms of degradation rates and operational duration with those of reported PCECs with the planar contact air electrode design.
The reported PCECs consist of various air electrodes/electrolytes combinations including BCFN/BHCYb (Ba0.9Co0.7Fe0.2Nb0.1O3−δ/BaHf0.1Ce0.7Yb0.2O3−δ), BCFZY/BZCYYb4411 (BaCo0.4Fe0.4Zr0.1Y0.1O3−δ/BaZr0.4Ce0.4Y0.1Yb0.1O3−δ), BCFN/BZCYYb1711 (Ba0.9Co0.7Fe0.2Nb0.1O3−δ/BaZr0.1Ce0.7Y0.1Yb0.1O3−δ), PBSLCC/BZCYYb1711 (Pr0.2Ba0.2Sr0.2La0.2Ca0.2CoO3−δ/BaZr0.1Ce0.7Y0.1Yb0.1O3−δ), PBCC/BNCYb (PrBa0.2Ca0.2Co2O5+δ/BaNb0.05Ce0.7Yb0.25O3–δ), PBCC/BTCYb (PrBa0.2Ca0.2Co2O5+δ/BaTa0.05Ce0.7Yb0.25O3–δ), SCFN/BZCYYb1711 (SrxCeyFemNinO3−δ/BaZr0.1Ce0.7Y0.1Yb0.1O3−δ), PBC-PCO/BZCYYb1711 (PrBaCo2O5+δ-Pr0.1Ce0.9O2+δ/BaZr0.1Ce0.7Y0.1Yb0.1O3−δ), LSCF-BCO/BZCYYb1711 (La0.6Sr0.4Co0.2Fe0.8O3–δ-BaCoO3−δ/BaZr0.1Ce0.7Y0.1Yb0.1O3−δ), BGPC-GCO/BZCYYb1711 (Ba0.8Gd0.8Pr0.4Co2O5+δ-GdxCoyO3−δ/BaZr0.1Ce0.7Y0.1Yb0.1O3−δ), PBCC/BMWCY (PrBa0.2Ca0.2Co2O5+δ/BaMo(W)0.03Ce0.71Yb0.26O3−δ), BCFZYN/BZCYYb1711 (Ba0.95(Co0.4Fe0.4Zr0.1Y0.1)0.95Ni0.05O3−δ/BaZr0.1Ce0.7Y0.1Yb0.1O3−δ), NCC/BZCYYb1711 (Na0.15Ca2.85Co4O9–δ/BaZr0.1Ce0.7Y0.1Yb0.1O3−δ) (refs. 16,17,19,21,23,24,31,36,38,49,50,51). The FC stability and composition of the compared devices are detailed in Supplementary Table 4.
Extended Data Fig. 2 Comparison of the electrolysis cell stability of the CCS-based PCECs in terms of degradation rates and operational duration with those of reported PCECs with the planar contact air electrode design.
PBCC/BZCYYb1711 (PrBa0.2Ca0.2Co2O5+δ/BaZr0.1Ce0.7Y0.1Yb0.1O3−δ), GCCC/BZCYYb1711 (Gd0.3Ca2.7Co3.82Cu0.18O9−δ/BaZr0.1Ce0.7Y0.1Yb0.1O3−δ), LSCF-PBC-BCO/BZCYYb1711 ((La0.6Sr0.4)0.95Co0.2Fe0.8O3−δ-Pr1−xBaxCoO3−δ-BaCoO3−δ/BaZr0.1Ce0.7Y0.1Yb0.1O3−δ) (refs. 3,13,14,16,19,21,22,23,25,26,36,49,50,51). The EC stability and composition of the compared devices are detailed in Supplementary Table 5.
Extended Data Fig. 4 Simulated electrolysis polarization curves under different conditions at 600 °C.
a, Comparison of simulated electrolysis polarization curves of the PC and CCS electrodes with different grain particle diameters. The experimental polarization curve of CCS electrode with a grain diameter of 0.3–0.4 µm is shown for validation of the simulated one. b, Simulated electrolysis polarization curve of the CCS electrode as a function of relative hydration capability. c, Simulated electrolysis polarization curve of the CCS electrode as a function of relative proton diffusivity.
Supplementary information
Supplementary Information
Supplementary Methods, Figs. 1–97, Tables 1–18, Notes 1–18 and References.
Supplementary Video 1
The conduction of protons with and without the trapping effect of interstitial O was simulated using AIMD simulations. The two proton defects generated by the dissociated H2O in separated rock salt layers represent a proton adjacent to an interstitial O and a proton near an apical lattice O away from the interstitial O. The trajectories of these two protons are shown in the video.
Supplementary Video 2
The UR-PCEC prototype system was operated in EC mode at −1 A cm−2 for 12 h, representing the storage of electricity as H2 fuel at off-peak demand time, and then switched to the FC mode at 0.5 A cm−2 for 12 h, representing electricity generation using the stored H2 fuel at on-peak demand time. A typical 12/12 h cycle is shown in the video.
Supplementary Data 1
PBNO and PNO configurations for Supplementary Fig. 72, crystallographic information files for Supplementary Figs. 73 and 74c,d, Slab_figures_Ba_seg for Supplementary Fig. 75 and crystallographic configurations for Supplementary Figs. 83 and 85.
Source data
Source Data Fig. 3
Source data for Fig. 3.
Source Data Fig. 4
Source data for Fig. 4c–f,h.
Source Data Fig. 5
Source data for Fig. 5c–h and inset of h.
Source Data Extended Data Fig. 1
Source data for Extended Data Fig. 1.
Source Data Extended Data Fig. 2
Source data for Extended Data Fig. 2.
Source Data Extended Data Fig. 3
Source data for Extended Data Fig. 3.
Source Data Extended Data Fig. 4
Source data for Extended Data Fig. 4.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tian, H., Li, W., Lee, YL. et al. Conformally coated scaffold design using water-tolerant Pr1.8Ba0.2NiO4.1 for protonic ceramic electrochemical cells with 5,000-h electrolysis stability. Nat Energy 10, 890–903 (2025). https://doi.org/10.1038/s41560-025-01800-1
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41560-025-01800-1
This article is cited by
-
Full steam ahead
Nature Energy (2025)