Abstract
Permafrost regions contain a substantial fraction of Earth’s soil nitrogen, which is vulnerable to climate change. The response of this crucial N stock to warming could impact the permafrost–climate feedback by altering plant productivity, microbial decomposition and nitrous oxide emissions. However, the long-term trajectory of soil N stocks in response to warming remains unclear. Here we present results from a ten-year field warming experiment in a permafrost ecosystem on the Tibetan Plateau. We made repeated measurements of soil N stocks to 50 cm depth and assessed 28 N-cycling variables to explore three primary pathways affecting soil N stocks, including N inputs, microbial N transformations and N losses. Our results reveal that, despite no changes being observed during the initial years of the experiment, warmed plots experienced a decline in surface soil N stocks (an average 7.7% reduction relative to control plots) after eight years of warming. This decrease is associated with the enhanced N sequestration in perennial plant biomass, increased ecosystem N leaching and gaseous N losses from soils. Our findings underscore the vulnerability of soil N stocks in permafrost regions to ongoing warming, and suggest that the potential permafrost–climate feedback may be stronger than previously anticipated.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
Data used in this study are available from the figshare data repository https://doi.org/10.6084/m9.figshare.29468852 (ref. 83). Source data are provided with this paper.
References
Obu, J. How much of the earth’s surface is underlain by permafrost? J. Geophys. Res. Earth Surf. 126, e2021JF006123 (2021).
Mishra, U. et al. Spatial heterogeneity and environmental predictors of permafrost region soil organic carbon stocks. Sci. Adv. 7, eaaz5236 (2021).
Schuur, E. A. G. et al. Permafrost and climate change: carbon cycle feedbacks from the warming arctic. Annu. Rev. Environ. Resour. 47, 343–371 (2022).
Rantanen, M. et al. The Arctic has warmed nearly four times faster than the globe since 1979. Commun. Earth Environ. 3, 168 (2022).
Schuur, E. A. G. et al. Climate change and the permafrost carbon feedback. Nature 520, 171–179 (2015).
McGuire, A. D. et al. Dependence of the evolution of carbon dynamics in the northern permafrost region on the trajectory of climate change. Proc. Natl Acad. Sci. USA 115, 3882–3887 (2018).
Maes, S. L. et al. Environmental drivers of increased ecosystem respiration in a warming tundra. Nature 629, 105–113 (2024).
See, C. R. et al. Decadal increases in carbon uptake offset by respiratory losses across northern permafrost ecosystems. Nat. Clim. Change 14, 853–862 (2024).
Harden, J. W. et al. Field information links permafrost carbon to physical vulnerabilities of thawing. Geophys. Res. Lett. 39, L15704 (2012).
Strauss, J. et al. A globally relevant stock of soil nitrogen in the Yedoma permafrost domain. Nat. Commun. 13, 6074 (2022).
Ramm, E. et al. A review of the importance of mineral nitrogen cycling in the plant–soil–microbe system of permafrost-affected soils—changing the paradigm. Environ. Res. Lett. 17, 013004 (2022).
Strauss, J. et al. Potential nitrogen mobilisation from the Yedoma permafrost domain. Environ. Res. Lett. 19, 043002 (2024).
Salmon, V. G. et al. Nitrogen availability increases in a tundra ecosystem during five years of experimental permafrost thaw. Glob. Change Biol. 22, 1927–1941 (2016).
Keuper, F. et al. Experimentally increased nutrient availability at the permafrost thaw front selectively enhances biomass production of deep-rooting subarctic peatland species. Glob. Change Biol. 23, 4257–4266 (2017).
Knorr, M. A. et al. Unexpected sustained soil carbon flux in response to simultaneous warming and nitrogen enrichment compared with single factors alone. Nat. Ecol. Evol. 8, 2277–2285 (2024).
Voigt, C. et al. Nitrous oxide emissions from permafrost-affected soils. Nat. Rev. Earth Environ. 1, 420–434 (2020).
Voigt, C. et al. Warming of subarctic tundra increases emissions of all three important greenhouse gases carbon dioxide, methane, and nitrous oxide. Glob. Change Biol. 23, 3121–3138 (2017).
Gundale, M. J., Nilsson, M., Bansal, S. & Jäderlund, A. The interactive effects of temperature and light on biological nitrogen fixation in boreal forests. New Phytol. 194, 453–463 (2012).
Schaeffer, S. M., Sharp, E., Schimel, J. P. & Welker, J. M. Soil–plant N processes in a High Arctic ecosystem, NW Greenland are altered by long-term experimental warming and higher rainfall. Glob. Change Biol. 19, 3529–3539 (2013).
Bouskill, N. J., Riley, W. J., Zhu, Q., Mekonnen, Z. A. & Grant, R. F. Alaskan carbon–climate feedbacks will be weaker than inferred from short-term experiments. Nat. Commun. 11, 5798 (2020).
Salazar, A., Rousk, K., Jónsdóttir, I. S., Bellenger, J. & Andrésson, Ó. S. Faster nitrogen cycling and more fungal and root biomass in cold ecosystems under experimental warming: a meta-analysis. Ecology 101, e02939 (2020).
Blok, D. et al. Contrasting above- and belowground organic matter decomposition and carbon and nitrogen dynamics in response to warming in High Arctic tundra. Glob. Change Biol. 24, 2660–2672 (2018).
Plaza, C. et al. Direct observation of permafrost degradation and rapid soil carbon loss in tundra. Nat. Geosci. 12, 627–631 (2019).
Mason, R. E. et al. Evidence, causes, and consequences of declining nitrogen availability in terrestrial ecosystems. Science 376, eabh3767 (2022).
Wan, L. et al. Global warming changes biomass and C:N:P stoichiometry of different components in terrestrial ecosystems. Glob. Change Biol. 29, 7102–7116 (2023).
Dawes, M. A., Schleppi, P., Hättenschwiler, S., Rixen, C. & Hagedorn, F. Soil warming opens the nitrogen cycle at the alpine treeline. Glob. Change Biol. 23, 421–434 (2017).
Luo, Y. et al. Progressive nitrogen limitation of ecosystem responses to rising atmospheric carbon dioxide. BioScience 54, 731–739 (2004).
Christiansen, C. T., Lafreniére, M. J., Henry, G. H. R. & Grogan, P. Long-term deepened snow promotes tundra evergreen shrub growth and summertime ecosystem net CO2 gain but reduces soil carbon and nutrient pools. Glob. Change Biol. 24, 3508–3525 (2018).
Tian, Y. et al. Long-term warming of a forest soil reduces microbial biomass and its carbon and nitrogen use efficiencies. Soil Biol. Biochem. 184, 109109 (2023).
Sistla, S. A. et al. Long-term warming restructures Arctic tundra without changing net soil carbon storage. Nature 497, 615–618 (2013).
Klarenberg, I. J. et al. Long-term warming effects on the microbiome and nifH gene abundance of a common moss species in sub-Arctic tundra. New Phytol. 234, 2044–2056 (2021).
Gundale, M. J., DeLuca, T. H. & Nordin, A. Bryophytes attenuate anthropogenic nitrogen inputs in boreal forests. Glob. Change Biol. 17, 2743–2753 (2011).
Maxwell, T. L. et al. Contrasting drivers of belowground nitrogen cycling in a montane grassland exposed to a multifactorial global change experiment with elevated CO2, warming, and drought. Glob. Change Biol. 28, 2425–2441 (2022).
Wanek, W., Mooshammer, M., Blöchl, A., Hanreich, A. & Richter, A. Determination of gross rates of amino acid production and immobilization in decomposing leaf litter by a novel 15N isotope pool dilution technique. Soil Biol. Biochem. 42, 1293–1302 (2010).
Wang, C. et al. Climate change amplifies gross nitrogen turnover in montane grasslands of Central Europe both in summer and winter seasons. Glob. Change Biol. 22, 2963–2978 (2016).
Jan, M. T., Roberts, P., Tonheim, S. K. & Jones, D. L. Protein breakdown represents a major bottleneck in nitrogen cycling in grassland soils. Soil Biol. Biochem. 41, 2272–2282 (2009).
Kuypers, M. M., Marchant, H. K. & Kartal, B. The microbial nitrogen-cycling network. Nat. Rev. Microbiol. 16, 263–276 (2018).
Kraiser, T., Gras, D. E., Gutiérrez, A. G., González, B. & Gutiérrez, R. A. A holistic view of nitrogen acquisition in plants. J. Exp. Bot. 62, 1455–1466 (2011).
Aerts, R. & Chapin, F. S. The mineral nutrition of wild plants revisited: a re-evaluation of processes and patterns. Adv. Ecol. Res. 30, 1–67 (1999).
Lugli, L. F. et al. Rapid responses of root traits and productivity to phosphorus and cation additions in a tropical lowland forest in Amazonia. New Phytol. 230, 116–128 (2021).
Aber, J. et al. Nitrogen saturation in temperate forest ecosystems. BioScience 48, 921–934 (1998).
Wagner-Riddle, C. et al. Globally important nitrous oxide emissions from croplands induced by freeze–thaw cycles. Nat. Geosci. 10, 279–283 (2017).
Schmidt, S. K. et al. Biogeochemical consequences of rapid microbial turnover and seasonal succession in soil. Ecology 88, 1379–1385 (2007).
Butterbach-Bahl, K., Baggs, E. M., Dannenmann, M., Kiese, R. & Zechmeister-Boltenstern, S. Nitrous oxide emissions from soils: how well do we understand the processes and their controls? Philos. Trans. R. Soc. B 368, 20130122 (2013).
Gil, J., Pérez, T., Boering, K., Martikainen, P. J. & Biasi, C. Mechanisms responsible for high N2O emissions from subarctic permafrost peatlands studied via stable isotope techniques. Glob. Biogeochem. Cycles 31, 172–189 (2017).
Tank, S. E. et al. Recent trends in the chemistry of major northern rivers signal widespread Arctic change. Nat. Geosci. 16, 789–796 (2023).
Chen, L. et al. Permafrost carbon cycle and its dynamics on the Tibetan Plateau. Sci. China Life Sci. 67, 1833–1848 (2024).
Goldberg, S. D., Knorr, K. H., Blodau, C., Lischeid, G. & Gebauer, G. Impact of altering the water table height of an acidic fen on N2O and NO fluxes and soil concentrations. Glob. Change Biol. 16, 220–233 (2010).
Bhattarai, H. R. et al. Germinating wheat promotes the emission of atmospherically significant nitrous acid (HONO) gas from soils. Soil Biol. Biochem. 136, 107518 (2019).
Hu, X. et al. Warming causes contrasting spider behavioural responses by changing their prey size spectra. Nat. Clim. Change 14, 190–197 (2024).
Wei, B. et al. Experimental warming altered plant functional traits and their coordination in a permafrost ecosystem. New Phytol. 240, 1802–1816 (2023).
Li, F. et al. Warming effects on permafrost ecosystem carbon fluxes associated with plant nutrients. Ecology 98, 2851–2859 (2017).
Kou, D. et al. Progressive nitrogen limitation across the Tibetan alpine permafrost region. Nat. Commun. 11, 3331 (2020).
Rousk, K., Sorensen, P. L. & Michelsen, A. Nitrogen transfer from four nitrogen-fixer associations to plants and soils. Ecosystems 19, 1491–1504 (2016).
Liengen, T. Conversion factor between acetylene reduction and nitrogen fixation in free-living cyanobacteria from high arctic habitats. Can. J. Microbiol. 45, 223–229 (1999).
Hobbie, S. E. Effects of plant species on nutrient cycling. Trends Ecol. Evol. 7, 336–339 (1992).
Vitousek, P. M., Turner, D. R., Parton, W. J. & Sanford, R. L. Litter decomposition on the Mauna Loa environmental matrix, Hawai’i: patterns, mechanisms, and models. Ecology 75, 418–429 (1994).
Sun, T. et al. Contrasting dynamics and trait controls in first-order root compared with leaf litter decomposition. Proc. Natl Acad. Sci. USA 115, 10392–10397 (2018).
Dong, L., Berg, B., Sun, T., Wang, Z. & Han, X. Response of fine root decomposition to different forms of N deposition in a temperate grassland. Soil Biol. Biochem. 147, 107845 (2020).
Luan, J. et al. Biodiversity mitigates drought effects in the decomposer system across biomes. Proc. Natl Acad. Sci. USA 121, e2313334121 (2024).
Darrouzet-Nardi, A., Ladd, M. P. & Weintraub, M. N. Fluorescent microplate analysis of amino acids and other primary amines in soils. Soil Biol. Biochem. 57, 78–82 (2013).
Yuan, H. et al. Rapid, sensitive analysis method for determining the nitrogen stable isotope ratio of total free amino acids in soil. Rapid Commun. Mass Spectrom. 36, e9390 (2022).
Kirkham, D. O. N. & Bartholomew, W. V. Equations for following nutrient transformations in soil, utilizing tracer data. Soil Sci. Soc. Am. J. 18, 33–34 (1954).
Smith, M. S. & Tiedje, J. M. Phases of denitrification following oxygen depletion in soil. Soil Biol. Biochem. 11, 261–267 (1979).
Groffman, P. M. et al. Methods for measuring denitrification: diverse approaches to a difficult problem. Ecol. Appl. 16, 2091–2122 (2006).
Felber, R., Conen, F., Flechard, C. R. & Neftel, A. Theoretical and practical limitations of the acetylene inhibition technique to determine total denitrification losses. Biogeosciences 9, 4125–4138 (2012).
Houlton, B. Z. & Bai, E. Imprint of denitrifying bacteria on the global terrestrial biosphere. Proc. Natl Acad. Sci. USA 160, 21713–21716 (2009).
Oulehle, F. et al. Dissolved and gaseous nitrogen losses in forests controlled by soil nutrient stoichiometry. Environ. Res. Lett. 16, 064025 (2021).
Yang, Y., Fang, J., Ma, W., Guo, D. & Mohammat, A. Large-scale pattern of biomass partitioning across China’s grasslands. Glob. Ecol. Biogeogr. 19, 268–277 (2010).
Miller, A. E. et al. Seasonal variation in nitrogen uptake and turnover in two high-elevation soils: mineralization responses are site-dependent. Biogeochemistry 93, 253–270 (2009).
Jiang, L. et al. Plant organic N uptake maintains species dominance under long-term warming. Plant Soil 433, 243–255 (2018).
Xu, X. et al. Spatio-temporal variations determine plant–microbe competition for inorganic nitrogen in an alpine meadow. J. Ecol. 99, 563–571 (2011).
Saiya-Cork, K. R., Sinsabaugh, R. L. & Zak, D. R. The effects of long term nitrogen deposition on extracellular enzyme activity in an Acer saccharum forest soil. Soil Biol. Biochem. 34, 1309–1315 (2002).
Yang, H., Guo, Y., Fang, N., Dong, B. & Wu, X. Greenhouse gas emissions of sewage sludge land application in urban green space: a field experiment in a Bermuda grassland. Sci. Total Environ. 926, 172106 (2024).
Gao, S. et al. Influence of woodchip size and nitrogen fertilization on carbon dioxide and nitrous oxide emissions from soils amended with orchard biomass. Soil Sci. Soc. Am. J. 88, 803–815 (2024).
Lewicka-Szczebak, D., Augustin, J., Giesemann, A. & Well, R. Quantifying N2O reduction to N2 based on N2O isotopocules—validation with independent methods (helium incubation and 15N gas flux method). Biogeosciences 14, 711–732 (2017).
Harris, E. et al. Denitrifying pathways dominate nitrous oxide emissions from managed grassland during drought and rewetting. Sci. Adv. 7, eabb7118 (2021).
Brookes, P. C., Landman, A., Pruden, G. & Jenkinson, D. S. Chloroform fumigation and the release of soil nitrogen: a rapid direct extraction method to measure microbial biomass nitrogen in soil. Soil Biol. Biochem. 17, 837–842 (1985).
Joergensen, R. G. & Mueller, T. The fumigation–extraction method to estimate soil microbial biomass: calibration of the kEN value. Soil Biol. Biochem. 28, 33–37 (1996).
Wu, Z., Dijkstra, P., Koch, G. W. & Hungate, B. A. Biogeochemical and ecological feedbacks in grassland responses to warming. Nat. Clim. Change 2, 458–461 (2012).
Bates, D., Maechler, M., Bolker, B. & Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48 (2015).
R Core Team R: a Language and Environment for Statistical Computing (R Foundation for Statistical Computing, 2023).
Wei, B. et al. Progressive decline in soil nitrogen stocks with warming in a Tibetan permafrost ecosystem. figshare https://doi.org/10.6084/m9.figshare.29468852 (2025).
Obu, J. et al. Northern Hemisphere permafrost map based on TTOP modelling for 2000–2016 at 1 km2 scale. Earth Sci. Rev. 193, 299–316 (2019).
Acknowledgements
We thank D. Li from the Institute of Subtropical Agriculture, Chinese Academy of Sciences, for his assistance with protein depolymerization determination, and X. Xu from the Institute of Geographic Sciences and Natural Resources Research, Chinese Academy of Sciences, for his guidance in 15N-labelling experimental design. We also thank Y. Fang from the Institute of Applied Ecology, C. Liu and W. Zhang from the Institute of Atmospheric Physics, Chinese Academy of Sciences, and J. Chang from Zhejiang University for their suggestions during the paper revision. This work was supported by the National Natural Science Foundation of China (32425004 and 32588202), the National Key Research and Development Program of China (2022YFF0801901 and 2022YFF0801902) and the New Cornerstone Science Foundation through the XPLORER PRIZE. C.V. was supported by the project MOMENT (grant number 03F0931A) funded by the German Federal Ministry of Education and Research (BMBF) and the European Research Council Starting Grant COLDSPOT (101163177).
Author information
Authors and Affiliations
Contributions
Y.Y. and B.W. designed the research. B.W., W.Z., Y.B., Z.Z., Y.X., C.Z., F.W. and L.H. performed the experiments. B.W. analysed the data. B.W. and Y.Y. wrote the paper with inputs from D.Z., C.V., Y.B., G.Y., D.K., Y.P., Y.L. and J.P.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Geoscience thanks Birgit Wild, Petr Capek and Kate Buckeridge for their contribution to the peer review of this work. Primary handling editors: Xujia Jiang and Carolina Ortiz Guerrero, in collaboration with the Nature Geoscience team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Overview of the study site and design of the warming experiment.
a, The field warming experiment was established within a 50 × 50 m fenced area in June 2013 (photo credit: Bin Wei). b, Design of open-top chamber warming devices. The manipulative warming experiment followed a paired design and comprised ten 4 × 4 m blocks. In each block, a hexagonal open-top chamber and a 0.8 × 1.2 m plot were diagonally arranged as the warming and control plot, respectively. The warming device elevated topsoil temperature by an average of 1.3 °C over the past decade. See Supplementary Fig. 1 for details.
Extended Data Fig. 2 A schematic diagram of ecosystem nitrogen cycle and 28 nitrogen-cycling parameters measured in this study.
The N cycle usually consists of an external (that is, atmospheric N deposition, biological N2 fixation, ecosystem N leaching and gaseous losses) and an internal cycle (that is, litter N return, microbial N transformation and plant N uptake). Yellowish-brown, reddish-brown, green and blue arrows denote biological N2 fixation, microbial N transformation, plant N uptake, N leaching and gaseous emissions, respectively. Abbreviations: \({{\rm{NH}}}_{4}^{+}-{\rm{N}}\), ammonium; \({{\rm{NO}}}_{3}^{-}-{\rm{N}}\), nitrate; NO, nitric oxide; N2O, nitrous oxide; N2, dinitrogen gas.
Extended Data Fig. 3 Warming effects on total nitrogen stocks and content in 10-30 cm soils from 2014 to 2023.
a,b, Total N stocks (a) and content (b) in 10-30 cm soil. In a,b, data are visualized as split violin plots with the outline denoting the kernel density estimation of the underlying distributions, and dashed lines denoting the average in control and warming plots (n = 10 biologically independent samples). These comparisons were conducted using the paired-samples t-tests (two sided).
Extended Data Fig. 4 Warming effects on total nitrogen stocks and content in 30-50 cm soils from 2018 to 2023.
a,b, total N stocks (a) and content (b) in 30-50 cm soil. In a,b, data are visualized as split violin plots with the outline denoting the kernel density estimation of the underlying distributions, and dashed lines denote the average in control and warming plots (n = 10 biologically independent samples). Paired-samples t-tests (two sided) were performed to compare the difference in soil N stocks and content between warming and control plots. Deeper soil samples (that is, 30-40 and 40-50 cm) were collected from 2018 onwards.
Extended Data Fig. 5 Warming effects on topsoil bioavailable nitrogen and microbial biomass nitrogen.
a-d, The content of \({{\rm{NO}}}_{4}^{+}-{\rm{N}}\) (a), \({{\rm{NO}}}_{3}^{-}-{\rm{N}}\) (b), dissolved organic N (c) and microbial biomass N (d) in the top 10 cm soil. In a-d, data are shown as the mean ± s.d. (n = 10 biologically independent samples). Each diamond represents the content of soil variables in control and warming plots. Paired-samples t-tests (two sided) were performed to compare differences in these topsoil variables between warming and control treatment. Soil abiotic and biotic parameters in the top 10 cm were determined in 2021. Abbreviations: \({{\rm{NH}}}_{4}^{+}-{\rm{N}}\), ammonium; \({{\rm{NO}}}_{3}^{-}-{\rm{N}}\), nitrate.
Extended Data Fig. 6 The relative contribution of three potential pathways to the observed declines in soil nitrogen stocks.
See Supplementary Table 2 for model details.
Extended Data Fig. 7 Warming effects on cumulative amount of nitrogen losses during the initial 8 years of experiment based on the DeNitrification–DeComposition model.
The green, purple and red areas indicate the primary pathways of N losses included plant N uptake, N leaching and gaseous N emissions from 2014 to 2021. The gaseous N refers to N2O, N2, nitric oxide and ammonia in the DNDC model.
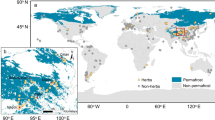
Extended Data Fig. 8 Locations of collected studies and soil nitrogen response to warming across the permafrost-affected regions.
a, Soil N response was collected in 38 datasets from 29 warming experiments at Arctic, subarctic and Tibetan alpine sites. Panel a adapted with permission from ref. 84, Elsevier. b, Mean soil N response to warming (Hedges’s SMD) for all sites, Arctic, subarctic and Tibetan alpine regions. In b, data were visualized as violin plots with outer shape denoting the kernel density estimation of underlying distributions. The white circle in the violin represents the metaregression model estimates, and the error bar denotes 95% confidence interval. The effect of warming was statistically significant if the 95% confidence interval did not overlap zero. Bubble sizes reflect the weight of the observation in the metaregression model. The sample size (n) for each region is indicated in the parenthesis. Abbreviations: Hedges’s SMD; the Hedges’ standardized mean difference. See Supplementary Table 4 for details.
Extended Data Fig. 9 Context-dependencies in the soil nitrogen response to warming.
a-d, Mean annual temperature (a), mean annual precipitation (b), soil pH (c) and soil C:N ratio (d) across Arctic, subarctic and Alpine regions. e-h, The single-factor metaregression modes were used to test the association of soil N Hedges’s SMD with these context-specific environmental conditions. In a-d, data are shown as the median values. In e-h, metaregression results are presented as the mean model estimate (solid line) and 95% confidence intervals grey area. Qm refer to the Q value of importance of the environmental and soil variables. Bubble sizes reflect weight of the observation in the metaregression, with larger bubbles denoting greater weights. In h, hollow point is the outlier identified through Boxplot Procedures and thus excluded from the fitting. Abbreviations: C:N ratios, carbon: nitrogen ratios; Hedges’s SMD; the Hedges’ standardized mean difference.
Extended Data Fig. 10 Trajectories of soil nitrogen stocks under two future climate change scenarios from 2024 to 2100.
The DNDC model was performed under two Shared Socioeconomic Pathway 1-2.6 (SSP1-2.6, low-carbon emissions) and SSP5-8.5 (high-carbon emissions) scenarios to simulate the dynamics of soil N stocks in the top 50 cm (detailed in Supplementary Text Note 5). Green and red lines are multi-model means under SSP1-2.6 and SSP5-8.5 scenarios, and shaded areas represent the standard deviation, calculated from modeling results using meteorological data from three General Circulation Models (that is, EC-Earth3-Veg-LR, ACCESS-CM2 and NorESM2-LM). Green and red donut charts denote that the relative proportions of plant uptake, N leaching and gaseous N emissions to total ecosystem N losses under SSP1-2.6 and SSP5-8.5 scenarios, respectively.
Supplementary information
Supplementary Information
Supplementary Notes 1–14, Tables 1–7, Figs. 1–19, Appendix and References. 1–72.
Source data
Extended Data Figs. 3–10
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wei, B., Zhang, D., Voigt, C. et al. Progressive decline in soil nitrogen stocks with warming in a Tibetan permafrost ecosystem. Nat. Geosci. (2025). https://doi.org/10.1038/s41561-025-01786-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41561-025-01786-1