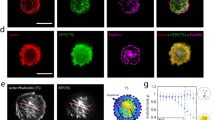
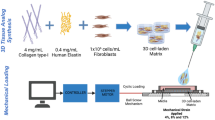
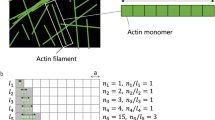
Abstract
Mechanical factors such as stress in the extracellular environment affect the phenotypic commitment of cells. Stress fields experienced by cells in tissues are multiaxial, but how cells integrate such information is largely unknown. Here we report that the anisotropy of stress fields is a critical factor triggering a phenotypic transition in fibroblast cells, outweighing the role of stress amplitude, a factor previously described to modulate such a transition. Combining experimental and computational approaches, we identified a self-reinforcing mechanism in which cellular protrusions interact with collagen fibres to establish tension anisotropy. This anisotropy, in turn, stabilizes the protrusions and enhances their contractile forces. Disruption of this self-reinforcing process, either by reducing tension anisotropy or by inhibiting contractile protrusions, prevents the phenotypic conversion of fibroblasts to contractile myofibroblasts. Overall, our findings support stress anisotropy as a factor modulating cellular responses, expanding our understanding of the role of mechanical forces in biological processes.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Data supporting the findings of this paper are available within the article and its Supplementary Information files. The data can also be obtained from the corresponding authors upon request. Source data are provided with this paper.
Code availability
The custom code used in this study is available on GitHub at https://github.com/Farid-Alisafaei/Tension-Anisotropy and on Zenodo at https://doi.org/10.5281/zenodo.14556939 (ref. 50).
References
Engler, A. J., Sen, S., Sweeney, H. L. & Discher, D. E. Matrix elasticity directs stem cell lineage specification. Cell 126, 677–689 (2006).
Mondrinos, M. J. et al. Surface-directed engineering of tissue anisotropy in microphysiological models of musculoskeletal tissue. Sci. Adv. 7, eabe9446 (2021).
Chaudhuri, O., Cooper-White, J., Janmey, P. A., Mooney, D. J. & Shenoy, V. B. Effects of extracellular matrix viscoelasticity on cellular behaviour. Nature 584, 535–546 (2020).
Lodyga, M. & Hinz, B. TGF-β1—a truly transforming growth factor in fibrosis and immunity. Semin. Cell Dev. Biol. 101, 123–139 (2020).
Li, Z. et al. Transforming growth factor-β and substrate stiffness regulate portal fibroblast activation in culture. Hepatology 46, 1246–1256 (2007).
Humphrey, J. D., Dufresne, E. R. & Schwartz, M. A. Mechanotransduction and extracellular matrix homeostasis. Nat. Rev. Mol. Cell Biol. 15, 802–812 (2014).
Legant, W. R. et al. Measurement of mechanical tractions exerted by cells in three-dimensional matrices. Nat. Methods 7, 969–971 (2010).
Oakes, P. W., Banerjee, S., Marchetti, M. C. & Gardel, M. L. Geometry regulates traction stresses in adherent cells. Biophys. J. 107, 825–833 (2014).
Hall, M. S. et al. Fibrous nonlinear elasticity enables positive mechanical feedback between cells and ECMs. Proc. Natl Acad. Sci. USA 113, 14043–14048 (2016).
van Helvert, S. & Friedl, P. Strain stiffening of fibrillar collagen during individual and collective cell migration identified by AFM nanoindentation. ACS Appl. Mater. Interfaces 8, 21946–21955 (2016).
Doyle, A. D., Carvajal, N., Jin, A., Matsumoto, K. & Yamada, K. M. Local 3D matrix microenvironment regulates cell migration through spatiotemporal dynamics of contractility-dependent adhesions. Nat. Commun. 6, 8720 (2015).
Kim, A. & Matthew Petroll, W. Microtubule regulation of corneal fibroblast morphology and mechanical activity in 3-D culture. Exp. Eye Res. 85, 546–556 (2007).
van Helvert, S., Storm, C. & Friedl, P. Mechanoreciprocity in cell migration. Nat. Cell Biol. 20, 8–20 (2018).
Shakiba, D. et al. The balance between actomyosin contractility and microtubule polymerization regulates hierarchical protrusions that govern efficient fibroblast–collagen interactions. ACS Nano 14, 7868–7879 (2020).
Kolodney, M. S. & Elson, E. L. Contraction due to microtubule disruption is associated with increased phosphorylation of myosin regulatory light chain. Proc. Natl Acad. Sci. USA 92, 10252–10256 (1995).
Feng, C., Cheng, Y. & Chao, P. G. The influence and interactions of substrate thickness, organization and dimensionality on cell morphology and migration. Acta Biomater. 9, 5502–5510 (2013).
Riching, K. M. et al. 3D collagen alignment limits protrusions to enhance breast cancer cell persistence. Biophys. J. 107, 2546–2558 (2014).
Yip, A. K. et al. Anisotropic traction stresses and focal adhesion polarization mediates topography-induced cell elongation. Biomaterials 181, 103–112 (2018).
Buskermolen, A. B. C. et al. Entropic forces drive cellular contact guidance. Biophys. J. 116, 1994–2008 (2019).
Kim, I. L., Khetan, S., Baker, B. M., Chen, C. S. & Burdick, J. A. Fibrous hyaluronic acid hydrogels that direct MSC chondrogenesis through mechanical and adhesive cues. Biomaterials 34, 5571–5580 (2013).
Baker, B. M. et al. Cell-mediated fibre recruitment drives extracellular matrix mechanosensing in engineered fibrillar microenvironments. Nat. Mater. 14, 1262–1268 (2015).
Wade, R. J., Bassin, E. J., Rodell, C. B. & Burdick, J. A. Protease-degradable electrospun fibrous hydrogels. Nat. Commun. 6, 6639 (2015).
Ban, E. et al. Mechanisms of plastic deformation in collagen networks induced by cellular forces. Biophys. J. 114, 450–461 (2018).
Kim, J. et al. Stress-induced plasticity of dynamic collagen networks. Nat. Commun. 8, 842 (2017).
Petroll, W. M., Cavanagh, H. D. & Jester, J. V. Dynamic three-dimensional visualization of collagen matrix remodeling and cytoskeletal organization in living corneal fibroblasts. Scanning 26, 1–10 (2004).
Grolman, J. M., Weinand, P. & Mooney, D. J. Extracellular matrix plasticity as a driver of cell spreading. Proc. Natl Acad. Sci. USA 117, 25999–26007 (2020).
Nam, S., Lee, J., Brownfield, D. G. & Chaudhuri, O. Viscoplasticity enables mechanical remodeling of matrix by cells. Biophys. J. 111, 2296–2308 (2016).
Mohammadi, H., Arora, P. D., Simmons, C. A., Janmey, P. A. & McCulloch, C. A. Inelastic behaviour of collagen networks in cell–matrix interactions and mechanosensation. J. R. Soc. Interface 12, 20141074 (2015).
Boyle, J. J. et al. Regularization-free strain mapping in three dimensions, with application to cardiac ultrasound. J. Biomech. Eng. 141, 011010 (2019).
Boyle, J. J. et al. Simple and accurate methods for quantifying deformation, disruption, and development in biological tissues. J. R. Soc. Interface 11, 20140685 (2014).
Alisafaei, F., Jokhun, D. S., Shivashankar, G. V. & Shenoy, V. B. Regulation of nuclear architecture, mechanics, and nucleocytoplasmic shuttling of epigenetic factors by cell geometric constraints. Proc. Natl Acad. Sci. USA 116, 13200–13209 (2019).
Discher, D. E., Janmey, P. & Wang, Y. Tissue cells feel and respond to the stiffness of their substrate. Science 310, 1139–1143 (2005).
Jain, N., Iyer, K. V., Kumar, A. & Shivashankar, G. V. Cell geometric constraints induce modular gene-expression patterns via redistribution of HDAC3 regulated by actomyosin contractility. Proc. Natl Acad. Sci. USA 110, 11349–11354 (2013).
Alisafaei, F. et al. Vimentin is a key regulator of cell mechanosensing through opposite actions on actomyosin and microtubule networks. Commun. Biol. 7, 1–13 (2024).
Versaevel, M., Grevesse, T. & Gabriele, S. Spatial coordination between cell and nuclear shape within micropatterned endothelial cells. Nat. Commun. 3, 671 (2012).
Achterberg, V. F. et al. The nano-scale mechanical properties of the extracellular matrix regulate dermal fibroblast function. J. Invest. Dermatol. 134, 1862–1872 (2014).
Pailler-Mattei, C., Bec, S. & Zahouani, H. In vivo measurements of the elastic mechanical properties of human skin by indentation tests. Med. Eng. Phys. 30, 599–606 (2008).
Reynolds, N. H., McEvoy, E., Panadero Pérez, J. A., Coleman, R. J. & McGarry, J. P. Influence of multi-axial dynamic constraint on cell alignment and contractility in engineered tissues. J. Mech. Behav. Biomed. Mater. 112, 104024 (2020).
Thavandiran, N. et al. Design and formulation of functional pluripotent stem cell-derived cardiac microtissues. Proc. Natl Acad. Sci. USA 110, E4698–E4707 (2013).
Zheng, L.-H., Cai, F.-F., Ge, I., Biskup, E. & Cheng, Z.-P. Stromal fibroblast activation and their potential association with uterine fibroids (review). Oncol. Lett. 8, 479–486 (2014).
Martin-Martin, B., Tovell, V., Dahlmann-Noor, A. H., Khaw, P. T. & Bailly, M. The effect of MMP inhibitor GM6001 on early fibroblast-mediated collagen matrix contraction is correlated to a decrease in cell protrusive activity. Eur. J. Cell Biol. 90, 26–36 (2011).
Madl, C. M. et al. Maintenance of neural progenitor cell stemness in 3D hydrogels requires matrix remodelling. Nat. Mater. 16, 1233–1242 (2017).
Khetan, S. et al. Degradation-mediated cellular traction directs stem cell fate in covalently crosslinked three-dimensional hydrogels. Nat. Mater. 12, 458–465 (2013).
Legant, W. R. et al. Microfabricated tissue gauges to measure and manipulate forces from 3D microtissues. Proc. Natl Acad. Sci. USA 106, 10097–10102 (2009).
Wade, R. J., Bassin, E. J., Gramlich, W. M. & Burdick, J. A. Nanofibrous hydrogels with spatially patterned biochemical signals to control cell behavior. Adv. Mater. 27, 1356–1362 (2015).
Gramlich, W. M., Kim, I. L. & Burdick, J. A. Synthesis and orthogonal photopatterning of hyaluronic acid hydrogels with thiol-norbornene chemistry. Biomaterials 34, 9803–9811 (2013).
Jiang, S. et al. An ex vivo culture model of kidney podocyte injury reveals mechanosensitive, synaptopodin-templating, sarcomere-like structures. Sci. Adv. 8, eabn6027 (2022).
Sander, E. A., Stylianopoulos, T., Tranquillo, R. T. & Barocas, V. H. Image-based multiscale modeling predicts tissue-level and network-level fiber reorganization in stretched cell-compacted collagen gels. Proc. Natl Acad. Sci. USA 106, 17675–17680 (2009).
Cross, V. L. et al. Dense type I collagen matrices that support cellular remodeling and microfabrication for studies of tumor angiogenesis and vasculogenesis in vitro. Biomaterials 31, 8596–8607 (2010).
Alisafaei, F. Tension anisotropy drives fibroblast phenotypic transition by self-reinforcing cell-extracellular matrix mechanical feedback. Zenodo https://doi.org/10.5281/zenodo.14556939 (2024).
Acknowledgements
D.S. acknowledges an NIH training grant (no. 5-T32-HL07081-38). F.A. acknowledges startup funding and a seed grant provided by NJIT. This work was supported by the National Science Foundation Center for Engineering Mechanobiology grant no. CMMI-154857 (G.M.G., V.B.S. and J.A.B.); National Cancer Institute awards R01CA232256 (V.B.S.) and U54CA261694 (V.B.S.); National Institute of Biomedical Imaging and Bioengineering awards R01EB017753 (V.B.S.) and R01EB030876 (V.B.S.); National Institute of Arthritis and Musculoskeletal and Skin Diseases award R01AR077793 (G.M.G.); National Heart, Lung, and Blood Institute award no. R01HL159094 (N.H.); and National Science Foundation grant nos. MRSEC/DMR-1720530 (V.B.S. and J.A.B.) and DMS-1953572 (V.B.S.). We thank A. J. Hartz, the Medical College of Wisconsin, for assistance with the statistical analysis.
Author information
Authors and Affiliations
Contributions
F.A., D.S., E.L.E. and G.M.G. designed the research. D.S., F.A., Y. Hong, G.R., Y. Huang, L.E.I., M.D.D., J.Q., C.Q., D.J., D.R.F., Y.-Y.H., P.G., S.J., A.M., S.S., K.M.P., P.-h.G.C., J.A.B., S.P.L., E.L.E., N.H. and G.M.G. performed, supervised and analysed the experiments. F.A., M.J., Y. Hong and V.B.S. developed the theoretical models and performed the numerical simulations. F.A., D.S. and G.M.G. wrote the paper. All authors discussed the results and commented on the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Materials thanks Manuel Salmeron-Sanchez and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
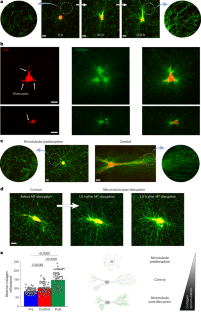
Extended Data Fig. 1 Pre-treatment of fibroblasts with inhibitors of actomyosin contractility further showed the key role of microtubules in the formation of cellular protrusions.
Pre-treatment of cells with actomyosin inhibitors (cytochalasin D, blebbistatin, and Y-27632) caused cells to develop more and longer protrusions. These protrusions exhibited accumulations of tubulin signals, indicative of a microtubule-rich core. Experiments were conducted with three independent biological replicates, all of which yielded similar results. Scale bar: 10 μm.
Extended Data Fig. 2 Collagen matrices with pre-aligned fibres exhibited an anisotropic stiffness.
Fig. 2a shows a high degree of collagen alignment in pre-aligned matrices. To test whether this alignment resulted in an anisotropic stiffness, we used a rotational shear rheometer to measure matrix stiffness along and perpendicular to the collagen alignment. We positioned the matrix sample off-center within the rheometer, as illustrated in schematics (A) and (B) showing side and top views, respectively. First, we measured the stiffness of pre-aligned matrices along the direction of alignment. This was achieved by ensuring the collagen alignment direction coincided with the rheometer movement direction, aligning the applied forces with the collagen fibres (left panel in B; green lines represent the collagen fibre orientation and black arrows indicate the direction of rheometer movement). Subsequently, we rotated the sample by 90 degrees, making the collagen alignment direction perpendicular to the rheometer movement (right panel in B). As described in Methods, torque and shear stress were continuously recorded as the sample underwent shear strain up to 5% (D). The ratio σa / σp served as an indicator of stiffness anisotropy, with σa and σp denoting the stress required for a 5% strain along and perpendicular to the collagen orientation, respectively. Pre-aligned matrices were stiffer in the direction of collagen alignment (F). To validate our approach, we conducted similar experiments with isotropic collagen matrices (C). Consistent with Fig. 2a, illustrating a uniform distribution of collagen fibres in isotropic matrices, our results here showed that isotropic matrices exhibited similar stiffness in different directions (E-F). Three matrices (n = 3) were tested for both isotropic and aligned collagen in panel (F). The height of the bars and the error bars indicate the mean and the standard error, respectively. Statistical analysis was performed using the two-sided unpaired Student’s t-test.
Extended Data Fig. 3 Effect of collagen pre-alignment on fibroblast morphology.
Representative image selected from over 30 cells imaged in a collagen ECM with an initial cell density of 2×104 cells/ml. When cultured within a pre-aligned collagen matrix, fibroblasts predominantly aligned with the collagen fibres and elongated in that direction. Green: collagen (confocal reflectance); Blue: nuclei (DAPI); Orange: tubulin. Scale bar: 50 μm.
Extended Data Fig. 4 Collagen accumulation reached a plateau over time.
To measure collagen accumulation around a cell, we performed confocal fluorescence and reflectance imaging in three-dimensional volumetric regions surrounding each cell at various time points. We then quantified the degree of collagen accumulation using a custom MATLAB script that averaged the confocal reflectance pixel intensity within volumetric shells surrounding each cell (A). The collagen density in different regions shown in (A) was quantified in (B) where the distance of each region from the cell membrane was presented next to its curve. The highest level of collagen accumulation was observed near the cell (blue line in (B) with 0.4 μm distance from the cell membrane). Also, our results showed that collagen accumulation in all surrounding regions reached a plateau over a time scale of hundreds of minutes.
Extended Data Fig. 5 Polarized cells generate anisotropic densification of the extracellular matrix.
To test whether polarized cells induced an anisotropic mechanical environment consistent with increased tension in their polarization direction, we adapted our collagen density quantification methodology (see Methods). For polarized cells, we measured collagen density in directions parallel and perpendicular to the axis of cell polarization (A). Results showed that, in regions distant from the cell (the far-field), collagen density remained the same in both orientations. However, in the proximity of the cells, significantly higher collagen accumulation was observed along the cell’s polarization direction than perpendicular to it (n = 6 cells) (B-C). In contrast, in nocodazole-treated, round cells, collagen density was the same in all directions both near the cells and far from them (far-field), indicating that round cells generate an isotropic stress field (D). The height of the bars and the error bars indicate the mean and the standard error, respectively. Statistical analysis was performed using the two-sided unpaired Student’s t-test.
Extended Data Fig. 6 The membrane area over which ECM resists cell contraction is lower for polarized contraction than for isotropic contraction.
To investigate the effect of stress field directionality on cell activation, we simulated isotropic and anisotropic contraction of a circular cell within a three-dimensional collagen matrix. (A) In isotropic contraction, cells contract with the same initial contractility \({\rho }_{0}\) in all directions, while in polarized contraction, cells contract with the initial contractility \({\rho }_{0}\) only in the direction of polarity. Therefore, the contact area at the cell-matrix interface resisting cell contraction is higher for isotropic contraction than for anisotropic contraction. (B) The model predicts that both the magnitude and the anisotropy of the tensile stress field generated in the cell cytoskeleton promote actomyosin contractility. (C) Without accounting for the effect of tension anisotropy (fa = 0), the theoretical model predicts that isotropic contraction would lead to a greater actomyosin contractility, higher tension in the cytoskeleton, and higher force transmission within the matrix, analogous to predictions from pre-stressed/pre-strained cell models and other thermoelastic-based classical cell models that do not account for tension anisotropy. (D) However, when tension anisotropy is taken into account in our model, the model predicts that anisotropic contraction, despite applying traction over a smaller fraction of the cell membrane, results in greater actomyosin contractility and tension in the cytoskeleton, and higher force transmission within the matrix.
Extended Data Fig. 7 Schematic of the stress field in the cruciform specimens.
Cells constrict against the attachment bars, leading to frustrated contraction and stress. In the middle of the arms, stress is directed along the centerline. In the center of the specimen, the stresses are added so that the stress state is equal in both directions; this means that the in-plane stress tensor is spherical and no preferred stress direction exists, whereas in the middle of the arms, vertical constriction is not resisted resulting in an anisotropic stress field in the horizontal direction. Near the attachment bars, contact with the bars causes a degree of constraint against vertical contraction. This frustrated contraction leads to stress, causing the stress field to be less anisotropic near the attachment bars.
Extended Data Fig. 8 Tension anisotropy promotes fibroblast activation level.
(a) Cell-induced contraction of a cruciform tissue, anchored at the ends of its four arms (Extended Data Figure 7), caused cells in the center of the specimen to experience equibiaxial isotropic tension, and cells in the arms to experience anisotropic (polarized) tension (Fig. 5a). (b-c) Cells subjected to anisotropic stress fields showed higher levels of F-actin and \({\rm{\alpha }}\)-smooth muscle actin (\({\rm{\alpha }}\)-SMA), indicating that anisotropic stress fields significantly increased activation of fibroblasts into myofibroblasts. Data in panels B and C represents the intensity of F-actin and \({\rm{\alpha }}\)-SMA per cell. The height of the bars and the error bars indicate the mean and the standard error, respectively (n = 9 images in (B) and (C)). Statistical analysis was performed using the two-sided unpaired Student’s t-test.
Extended Data Fig. 9 Tension anisotropy promotes fibroblast activation levels even in the presence of matrix metalloproteinase inhibitors.
To inhibit the degradation of collagen fibres by fibroblasts, we treated fibroblasts with GM6001, a well-known inhibitor of matrix metalloproteinases (MMPs). Similar to the control cells in Fig. 5, fibroblasts treated with the MMP inhibitor exhibited higher activation levels in the anisotropic region compared to the isotropic region. Data represents the intensity of F-actin per cell. The height of the bars and the error bars indicate the mean and the standard error, respectively (n = 40 images). Statistical analysis was performed using the two-sided unpaired Student’s t-test.
Extended Data Fig. 10 Tension anisotropy promotes fibroblast activation levels independent of extracellular collagen concentration.
To examine whether tension anisotropy enhances fibroblast activation within tissues of higher collagen concentrations, we increased collagen concentration from 1 mg/ml (as used in Fig. 5) to 2 mg/ml. Similar to cells in tissues with low collagen concentration (Fig. 5), fibroblasts exhibited higher activation levels in the anisotropic region compared to the isotropic region, indicating that tension anisotropy can activate fibroblasts within matrices of varying collagen concentrations. Data represents the intensity of F-actin per cell. The height of the bars and the error bars indicate the mean and the standard error, respectively (n = 44 and 40 images). Statistical analysis was performed using the two-sided unpaired Student’s t-test.
Supplementary information
Supplementary Information
Supplementary Note 1, caption for Supplementary Video 1, Figs. 1–18, Table 1 and References.
Supplementary Video 1
Two-way feedback mechanism during cycles of protrusion extension and retraction: cell protrusions align collagen fibres in their vicinity, and these aligned fibres stabilize the protrusion direction, promoting further growth in that direction.
Source data
Source Data Figs. 1–6 and Extended Data Figs. 2, 4–6 and 8–10
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Alisafaei, F., Shakiba, D., Hong, Y. et al. Tension anisotropy drives fibroblast phenotypic transition by self-reinforcing cell–extracellular matrix mechanical feedback. Nat. Mater. 24, 955–965 (2025). https://doi.org/10.1038/s41563-025-02162-5
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41563-025-02162-5
This article is cited by
-
A comprehensive framework for computational modeling of growth and remodeling in tissue-engineered soft collagenous materials
Biomechanics and Modeling in Mechanobiology (2025)