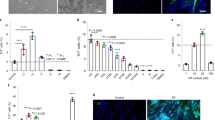
Abstract
Stem cell therapy is a promising approach for tissue regeneration after traumatic injury, yet current applications are limited by inadequate control over the fate of stem cells after transplantation. Here we introduce a bioconstruct engineered for the staged release of growth factors, tailored to direct different phases of muscle regeneration. The bioconstruct is composed of a decellularized extracellular matrix containing polymeric nanocapsules sequentially releasing basic fibroblast growth factor and insulin-like growth factor 1, which promote the proliferation and differentiation of muscle stem cells, respectively. When applied to a volumetric muscle loss defect in an animal model, the bioconstruct enhances myofibre formation, angiogenesis, innervation and functional restoration. Further, it promotes functional muscle formation with human or aged murine muscle stem cells, highlighting the translational potential of this bioconstruct. Overall, these results highlight the potential of bioconstructs with orchestrated growth factor release for stem cell therapies in traumatic injury.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The data that support the findings of this study are available within the article and its Supplementary Information and can be obtained from the corresponding author upon request. Source data are provided with this paper.
References
Discher, D. E., Mooney, D. J. & Zandstra, P. W. Growth factors, matrices, and forces combine and control stem cells. Science 324, 1673–1677 (2009).
Lepper, C., Partridge, T. A. & Fan, C.-M. An absolute requirement for Pax7-positive satellite cells in acute injury-induced skeletal muscle regeneration. Development 138, 3639–3646 (2011).
Sambasivan, R. et al. Pax7-expressing satellite cells are indispensable for adult skeletal muscle regeneration. Development 138, 3647–3656 (2011).
Wosczyna, M. N. & Rando, T. A. A muscle stem cell support group: coordinated cellular responses in muscle regeneration. Dev. Cell 46, 135–143 (2018).
Eugenis, I., Wu, D. & Rando, T. A. Cells, scaffolds, and bioactive factors: engineering strategies for improving regeneration following volumetric muscle loss. Biomaterials 278, 121173 (2021).
Mitrousis, N., Fokina, A. & Shoichet, M. S. Biomaterials for cell transplantation. Nat. Rev. Mater. 3, 174–195 (2018).
Kharbikar, B. N., Mohindra, P. & Desai, T. A. Biomaterials to enhance stem cell transplantation. Cell Stem Cell 29, 692–721 (2022).
Quarta, M. et al. Bioengineered constructs combined with exercise enhance stem cell-mediated treatment of volumetric muscle loss. Nat. Commun. 8, 15613 (2017).
Syverud, B. C., VanDusen, K. W. & Larkin, L. M. Growth factors for skeletal muscle tissue engineering. Cells Tissues Organs 202, 169–179 (2016).
Lee, K., Silva, E. A. & Mooney, D. J. Growth factor delivery-based tissue engineering: general approaches and a review of recent developments. J. R. Soc. Interface 8, 153–170 (2011).
Li, J. & Mooney, D. J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 1, 16071 (2016).
Richardson, T. P., Peters, M. C., Ennett, A. B. & Mooney, D. J. Polymeric system for dual growth factor delivery. Nat. Biotechnol. 19, 1029–1034 (2001).
Pakulska, M. M. et al. Encapsulation-free controlled release: electrostatic adsorption eliminates the need for protein encapsulation in PLGA nanoparticles. Sci. Adv. 2, e1600519 (2016).
Gaharwar, A. K., Singh, I. & Khademhosseini, A. Engineered biomaterials for in situ tissue regeneration. Nat. Rev. Mater. https://doi.org/10.1038/s41578-020-0209-x (2020).
Pawlikowski, B., Vogler, T. O., Gadek, K. & Olwin, B. B. Regulation of skeletal muscle stem cells by fibroblast growth factors. Dev. Dyn. 246, 359–367 (2017).
Yoshida, T. & Delafontaine, P. Mechanisms of IGF-1-mediated regulation of skeletal muscle hypertrophy and atrophy. Cells 9, 1970 (2020).
Greising, S. M., Corona, B. T., McGann, C., Frankum, J. K. & Warren, G. L. Therapeutic approaches for volumetric muscle loss injury: a systematic review and meta-analysis. Tissue Eng. Part B Rev. 25, 510–525 (2019).
Hu, C. et al. A mouse model of volumetric muscle loss and therapeutic scaffold implantation. Nat. Protoc. https://doi.org/10.1038/s41596-024-01059-y (2024).
Xiao, W. et al. Time-dependent gene expression analysis after mouse skeletal muscle contusion. J. Sport Health Sci. 5, 101–108 (2016).
Mehiri, S. N. et al. Time-based gene expression programme following diaphragm injury in a rat model. Eur. Respir. J. 25, 422–430 (2005).
Cezar, C. A. & Mooney, D. J. Biomaterial-based delivery for skeletal muscle repair. Adv. Drug Deliv. Rev. 84, 188–197 (2015).
Qazi, T. H., Mooney, D. J., Pumberger, M., Geissler, S. & Duda, G. N. Biomaterials based strategies for skeletal muscle tissue engineering: existing technologies and future trends. Biomaterials 53, 502–521 (2015).
Larouche, J., Greising, S. M., Corona, B. T. & Aguilar, C. A. Robust inflammatory and fibrotic signaling following volumetric muscle loss: a barrier to muscle regeneration. Cell Death Dis. 9, 409 (2018).
Hurtgen, B. J. et al. Severe muscle trauma triggers heightened and prolonged local musculoskeletal inflammation and impairs adjacent tibia fracture healing. J. Musculoskelet. Neuronal Interact. 16, 122–134 (2016).
Corona, B. T. et al. The promotion of a functional fibrosis in skeletal muscle with volumetric muscle loss injury following the transplantation of muscle-ECM. Biomaterials 34, 3324–3335 (2013).
Murakami, M. & Simons, M. Fibroblast growth factor regulation of neovascularization. Curr. Opin. Hematol. 15, 215–220 (2008).
Cross, M. J. & Claesson-Welsh, L. FGF and VEGF function in angiogenesis: signalling pathways, biological responses and therapeutic inhibition. Trends Pharmacol. Sci. 22, 201–207 (2001).
Lin, S. et al. IGF-1 promotes angiogenesis in endothelial cells/adipose-derived stem cells co-culture system with activation of PI3K/Akt signal pathway. Cell Prolif. 50, e12390 (2017).
Huang, Q., Liu, B. & Wu, W. Biomaterial-based bFGF delivery for nerve repair. Oxid. Med. Cell. Longev. 2023, 8003821 (2023).
Rabinovsky, E. D. The multifunctional role of IGF-1 in peripheral nerve regeneration. Neurol. Res. 26, 204–210 (2004).
Brunet, A., Goodell, M. A. & Rando, T. A. Ageing and rejuvenation of tissue stem cells and their niches. Nat. Rev. Mol. Cell Biol. 24, 45–62 (2023).
Conboy, I. M., Conboy, M. J., Smythe, G. M. & Rando, T. A. Notch-mediated restoration of regenerative potential to aged muscle. Science 302, 1575–1577 (2003).
Reed, M. J. & Edelberg, J. M. Impaired angiogenesis in the aged. Sci. Aging Knowledge Environ. 2004, pe7 (2004).
Hepple, R. T. & Rice, C. L. Innervation and neuromuscular control in ageing skeletal muscle. J. Physiol. 594, 1965–1978 (2016).
Verdú, E., Ceballos, D., Vilches, J. J. & Navarro, X. Influence of aging on peripheral nerve function and regeneration. J. Peripher. Nerv. Syst. 5, 191–208 (2000).
Apel, P. J. et al. Effect of locally delivered IGF-1 on nerve regeneration during aging: an experimental study in rats. Muscle Nerve 41, 335–341 (2010).
Alcazar, C. A., Hu, C., Rando, T. A., Huang, N. F. & Nakayama, K. H. Transplantation of insulin-like growth factor-1 laden scaffolds combined with exercise promotes neuroregeneration and angiogenesis in a preclinical muscle injury model. Biomater. Sci. 8, 5376–5389 (2020).
Hu, C. et al. Comparative effects of basic fibroblast growth factor delivery or voluntary exercise on muscle regeneration after volumetric muscle loss. Bioengineering 9, 37 (2022).
Baker, H. B. et al. Cell and growth factor-loaded keratin hydrogels for treatment of volumetric muscle loss in a mouse model. Tissue Eng. Part A 23, 572–584 (2017).
Passipieri, J. A. et al. Keratin hydrogel enhances in vivo skeletal muscle function in a rat model of volumetric muscle loss. Tissue Eng. Part A 23, 556–571 (2017).
Zhang, F. & King, M. W. Biodegradable polymers as the pivotal player in the design of tissue engineering scaffolds. Adv. Healthc. Mater. 9, e1901358 (2020).
Okada-Ban, M., Thiery, J. P. & Jouanneau, J. Fibroblast growth factor-2. Int. J. Biochem. Cell Biol. 32, 263–267 (2000).
Ahmad, S. S., Ahmad, K., Lee, E. J., Lee, Y.-H. & Choi, I. Implications of insulin-like growth factor-1 in skeletal muscle and various diseases. Cells 9, 1773 (2020).
Konttinen, Y. T. et al. Fibroblast biology. Signals targeting the synovial fibroblast in arthritis. Arthritis Res. 2, 348–355 (2000).
Wabitsch, M., Hauner, H., Heinze, E. & Teller, W. M. The role of growth hormone/insulin-like growth factors in adipocyte differentiation. Metab. Clin. Exp. 44, 45–49 (1995).
Zittermann, S. I. & Issekutz, A. C. Basic fibroblast growth factor (bFGF, FGF-2) potentiates leukocyte recruitment to inflammation by enhancing endothelial adhesion molecule expression. Am. J. Pathol. 168, 835–846 (2006).
Smith, T. J. Insulin-like growth factor-I regulation of immune function: a potential therapeutic target in autoimmune diseases? Pharmacol. Rev. 62, 199–236 (2010).
Charville, G. W. et al. Ex vivo expansion and in vivo self-renewal of human muscle stem cells. Stem Cell Rep. 5, 621–632 (2015).
Zhao, S. et al. Induced pluripotent stem cells for tissue-engineered skeletal muscles. Int. J. Mol. Sci. 24, 11520 (2023).
Eugenis, I. et al. Scalable macroporous hydrogels enhance stem cell treatment of volumetric muscle loss. Biomaterials 290, 121818 (2022).
Liu, L., Cheung, T. H., Charville, G. W. & Rando, T. A. Isolation of skeletal muscle stem cells by fluorescence-activated cell sorting. Nat. Protoc. 10, 1612–1624 (2015).
Kang, J. et al. Depletion of SAM leading to loss of heterochromatin drives muscle stem cell ageing. Nat. Metab. 6, 153–168 (2024).
Conchinha, N. V. et al. Protocols for endothelial cell isolation from mouse tissues: brain, choroid, lung, and muscle. STAR Protoc. 2, 100508 (2021).
Tabula Sapiens Consortium et al. The Tabula Sapiens: a multiple-organ, single-cell transcriptomic atlas of humans. Science 376, eabl4896 (2022).
Acknowledgements
We thank the members of the Rando laboratory for comments and discussions. This work was supported by grants from the US Department of Veterans Affairs (DVA) (1I01BX004259) to N.F.H. and from the US National Institutes of Health (P01 AG036695 and R01 AG068667) and the DVA (I01 RX001222) to T.A.R. N.F.H. is a recipient of a DVA Research Career Scientist award (IK6 BX006309).
Author information
Authors and Affiliations
Contributions
D.W., I.E. and T.A.R. conceived and designed the experiments. D.W., I.E., C.H., S.K., A.K. and S.Y. performed the experiments. D.W., S.K., S.Y. and S.F. analysed the data. J.R.W., I.F. and J.B.S. provided the human skeletal muscle biopsy. N.F.H. and T.A.R. supervised the project. D.W. and T.A.R. wrote the paper. D.W., S.Y. and T.A.R. edited the paper. All authors discussed the results and commented on the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Materials thanks Pedro Baptista, Francesco Tedesco and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Fabrication of bioconstructs for VML therapy.
a, Surgical procedure of the VML model. (i) TA muscles were exposed, (ii) 7 × 2 × 2 mm (length × width × depth) defects were created, (iii) scaffolds were implanted, and (iv) muscles were closed. b, Quantification of the weight of TA muscles with and without VML surgery (n = 6 biologically independent experiments). c, d, Quantification of TA muscle weight (c) and maximum tetanic force (d) 6 weeks following VML surgery (n = 6 and 4 biologically independent experiments for c and d, respectively). e, H&E and immunofluorescence staining of muscles with and without decellularization. Scale bar, 50 μm. f, Porosity of dECM scaffolds. Representative images of H&E staining (i), quantification of porosity (ii), and size distribution of the pores (iii) of dECM scaffold before and after lyophilization. (iv) A representative SEM image of lyophilized dECM scaffold. Scale bar, 25 μm. g, Quantification the average size (i) and size distribution of NCLy(Low) (ii), NCLy(Medium) (iii), and NCLy(High) (iv) constructs with and without PEGylation compared with Lysozyme (n = 6 independent synthesis experiments). h, A representative TEM image of Lysozyme, labeled with one 5-nm gold nanoparticle per protein. Scale bar, 25 nm. i, Relative cell viability of C2C12 cells treated with different NCs (n = 5 independent synthesis experiments). j, Loading efficiency of NCs on dECM scaffold. (i) The concentration of DBCO moieties per scaffold with varying DBCO-PEG4-NHS ester amounts (left) and the conjugation efficiency (right) (n = 3 independent synthesis experiments). (ii) The concentration of NCLy(low) constructs per scaffold with the maximum DBCO number (left) and the conjugation efficiency (right) (n = 6 independent synthesis experiments). Data in b-d, f, g, i, j are presented as the mean ± SEM. P values in c, d, f, g were determined by two-sided t test. **P ≤ 0.01, ***P ≤ 0.001, ****P ≤ 0.0001; ns, not significant.
Extended Data Fig. 2 NCs control the release kinetics and improve the stability of GFs.
a, The remaining amount of protein in the supernatant after incubation of NCs (NCFGF (left); NCIGF (right)) with mouse serum, compared with their native counterparts (n = 6 independent synthesis experiments). b, Representative images (left) and quantification (right) of cell uptake of fluorophore-labeled bFGF, without and with NC encapsulation, by the J774A.1 macrophages (n = 3 independent synthesis experiments). Scale bar, 50 μm. c, Representative FACS plots of MuSC isolation. d, Representative images of Pax7 staining of isolated MuSCs. Scale bar, 100 μm. e, Effect of macrophage incubation of bFGF activity with and without encapsulation (n = 6 independent synthesis experiments). f, Effect of trypsin degradation on bFGF activity with and without encapsulation (n = 6 independent synthesis experiments). g, h, Release kinetics of bFGF (g) and IGF-1 (h) that had been physically adsorbed to dECM scaffolds (n = 6 technical replicates). i, j, Dose-response curves of bFGF or IGF with and without encapsulation. Cell proliferation (i) and differentiation (j) of MuSCs treated with a series of concentrations of bFGF and IGF-1 with and without encapsulation (n = 3 independent experiments with MuSCs pooled from four mice). Data in a, b, e-j are presented as the mean ± SEM. P values in a, e, f were determined by two-sided t test. **P ≤ 0.01, ****P ≤ 0.0001; ns, not significant.
Extended Data Fig. 3 Characterization of bioconstructs in vitro and in vivo.
a, A representative image (left) and quantification (right) of the distribution of MuSCs within dECM scaffolds and cultured 24 h in vitro after cell seeding. Scale bar, 100 μm. b–e, Characterization of muscle formation, inflammation, and cytotoxicity of muscles subjected to VML lesions two weeks after treatment with Ctrl (dECM only), NCFGF(E), or NCFGF(E)/IGF(L) scaffolds. b, (left) Representative images of Gomori trichrome staining of muscles subjected to VML lesions. The dashed yellow line indicates the edge between the scaffold (above the line) and host tissue (below the line). Scale bar, 50 μm. (right) Quantification of centrally nucleated fibers (CNFs) based on trichrome stained images (n = 4 biologically independent experiments). c, Representative H&E stained images of muscles subjected to VML lesions. The dashed yellow line indicates the edge between the scaffold (above the line) and host tissue (below the line). Blood vessels are highlighted by the arrows. Scale bar, 100 μm. d, Representative images (left) and quantifications (right) of CD45+ cells in muscles subjected to VML lesions (n = 6 biologically independent experiments). Scale bar, 25 μm. e, Representative images (left) and quantifications (right) of TUNEL staining of muscles subjected to VML lesions (n = 6 biologically independent experiments). Scale bar, 50 μm. f, Representative images (left) and quantifications (right) of Raw264.7 macrophage polarization into M1 (iNOS+) and M2 (CD206+) macrophages following treatment with PBS (Ctrl), NCFGF(E), NCIGF(L), or NCFGF(E)/NCIGF(L) constructs (n = 5 independent synthesis experiments). Scale bar, 50 μm. Data in b, d-f are presented as the mean ± SEM. P values were determined by one-way ANOVA. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, ****P ≤ 0.0001; ns, not significant.
Extended Data Fig. 4 Muscle regeneration with angiogenesis and innervation.
a, Representative images (left) and quantification (right) of self-renewed transplanted cells (RFP+Pax7+) within the region of regenerated myofibers (n = 6 biologically independent experiments). Scale bar, 10 μm. b, Representative FACS plots of endothelial cell isolation. c, A representative image demonstrating the purity of isolated endothelial cells. Scale bar, 20 μm. d–f, Representative images (d) and quantification of the number (e) and length (f) of tube formation by endothelial cells treated with PBS, NCFGF(E), or NCFGF(E)/NCIGF(L) constructs (n = 4 independent synthesis experiments with endothelial cells pooled from three mice). Scale bar, 100 μm. g, h, Representative images (g) and quantification (h) of endothelial cell infiltration into different scaffolds transplanted into VML defects without MuSCs 2-week post-transplantation (n = 4 biologically independent experiments). Dashed yellow line indicates the edge between host tissue (left of the line) and scaffold (right of the line). Scale bar, 100 μm. i–k, Quantification of the density of endothelial cells (i), functional capillaries (j), and the number of capillaries per myofiber within the region of donor-derived fibers 6-week post-transplantation of RFP+ MuSCs seeded onto different bioconstructs (n = 6 biologically independent experiments). l, Representative images (left) and quantifications (right) of neovessels surrounded by pericytes (PDGFRβ+) and vascular smooth muscle cells (α-SMA+) within the region of RFP+ regenerated myofibers (n = 3 biologically independent experiments). Scale bar, 10 μm. m, Representative images (left) and quantification (right) of Neuro2a neuroblast differentiation after treatment with PBS, NCFGF(E), or NCFGF(E)/NCIGF(L) constructs. The arrows indicate neurites of differentiated Neuro2a cells (n = 3 independent synthesis experiments). Scale bar, 25 μm. Data in a, e, f, h-m are presented as the mean ± SEM. P values in a, e, f, h-k, m were determined by one-way ANOVA. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, ****P ≤ 0.0001; ns, not significant.
Extended Data Fig. 5 Gait analysis of mice treated with different bioconstructs.
a, Schematic of the experimental setup during Digigait data collection. The gait of mice was continuously imaged while they walked on a transparent treadmill. b, (top) Representative images of a walking mouse captured in (a), and (bottom) the digital paw prints generated by the Digigait analysis software. Each paw of the mice was recognized and labelled in a different color. c, (left) Schematic of digital paw print representing the indices measured in gait analysis. (right) A complete stride consisted of two phases: a stance phase and a swing phase. The stance phase could be further divided into a brake phase and a propel phase. d–n, Gait indices notably changed 6 weeks following VML injury. Compared with uninjured muscles, muscles with VML defects demonstrated gait abnormalities in brake time (d), max dA/dt (e), propel time (f), min dA/dt (g), stance time (h), swing time (i), stride time (j), stride frequency (k), stride length (l), paw area (m), and ataxia coefficient (n), which describes the step-to-step gait variability (n = 12 biologically independent experiments). o-r, Quantification of gait indices, including the stride time (o), the stride frequency (p), the stride length (q), and the paw area (r) after transplantation of MuSCs using Ctrl (dECM only), NCFGF(E), or NCFGF(E)/NCIGF(L) scaffolds (n = 12 biologically independent experiments). Data in d-r are presented as the mean ± SEM. P values were determined by two-sided t test (d-n) or one-way ANOVA (o-r). *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, ****P ≤ 0.0001; ns, not significant.
Extended Data Fig. 6 Engraftment of aged murine MuSCs or human MuSCs.
a–c, Quantification of muscle area (a), the average CSA (b), and the RFP+ fiber number (c) of aged TA muscles transplanted with Ctrl (dECM only) or NCFGF(E)/NCIGF(L) scaffolds seeded with young or aged RFP+ MuSCs 6 weeks following transplantation (n = 5 biologically independent experiments). d,e, Representative images (d) and quantification (e) of the density of the endothelial cells within the region of donor-derived RFP+ fibers in (a) (n = 5 biologically independent experiments). Scale bar, 50 μm. f, g, Representative images (f) and quantification (g) of NMJs within the regions of donor-derived RFP+ fibers in (a) (n = 5 biologically independent experiments). Scale bar, 50 μm. h, Representative FACS plots of hMuSC isolation. i, (left) Representative images of Pax7 staining of isolated hMuSCs and (right) quantification of the purity of the sorted populations (n = 9 technical replicates from single donor). Scale bar, 50 μm. j, k, Quantification of the CSA of hMuSC-derived myofibers (j) and the muscle area and unrepaired area (k) 6-week post-transplantation of Ctrl (dECM only) or NCFGF(E)/NCIGF(L) scaffolds (n = 4 replicates across three donors). l, Representative images (left) and quantification (right) of the area of fibrotic tissue in muscles treated with Ctrl (dECM only) or NCFGF(E)/NCIGF(L) scaffolds (n = 4 replicates across three donors). Scale bar, 50 μm. m, Representative images of self-renewed hMuSCs 6 weeks following the transplantation of dECM (Ctrl) or NCFGF(E)/ NCIGF(L) scaffolds. Scale bar, 25 μm. n, o, Representative images (left) and quantifications (right) of the density of the endothelial cells (n) and NMJs (o) within the region of hMuSC-derived fibers (n = 4 replicates across three donors). Scale bar, 50 μm. Data in a-c, e, g, i-l, n, and o are presented as the mean ± SEM. P values were determined by multiple unpaired two-sided t test. *P ≤ 0.05 **P ≤ 0.01; ns, not significant.
Supplementary information
Supplementary Information
Supplementary Notes 1–7, Discussions 1 and 2, Tables 1 and 2, Methods, and References.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 6
Statistical source data.
Source Data Extended Data Figs. 1–6
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wu, D., Eugenis, I., Hu, C. et al. Bioinstructive scaffolds enhance stem cell engraftment for functional tissue regeneration. Nat. Mater. 24, 1364–1374 (2025). https://doi.org/10.1038/s41563-025-02212-y
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41563-025-02212-y
This article is cited by
-
Orchestrated growth factor release for muscle restoration
Nature Materials (2025)
-
Hyaluronic Acid-Coated Melt Electrowritten Scaffolds Promote Myoblast Attachment, Alignment, and Differentiation
Cellular and Molecular Bioengineering (2025)