Abstract
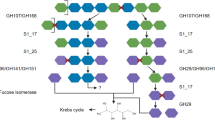
Most of Earth’s prokaryotes live under energy limitation, yet the full breadth of strategies that enable survival under such conditions remain poorly understood. Here we report the isolation of a bacterial strain, IA91, belonging to the candidate phylum Marine Group A (SAR406 or ‘Candidatus Marinimicrobia’) that is unable to synthesize the central cell wall compound peptidoglycan itself. Using cultivation experiments and microscopy, we show that IA91 growth and cell shape depend on other bacteria, deriving peptidoglycan, energy and carbon from exogenous muropeptide cell wall fragments released from growing bacteria. Reliance on exogenous muropeptides is traceable to the phylum’s ancestor, with evidence of vertical inheritance across several classes. This dependency may be widespread across bacteria (16 phyla) based on the absence of key peptidoglycan synthesis genes. These results suggest that uptake of exogenous cell wall components could be a relevant and potentially common survival strategy in energy-limited habitats like the deep biosphere.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
Sequence data that support the findings of this study have been deposited in NCBI Sequence Read Archive under Bioproject accession numbers PRJDB13945 (IA91 genome) and PRJDB17482 (RNA-seq). Other genome sequences used in this study are available in GTDB. All unique biological materials (that is, strains Acc8 and IA91) are available at Japan Collection of Microorganisms (JCM) in RIKEN-BRC under accession numbers JCM 39386 (Acc8) and JCM 39387 (IA91). Raw microscopic images are available via Zenodo at https://doi.org/10.5281/zenodo.10617219 (ref. 62). Source data are provided with this paper.
References
Bar-On, Y. M., Phillips, R. & Milo, R. The biomass distribution on Earth. Proc. Natl Acad. Sci. USA 115, 6506–6511 (2018).
Hoehler, T. M. & Jørgensen, B. B. Microbial life under extreme energy limitation. Nat. Rev. Microbiol. 11, 83–94 (2013).
Emilio, M. N., Michael, J. B., Natalia, G. Âl, Beatriz, M. Â. O. & Mikhail, V. Z. High variability of primary production in oligotrophic waters of the Atlantic Ocean: uncoupling from phytoplankton biomass and size structure. Mar. Ecol. Prog. Ser. 257, 1–11 (2003).
Lever, M. A. et al. Life under extreme energy limitation: a synthesis of laboratory- and field-based investigations. FEMS Microbiol. Rev. 39, 688–728 (2015).
Morris, J. J., Lenski, R. E. & Zinser, E. R. The Black Queen Hypothesis: evolution of dependencies through adaptive geneloss. mBio 3, e00036–12 (2012).
Swan, B. K. et al. Prevalent genome streamlining and latitudinal divergence of planktonic bacteria in the surface ocean. Proc. Natl Acad. Sci. USA 110, 11463–11468 (2013).
Giovannoni, S. J., Cameron Thrash, J. & Temperton, B. Implications of streamlining theory for microbial ecology. ISME J. 8, 1553–1565 (2014).
Parks, D. H. et al. A standardized bacterial taxonomy based on genome phylogeny substantially revises the tree of life. Nat. Biotechnol. 36, 996–1004 (2018).
Fry, J. C., Parkes, R. J., Cragg, B. A., Weightman, A. J. & Webster, G. Prokaryotic biodiversity and activity in the deep subseafloor biosphere. FEMS Microbiol. Ecol. 66, 181–196 (2008).
Lloyd, K. G., Steen, A. D., Ladau, J., Yin, J. & Crosby, L. Phylogenetically novel uncultured microbial cells dominate Earth microbiomes. mSystems 3, e00055–00018 (2018).
Hawley, A. K. et al. Diverse Marinimicrobia bacteria may mediate coupled biogeochemical cycles along eco-thermodynamic gradients. Nat. Commun. 8, 1507 (2017).
Lewis, W. H., Tahon, G., Geesink, P., Sousa, D. Z. & Ettema, T. J. G. Innovations to culturing the uncultured microbial majority. Nat. Rev. Microbiol. 19, 225–240 (2021).
Fuhrman, J. A., McCallum, K. & Davis, A. A. Phylogenetic diversity of subsurface marine microbial communities from the Atlantic and Pacific Oceans. Appl. Environ. Microbiol. 59, 1294–1302 (1993).
Gordon, D. A. & Giovannoni, S. J. Detection of stratified microbial populations related to Chlorobium and Fibrobacter species in the Atlantic and Pacific oceans. Appl. Environ. Microbiol. 62, 1171–1177 (1996).
Rinke, C. et al. Insights into the phylogeny and coding potential of microbial dark matter. Nature 499, 431–437 (2013).
Hirakata, Y. et al. Identification and cultivation of anaerobic bacterial scavengers of dead cells. ISME J. 17, 2279–2289 (2023).
Mayer, C. et al. Bacteria’s different ways to recycle their own cell wall. Int. J. Med. Microbiol. 309, 151326 (2019).
Borisova, M. et al. Peptidoglycan recycling in Gram-positive bacteria is crucial for survival in stationary phase. mBio 7, e00923–00916 (2016).
Schink, B. Energetics of syntrophic cooperation in methanogenic degradation. Microbiol. Mol. Biol. Rev. 61, 262–280 (1997).
Katayama, T. et al. Isolation of a member of the candidate phylum ‘Atribacteria’ reveals a unique cell membrane structure. Nat. Commun. 11, 6381 (2020).
Katayama, T. & Kamagata, Y. in Hydrocarbon and Lipid Microbiology Protocols (eds McGenity, T. J. et al.) 177–195 (Springer, 2015).
Imachi, H. et al. Isolation of an archaeon at the prokaryote–eukaryote interface. Nature 577, 519–525 (2020).
Brumm, P. J. et al. Complete genome sequence of Thermus aquaticus Y51MC23. PLoS ONE 10, e0138674 (2015).
Johnson, J. W., Fisher, J. F. & Mobashery, S. Bacterial cell-wall recycling. Ann. N. Y. Acad. Sci. 1277, 54–75 (2013).
Jacobs, C., Huang, L. J., Bartowsky, E., Normark, S. & Park, J. T. Bacterial cell wall recycling provides cytosolic muropeptides as effectors for beta-lactamase induction. EMBO J. 13, 4684–4694 (1994).
Dworkin, J. The medium is the message: interspecies and interkingdom signaling by peptidoglycan and related bacterial glycans. Annu. Rev. Microbiol. 68, 137–154 (2014).
Yoshimura, T. & Goto, M. D-amino acids in the brain: structure and function of pyridoxal phosphate-dependent amino acid racemases. FEBS J. 275, 3527–3537 (2008).
Löffler, F. E. et al. Dehalococcoides mccartyi gen. nov., sp. nov., obligately organohalide-respiring anaerobic bacteria relevant to halogen cycling and bioremediation, belong to a novel bacterial class, Dehalococcoidia classis nov., order Dehalococcoidales ord. nov. and family Dehalococcoidaceae fam. nov., within the phylum Chloroflexi. Int. J. Syst. Evol. Microbiol. 63, 625–635 (2013).
Kube, M. et al. Genome sequence of the chlorinated compound-respiring bacterium Dehalococcoides species strain CBDB1. Nat. Biotechnol. 23, 1269–1273 (2005).
Graf, J. & Ruby, E. G. Host-derived amino acids support the proliferation of symbiotic bacteria. Proc. Natl Acad. Sci. USA 95, 1818–1822 (1998).
Embree, M., Liu, J. K., Al-Bassam, M. M. & Zengler, K. Networks of energetic and metabolic interactions define dynamics in microbial communities. Proc. Natl Acad. Sci. USA 112, 15450–15455 (2015).
Croft, M. T., Lawrence, A. D., Raux-Deery, E., Warren, M. J. & Smith, A. G. Algae acquire vitamin B12 through a symbiotic relationship with bacteria. Nature 438, 90–93 (2005).
Hosokawa, T., Koga, R., Kikuchi, Y., Meng, X.-Y. & Fukatsu, T. Wolbachia as a bacteriocyte-associated nutritional mutualist. Proc. Natl Acad. Sci. USA 107, 769–774 (2010).
Stams, A. J. M. & Plugge, C. M. Electron transfer in syntrophic communities of anaerobic bacteria and archaea. Nat. Rev. Microbiol. 7, 568–577 (2009).
Renzi, F. et al. Glycan-foraging systems reveal the adaptation of Capnocytophaga canimorsus to the dog mouth. mBio 6, e02507 (2015).
Mayer, V. M. T. et al. Utilization of different MurNAc sources by the oral pathogen Tannerella forsythia and role of the inner membrane transporter AmpG. BMC Microbiol. 20, 352 (2020).
Hottmann, I., Borisova, M., Schäffer, C. & Mayer, C. Peptidoglycan salvage enables the periodontal pathogen Tannerella forsythia to survive within the oral microbial community. Microb. Physiol. 31, 123–134 (2021).
Sharma, A. Persistence of Tannerella forsythia and Fusobacterium nucleatum in dental plaque: a strategic alliance. Curr. Oral Health Rep. 7, 22–28 (2020).
Jørgensen, N. O., Stepanaukas, R., Pedersen, A. G., Hansen, M. & Nybroe, O. Occurrence and degradation of peptidoglycan in aquatic environments. FEMS Microbiol. Ecol. 46, 269–280 (2003).
Ramin, K. I. & Allison, S. D. Bacterial tradeoffs in growth rate and extracellular enzymes. Front. Microbiol. 10, 2956 (2019).
Lynch, M. & Marinov, G. K. The bioenergetic costs of a gene. Proc. Natl Acad. Sci. USA 112, 15690–15695 (2015).
Schönheit, P., Buckel, W. & Martin, W. F. On the origin of heterotrophy. Trends Microbiol. 24, 12–25 (2016).
Sajed, T. et al. ECMDB 2.0: a richer resource for understanding the biochemistry of E. coli. Nucleic Acids Res. 44, D495–D501 (2016).
Bradbeer, C., Woodrow, M. L. & Khalifah, L. I. Transport of vitamin B12 in Escherichia coli: common receptor system for vitamin B12 and bacteriophage BF23 on the outer membrane of the cell envelope. J. Bacteriol. 125, 1032–1039 (1976).
Katayama, T. et al. Physicochemical impacts associated with natural gas development on methanogenesis in deep sand aquifers. ISME J. 9, 436–446 (2015).
Komagata, K. & Suzuki, K.-I. in Methods in Microbiology Vol. 19 (eds Colwell, R. R. & Grigorova, R.) 161–207 (Academic Press, 1988).
Malac, M., Beleggia, M., Kawasaki, M., Li, P. & Egerton, R. F. Convenient contrast enhancement by a hole-free phase plate. Ultramicroscopy 118, 77–89 (2012).
Wick, R. R., Judd, L. M., Gorrie, C. L. & Holt, K. E. Unicycler: resolving bacterial genome assemblies from short and long sequencing reads. PLoS Comput. Biol. 13, e1005595 (2017).
Wick, R. R., Judd, L. M., Gorrie, C. L. & Holt, K. E. Completing bacterial genome assemblies with multiplex MinION sequencing. Microb. Genom. 3, e000132 (2017).
Seemann, T. Prokka: rapid prokaryotic genome annotation. Bioinformatics 30, 2068–2069 (2014).
Cantalapiedra, C. P., Hernández-Plaza, A., Letunic, I., Bork, P. & Huerta-Cepas, J. eggNOG-mapper v2: functional annotation, orthology assignments, and domain prediction at the metagenomic scale. Mol. Biol. Evol. 38, 5825–5829 (2021).
Lu, S. et al. CDD/SPARCLE: the conserved domain database in 2020. Nucleic Acids Res. 48, D265–d268 (2020).
Teufel, F. et al. SignalP 6.0 predicts all five types of signal peptides using protein language models. Nat. Biotechnol. 40, 1023–1025 (2022).
Pruesse, E. et al. SILVA: a comprehensive online resource for quality checked and aligned ribosomal RNA sequence data compatible with ARB. Nucleic Acids Res. 35, 7188–7196 (2007).
Schloss, P. D. et al. Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 75, 7537–7541 (2009).
Minh, B. Q. et al. IQ-TREE 2: new models and efficient methods for phylogenetic inference in the genomic era. Mol. Biol. Evol. 37, 1530–1534 (2020).
Katoh, K. & Standley, D. M. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol. Biol. Evol. 30, 772–780 (2013).
Capella-Gutiérrez, S., Sillaz-Martínez, J. M. & Gabaldón, T. trimAl: a tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinformatics 25, 1972–1973 (2009).
Lemoine, F. et al. Renewing Felsenstein’s phylogenetic bootstrap in the era of big data. Nature 556, 452–456 (2018).
Chen, S., Zhou, Y., Chen, Y. & Gu, J. fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 34, i884–i890 (2018).
Shah, I. M., Laaberki, M. H., Popham, D. L. & Dworkin, J. A eukaryotic-like Ser/Thr kinase signals bacteria to exit dormancy in response to peptidoglycan fragments. Cell 135, 486–496 (2008).
Katayama, T. Data: A Marine Group A isolate relies on other growing bacteria for cell wall formation Zenodo https://doi.org/10.5281/zenodo.10617219 (2024).
Acknowledgements
We acknowledge the Kanto Natural Gas Development Co., Ltd. for collecting samples at their facilities. We also thank C. Miyako, R. Iwanami and M. Ogawara for assistance in molecular analyses and F. Nozawa and S. Yamaoka for assistance in cultivation experiments. The ampG-deficient cells of E. coli K-12 BW25113 were provided by the National Bio-Resource Project (NIG, Japan): E. coli. This work was supported by JSPS KAKENHI grant numbers 17K15183 (T.K.), 18H03367 (M.K.N.), 18H02426 (H.T.), 18H05295 (Y.K.), 22H04985 (H.I.) and 23K18158 (T.K.).
Author information
Authors and Affiliations
Contributions
T.K., M.K.N. and H.T. designed the study. T.K., M.K.N., Y.K. and H.T. wrote the paper. T.K., H.Y. and H.I. performed the cultures, and T.K. isolated strains Acc8 and IA91. T.K. and M.K.N. performed bioinformatic analyses. T.K. and K.M. performed phase-contrast and fluorescence microscopy. X.-Y.M. performed scanning and transmission electron microscopy. N.H. performed cryo-electron microscopy. H.A.T. and H.Y. performed stable carbon isotopic analysis. All authors reviewed the results and approved the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Microbiology thanks the anonymous reviewers for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Phylogenetic tree of IA91 and MG-A members.
Phylogenomic tree (maximum-likelihood tree) of strain IA91 (bold red), clone MK-334 (bold) and relatives in Marine Group A based on a concatenated alignment of ribosomal proteins. Metagenome-assembled genomes of representatives in Genome Taxonomy Database with CheckM completeness ≥ 85% and contamination ≤ 5% were selected for the analysis. The previously-used clade names are indicated in parentheses. Asterisks in nodes denote low bootstrap values, that is, ultrafast bootstrap approximation < 95% or SH-like approximate likelihood ratio test (SH-aLRT) support < 80%.
Extended Data Fig. 2 Effect of ampicillin treatment on IA91 cell morphology.
Representative phase-contrast micrographs before treatment (after one week of cultivation) (a), after 24h treatment (b) and without treatment (c). SYBR Green I was used to stain DNA (right panels). n=3 independent experiments. (Scale bars: 5 µm).
Extended Data Fig. 3 Peptidoglycan staining of ampicillin-treated IA91 cells.
Peptidoglycan (PG) staining of IA91 cells showing the presence of PG layer in rod-shaped cells but not in coccoids. Ampicillin-treated cells from pure culture were stained with Alexa Flour 488 dye-labeled wheat germ agglutinin (WGA) and visualized using phase-contrast (left), fluorescent microscopy (middle) and overlay images (right). Cells were treated with ampicillin for shorter time frame (12 h) compared with those in Extended Data Fig. 2 so as to observe both rod- and sphere-shaped cells in the same condition. Coccoids with local staining are indicated by blue arrows, whereas non-stained coccoids are indicated by black arrows. n=3 independent experiments. (Scale bars: 5 µm).
Extended Data Fig. 4 The occurrence of IA91 growth in cultures supplemented with peptidoglycan-derived compounds.4.
The final concentration of each compound in the culture medium was 10 µg ml−1. IA91 growth was determined by CH4 production after one month of cultivation and microscopy after 10 days of cultivation. IA91 was cultured with H2-utilizing Methanothermobacter thermautotrophicus strain ΔH. For CH4 production, means ± standard deviation of triplicate cultures are shown (a). CH4 detection limit was 0.5 mM. Representative phase-contrast micrographs are shown (b-i). SYBR Green I was used to stain DNA (b-i). Cells of co-cultured M. thermautotrophicus strain ∆H are visible in cyan due to their F420 autofluorescence. n=3 independent experiments. Abbreviation; PG, peptidoglycan; MP, muropeptide; MurNAc, N-acetylmuramic acid; GlcNAc, N-acetylglucosamine; AA, PG amino acids (that is, D-Ala, D-Glu and L-Lys). (Scale bars: 5 µm, b-i).
Extended Data Fig. 5 The presence of PG synthesis and recycle proteins within the members of the classes UBA2242, AB16 and UBA8477.
Corresponding to each lineage, the presence/absence of specific proteins are indicated by the heatmap. Asterisk indicates the clade to which strain IA91 and clone MK-334 belongs.
Extended Data Fig. 6 Phylogenetic tree of MurB within MG-A.
Comparison of phylogenetic tree of MurB (left) and concatenated ribosomal proteins (right) within Marine Group A showing horizontal transfer of MurB gene to the classes SORT01, JAANXI01, UBA2242 and Ca. Marinisomatia. Nodes having low statistical support values (ultrabootstrap approximation < 95%) were removed from the tree.
Extended Data Fig. 7 Phylogenetic tree of MurA within MG-A.
Comparison of phylogenetic tree of MurA (left) and concatenated ribosomal proteins (right) within Marine Group A. Nodes having low statistical support values (ultrabootstrap approximation < 95%) were removed from the tree.
Extended Data Fig. 8 Phylogenetic tree of cytochrome c oxidase subunit 1 within MG-A.
Comparison of phylogenetic tree of cytochrome c oxidase subunit 1 (left) and concatenated ribosomal proteins (right) within Marine Group A showing horizontal transfer of cytochrome c oxidase subunit 1 gene to the classes SORT01, JAANXI01, UBA8477 and Ca. Marinisomatia. Nodes having low statistical support values (ultrabootstrap approximation < 95%) were removed from the tree.
Supplementary information
Supplementary Information
Supplementary Figs. 1–13 and Tables 1–4.
Supplementary Table 5
Phylogenetic distribution of genomes that lack MurA and MurB genes.
Supplementary Data 2
Statistical source data for Supplementary Figs. 5, 7 and 11.
Source data
Source Data Fig. 1
Statistical source data for Fig. 1.
Source Data Fig. 3
Statistical source data for Fig. 3.
Source Data Extended Data Fig. 5
Statistical source data for Extended Data Fig. 5.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Katayama, T., Nobu, M.K., Imachi, H. et al. A Marine Group A isolate relies on other growing bacteria for cell wall formation. Nat Microbiol 9, 1954–1963 (2024). https://doi.org/10.1038/s41564-024-01717-7
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41564-024-01717-7
This article is cited by
-
Ecological diversity and metabolic strategies of widespread Marinisomatota in global oceans
Marine Life Science & Technology (2025)
-
A year of microbiology
Nature Microbiology (2024)