Abstract
Joint pain and osteoarthritis can occur as coronavirus disease 2019 (COVID-19) sequelae after infection. However, little is known about the damage to articular cartilage. Here we illustrate knee joint damage after wild-type, Delta and Omicron variants of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in vivo. Rapid joint injury with cystic lesions at the osteochondral junction was observed in two patients with post-COVID osteoarthritis and recapitulated in a golden Syrian hamster model. SARS-CoV-2-activated endothelin-1 signalling increased vascular permeability and caused viral spike proteins leakage into the subchondral bone. Osteoclast activation, chondrocyte dropout and cyst formation were confirmed histologically. The US Food and Drug Administration-approved endothelin receptor antagonist, macitentan, mitigated cystic lesions and preserved chondrocyte number in the acute phase of viral infection in hamsters. Delayed macitentan treatment at post-acute infection phase alleviated chondrocyte senescence and restored subchondral bone loss. It is worth noting that it could also attenuate viral spike-induced joint pain. Our work suggests endothelin receptor blockade as a novel therapeutic strategy for post-COVID arthritis.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The authors declare that the data supporting the findings of this study are available within the Article and Supplementary Information tables. RNA-seq data are available under GEO Accession Viewer with GSE267009. All the raw data are also available on Sequence Read Archive with BioProject number PRJNA1104007. The graphs derived from the datasets that are used in the manuscript were also uploaded to GitHub at https://github.com/MZ1808/DataDeposit/tree/main. The public scRNA sequencing datasets used in this study can be downloaded from NCBI Gene Expression Omnibus (GEO) (https://www.ncbi.nlm.nih.gov/geo/) at GSE122960, GSE149878 and GSE171668. Source data are provided with this paper.
Code availability
Custom code for bulk RNA and single-cell sequencing analysis performed in this study are publicly available via Zenodo at https://zenodo.org/doi/10.5281/zenodo.12561715 (ref. 56) and https://zenodo.org/doi/10.5281/zenodo.12565023 (ref. 57).
Change history
16 September 2024
A Correction to this paper has been published: https://doi.org/10.1038/s41564-024-01826-3
References
Siva, C., Velazquez, C., Mody, A. & Brasington, R. Diagnosing acute monoarthritis in adults: a practical approach for the family physician. Am. Fam. Physician 68, 83–90 (2003).
Joo, Y. B., Lim, Y. H., Kim, K. J., Park, K. S. & Park, Y. J. Respiratory viral infections and the risk of rheumatoid arthritis. Arthritis Res. Ther. 21, 199 (2019).
Hoong, C. W. S., Amin, M., Tan, T. C. & Lee, J. E. Viral arthralgia a new manifestation of COVID-19 infection? A cohort study of COVID-19-associated musculoskeletal symptoms. Int. J. Infect. Dis. 104, 363–369 (2021).
Slouma, M. et al. Reactive arthritis occurring after COVID-19 infection: a narrative review. Infection https://doi.org/10.1007/s15010-022-01858-z (2022).
Honge, B. L., Hermansen, M. F. & Storgaard, M. Reactive arthritis after COVID-19. BMJ Case Rep. 14 https://doi.org/10.1136/bcr-2020-241375 (2021).
Kocyigit, B. F. & Akyol, A. Reactive arthritis after COVID-19: a case-based review. Rheumatol. Int. 41, 2031–2039 (2021).
Ono, K. et al. Reactive arthritis after COVID-19 infection. RMD Open 6 https://doi.org/10.1136/rmdopen-2020-001350 (2020).
Stein, S. R. et al. SARS-CoV-2 infection and persistence in the human body and brain at autopsy. Nature 612, 758–763 (2022).
Bussani, R. et al. Persistent SARS-CoV-2 infection in patients seemingly recovered from COVID-19. J. Pathol. 259, 254–263 (2023).
Swank, Z. et al. Persistent circulating severe acute respiratory syndrome coronavirus 2 spike is associated with post-acute coronavirus disease 2019 sequelae. Clin. Infect. Dis. 76, e487–e490 (2023).
Taha, S. I., Samaan, S. F., Ibrahim, R. A., El-Sehsah, E. M. & Youssef, M. K. Post-COVID-19 arthritis: is it hyperinflammation or autoimmunity? Eur. Cytokine Netw. 32, 83–88 (2021).
Son, K. et al. Circulating anti-nuclear autoantibodies in COVID-19 survivors predict long-COVID symptoms. Eur. Respir. J. https://doi.org/10.1183/13993003.00970-2022 (2022).
Mendez, R. et al. Acute and sustained increase in endothelial biomarkers in COVID-19. Thorax 77, 400–403 (2022).
Lampsas, S. et al. The role of endothelial related circulating biomarkers in COVID-19. A systematic review and meta-analysis. Curr. Med. Chem. 29, 3790–3805 (2022).
Willems, L. H. et al. Sustained inflammation, coagulation activation and elevated endothelin-1 levels without macrovascular dysfunction at 3 months after COVID-19. Thromb. Res. 209, 106–114 (2022).
Abraham, G. R. et al. Endothelin-1 is increased in the plasma of patients hospitalised with Covid-19. J. Mol. Cell. Cardiol. 167, 92–96 (2022).
Atar, M. O., Ozcakar, L., Gencturk, Z. & Aytur, Y. Serum endothelin-1 levels, radiographic and ultrasonographic evaluations, and clinical parameters in patients with knee and/or hand osteoarthritis. J. Back Musculoskelet. Rehabil. 32, 549–554 (2019).
Zhao, Z., Li, E., Cao, Q., Sun, J. & Ma, B. Endothelin-1 concentrations are correlated with the severity of knee osteoarthritis. J. Investig. Med. 64, 872–874 (2016).
Au, M., Liu, Z., Rong, L., Zheng, Y. & Wen, C. Endothelin-1 induces chondrocyte senescence and cartilage damage via endothelin receptor type B in a post-traumatic osteoarthritis mouse model. Osteoarthritis Cartilage https://doi.org/10.1016/j.joca.2020.08.006 (2020).
De-Melo, J. D., Tonussi, C. R., D’Orleans-Juste, P. & Rae, G. A. Effects of endothelin-1 on inflammatory incapacitation of the rat knee joint. J. Cardiovasc. Pharmacol. 31, S518–S520 (1998).
De-Melo, J. D., Tonussi, C. R., D’Orleans-Juste, P. & Rae, G. A. Articular nociception induced by endothelin-1, carrageenan and LPS in naive and previously inflamed knee-joints in the rat: inhibition by endothelin receptor antagonists. Pain 77, 261–269 (1998).
Roy-Beaudry, M. et al. Endothelin 1 promotes osteoarthritic cartilage degradation via matrix metalloprotease 1 and matrix metalloprotease 13 induction. Arthritis Rheum. 48, 2855–2864 (2003).
Kaufman, G. N., Zaouter, C., Valteau, B., Sirois, P. & Moldovan, F. Nociceptive tolerance is improved by bradykinin receptor B1 antagonism and joint morphology is protected by both endothelin type A and bradykinin receptor B1 antagonism in a surgical model of osteoarthritis. Arthritis. Res. Ther. 13, R76 (2011).
Imhof, A. K. et al. Potent anti-inflammatory and antinociceptive activity of the endothelin receptor antagonist bosentan in monoarthritic mice. Arthritis. Res. Ther. 13, R97 (2011).
Khodorova, A., Montmayeur, J. P. & Strichartz, G. Endothelin receptors and pain. J. Pain 10, 4–28 (2009).
Lei, Y. et al. SARS-CoV-2 spike protein impairs endothelial function via downregulation of ACE 2. Circ. Res. 128, 1323–1326 (2021).
Dupont, A. et al. Vascular endothelial damage in the pathogenesis of organ injury in severe COVID-19. Arterioscler. Thromb. Vasc. Biol. 41, 1760–1773 (2021).
Bonin, R. P., Bories, C. & De Koninck, Y. A simplified up-down method (SUDO) for measuring mechanical nociception in rodents using von Frey filaments. Mol. Pain. 10, 26 (2014).
Kuschner, Z., Ortega, A. & Mukherji, P. A case of SARS-CoV-2-associated arthritis with detection of viral RNA in synovial fluid. J. Am. Coll. Emerg. Physicians Open 2, e12452 (2021).
Lopez-Leon, S. et al. More than 50 long-term effects of COVID-19: a systematic review and meta-analysis. Sci. Rep. 11, 16144 (2021).
Evangelou, K. et al. Pulmonary infection by SARS-CoV-2 induces senescence accompanied by an inflammatory phenotype in severe COVID-19: possible implications for viral mutagenesis. Eur. Respir. J. https://doi.org/10.1183/13993003.02951-2021 (2022).
Lee, S. et al. Virus-induced senescence is driver and therapeutic target in COVID-19. Nature https://doi.org/10.1038/s41586-021-03995-1 (2021).
Gioia, U. et al. SARS-CoV-2 infection induces DNA damage, through CHK1 degradation and impaired 53BP1 recruitment, and cellular senescence. Nat. Cell Biol. 25, 550–564 (2023).
Camell, C. D. et al. Senolytics reduce coronavirus-related mortality in old mice. Science https://doi.org/10.1126/science.abe4832 (2021).
Hirosue, A. et al. Quantitative assessment of higher-order chromatin structure of the INK4/ARF locus in human senescent cells. Aging Cell 11, 553–556 (2012).
Jeon, O. H. et al. Local clearance of senescent cells attenuates the development of post-traumatic osteoarthritis and creates a pro-regenerative environment. Nat. Med. 23, 775–781 (2017).
Krishnamurthy, J. et al. Ink4a/Arf expression is a biomarker of aging. J. Clin. Invest. 114, 1299–1307 (2004).
Qiao, W. et al. SARS-CoV-2 infection induces inflammatory bone loss in golden Syrian hamsters. Nat. Commun. 13, 2539 (2022).
Queiroz-Junior, C. M. et al. Acute coronavirus infection triggers a TNF-dependent osteoporotic phenotype in mice. Life Sci. 324, 121750 (2023).
Kerschan-Schindl, K. et al. Moderate COVID-19 disease is associated with reduced bone turnover. J. Bone Miner. Res. 38, 943–950 (2023).
Chen, W. et al. Arthritogenic alphaviral infection perturbs osteoblast function and triggers pathologic bone loss. Proc. Natl Acad. Sci. USA 111, 6040–6045 (2014).
Mi, B. et al. SARS-CoV-2-induced overexpression of miR-4485 suppresses osteogenic differentiation and impairs fracture healing. Int. J. Biol. Sci. 17, 1277–1288 (2021).
Swank, Z. et al. Persistent circulating severe acute respiratory syndrome coronavirus 2 spike is associated with post-acute coronavirus disease 2019 sequelae. Clin. Infect. Dis. 76, e487–e490 (2022).
Patterson, B. K. et al. Persistence of SARS CoV-2 S1 protein in CD16+ monocytes in post-acute sequelae of COVID-19 (PASC) up to 15 months post-infection. Front. Immunol. 12, 746021 (2021).
Chu, H. et al. Comparative tropism, replication kinetics, and cell damage profiling of SARS-CoV-2 and SARS-CoV with implications for clinical manifestations, transmissibility, and laboratory studies of COVID-19: an observational study. Lancet Microbe 1, e14–e23 (2020).
Chan, J. F. et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet 395, 514–523 (2020).
Yuan, S. et al. Metallodrug ranitidine bismuth citrate suppresses SARS-CoV-2 replication and relieves virus-associated pneumonia in Syrian hamsters. Nat. Microbiol. 5, 1439–1448 (2020).
Yuan, S. et al. Pathogenicity, transmissibility, and fitness of SARS-CoV-2 Omicron in Syrian hamsters. Science 377, 428–433 (2022).
Nair, A. B. & Jacob, S. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm. 7, 27–31, (2016).
Kikinis, R., Pieper, S. D. & Vosburgh, K. G. in Intraoperative Imaging and Image-Guided Therapy (ed. F. A. Jolesz) 277–289 (Springer, 2014).
Hayer, S. et al. ‘SMASH’ recommendations for standardised microscopic arthritis scoring of histological sections from inflammatory arthritis animal models. Ann. Rheum. Dis. 80, 714–726 (2021).
Ma, C. H. et al. Protective effects of tumor necrosis factor-alpha blockade by adalimumab on articular cartilage and subchondral bone in a rat model of osteoarthritis. Braz. J. Med. Biol. Res. 48, 863–870 (2015).
To, K. K. et al. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect. Dis. 20, 565–574 (2020).
Luo, W., Friedman, M. S., Shedden, K., Hankenson, K. D. & Woolf, P. J. GAGE: generally applicable gene set enrichment for pathway analysis. BMC Bioinformatics 10, 161 (2009).
Yu, G., Wang, L. G., Han, Y. & He, Q. Y. clusterProfiler: an R package for comparing biological themes among gene clusters. OMICS 16, 284–287 (2012).
Au, M. T. et al. Analysis of hMSC-derived chondrocytes in response to stimulation with SARS-CoV-2 spike proteins. Zenodo https://doi.org/10.5281/zenodo.12561716 (2024).
Au, M. T. & Zhou, B. Analysis of public single-cell sequencing database of COVID lung samples. Zenodo https://doi.org/10.5281/zenodo.12565023 (2024).
Acknowledgements
This work was supported by Health and Medical Research Fund (16172691# to C.W. and 07210107# to S.Y.), the Food and Health Bureau, The Government of the Hong Kong Special Administrative Region; General Research Fund (PolyU 151061/20M, PolyU15100821M, 15100324 to C.W.), Collaborative Research Fund (C7060-21G and C7002-23Y to S.Y.) and Theme-Based Research Scheme (T11-709/21-N to S.Y.), the Research Grants Council of the Hong Kong Special Administrative Region; The National Natural Science Foundation of China (NSFC) and the Research Grants Council (RGC) (NFSC/RGC) schemes (N_PolyU 520/20 to C.W. and N_HKU767/22 to S.Y.); The Innovation and Technology Fund Mainland-Hong Kong Joint Funding Scheme (ITF MHKJFS) (MHP/011/20 to C.W.); the Hong Kong Polytechnic University (HKPU) Project of Strategic Importance (ZE2C to C.W.); Health@InnoHK, Innovation and Technology Commission, the Government of the Hong Kong Special Administrative Region; National Key Research and Development Program of China (2021YFC0866100 and 2023YFC3041600 to S.Y.); National Natural Science Foundation of China (32322087 and 32300134 to S.Y.); and PolyU SZRI Seed Fund (I2022A006 to C.W.). We thank D. Chan from the HKU, L. Rong from Sun Yat-Sen University and M. Yang from the HKPU for the cell lines used in this study. We also thank E. Lau and M. Yuen from the University Research Facility in Life Sciences of the HKPU for providing technical support of micro-MRI scanning and high-resolution microscopy. We likewise thank J. Liu and Y. Zhang from the Department of Biomedical Engineering of HKPU for providing imaging assistance for micro-MRI and µCT scanning.
Author information
Authors and Affiliations
Contributions
C.W., S.Y., M.T.A. and J.N. conceived and designed the experiments. M.T.A., J.N., K.T., H.W., F.Z., Z.L., C.L., L. Zhu, C.Y.Z., T.J. and M.L. performed experiments and analysed data. M.T.A., C.W., L.C.-M.L., S.Y. and K.T. wrote the manuscript. L.C.-M.L., W.W. and P.-K.C. did the retrospective clinical study. B.Z., L. Zhang and P.L. performed sequencing analysis. C.W., S.Y. and J.F.-W.C. oversaw the conception, supervised the study and provided writing advice. C.W. and S.Y. provided the grant support.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Microbiology thanks Suresh Mahalingam and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Clinical CT images showing cyst formation at the osteochondral junction of COVID patients.
X-ray images of knee joints from 5 individuals. Cysts formation (yellow rectangle) was observed in medial compartment of knee in COVID patients. Collapse of tibial plateau on medial side in the post-COVID OA patient was shown. Scale bar, 1 cm.
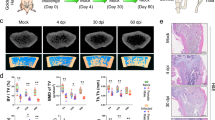
Extended Data Fig. 2 Structural change of knee joint and chondrocyte senescence upon infection of different SARS-CoV-2 variants.
a, Experimental design to study the effect of different variants of SARS-CoV-2 on knee joints of golden Syrian hamsters at 4 dpi. b, c, Representative MRI images and quantitative measurement of synovium area in mock and infected hamsters. Segmentation of each sample was shown at the bottom right corner. n = 3 for each group, both hind limbs were scanned excluding one leg from Delta group due to distorted joint capsule. The arrow indicates the hyperintensive region in synovium after virus infection. Scale bar, 1 mm. Mann-Whitney test. d, MicroCT and 3D rendering of microCT images showing bulges on the subchondral bone plate surface. Each data point represents the number of bulges per sample. n = 3 for each group. Data are mean ± S.E.M. Kruskal-Wallis test with Dunn’s multiple comparisons test. Scale bar, 200 µm. e, MicroCT images of knee joints at comparable positions showing no meniscal extrusion after SARS-CoV-2 infection. Scale bar, 1 mm. f, Representative immunostaining images and quantification for p15INK4b and p21 in mock-infected group and different time points after infection. n = 5 for each group. Data are mean ± S.E.M. One-way ANOVA with Tukey’s post hoc test unless otherwise specified.
Extended Data Fig. 3 Expression pattern of inflammatory cytokines at local joint tissue.
Representative immunostaining images for IL-1β, IL-6 and TNF-α at (a) articular cartilage, (b) subchondral bone and (c) synovium underwent mock or wildtype SARS-CoV-2 infection with macitentan treatment. Mock, n = 5; 4 dpi PBS, n = 5; 4 dpi Mac, n = 4; 30 dpi PBS, n = 5; 30 dpi Mac; n = 5 for IL-6 and TNF-α. Only n = 1 for each group was performed for IL-1β.
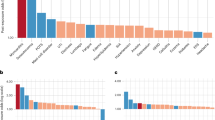
Extended Data Fig. 4 Integrative analysis of public scSeq datasets of lungs from normal subjects and COVID patients.
a, UMAP visualization of endothelial sub-groups from healthy subjects and COVID-19 patients. b, Boxplot showing individual proportions of endothelial sub-groups in healthy (n = 8) and COVID-19 patients (n = 11). c, Boxplot showing differential gene expression between healthy (n = 8) and COVID-19 patients (n = 11) in IL7R+ population. The boxplot compactly displays the distribution of a continuous variable. It visualises five summary statistics (the median, two hinges and two whiskers). The upper and lower hinges correspond to the first and third quartiles. The upper and lower whiskers extend from the hinge to the highest or lowest value that is within 1.5 * IQR of the hinge, where IQR is the inter-quartile range. The data in panels b and c are mean ± S.E.M. Wilcoxon rank-sum test.
Extended Data Fig. 5 SARS-CoV-2 viral proteins damage endothelial cells and accumulate near blood vessels.
a, b, Representative images of (a) CellROX and (b) MitoTracker staining showing accumulation of oxidative stress and changes in mitochondrial dynamics in HUVEC after SP RBD (10 µg/ml) treatment for 1 hour. Hydrogen peroxide at 100µM was used as positive control. Blob form structures were indicated by arrows. Three independent experiments. c, Representative immunostaining for vWF and SARS-CoV-2 nucleocapsid protein (NP) in showing nucleocapsid proteins located near dysfunctional blood vessels after infection. Arrows indicate nucleocapsid proteins. d, e, Representative images of (d) SARS-CoV-2 nucleocapsid protein (NP) (brown) and (e) SARS-CoV-2 spike protein (SP) (brown) in subchondral bone marrow cavity at different time points after infection. n = 5 per time point. Semi-quantification of NP and SP was performed based on integrated optical density (IOD). The grading method is shown in Supplementary Table 1.
Extended Data Fig. 6 Distribution of viral copies and components in different parts of the joint after SARS-CoV-2 infection.
a, b, c, Relative mRNA expression of (a) RdRp, (b) envelop gene and (c) nucleocapsid gene detected in different parts of a hamster knee joint after 4 days of mock or SARS-CoV-2 infection. Each data point represents sample obtained from one animal, except 3 animals were pooled for one data point for articular cartilage to get sufficient RNA. Multiple unpaired t-tests. d, Representative immunostaining images for spike protein in different regions of hamster knee joints with and without macitentan treatment after SARS-CoV-2 infection. Arrowheads indicate positive signals. Magnified figures are shown in the bottom left corner of each figure. Mock, n = 5; 4 dpi PBS, n = 5; 4 dpi Mac, n = 4; 30 dpi PBS, n = 5; 30 dpi Mac; n = 5.
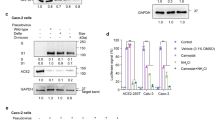
Extended Data Fig. 7 SARS-CoV-2 caused upregulation of ET-1 and oxidative stress in chondrocytes.
a, Representative immunostaining images and quantification for ET-1 at articular cartilage at different times of infection. Treatment effect with macitentan was also shown. Mock, n = 5; 4 dpi PBS, n = 5; 4 dpi Mac, n = 4; 30 dpi PBS, n = 5; 30 dpi Mac; n = 5. Data are mean ± S.E.M. Data were excluded when they fell out of 1.5 IQR. One-way ANOVA with Tukey’s multiple-comparison. b, c, Representative images of (b) CellROX and (c) MitoTracker staining showing accumulation of oxidative stress and changes in mitochondrial dynamics in C28/I2 after SP RBD (10 µg/ml) treatment for 1 hour. C28/I2 cells were pre-treated with macitentan for 30 minutes before stimulation by SP RBD for 1 h. Three independent biological repeats.
Extended Data Fig. 8 RNA sequencing of human mesenchymal stem cells-differentiated chondrocytes after SARS-CoV-2 spike protein stimulation.
a, b, Top upregulated KEGG pathways comparing (a) SAE or (b) SP-treated hMSCs-differentiated chondrocytes with control. n = 3 for each group. Cellular senescence pathway was upregulated only in SAE-treated cells. c, Heatmap showing selected differentially expressed genes (DEGs) related to cellular senescence with p < 0.05, highlighting p15 and p21. d, Heatmap showing upregulation of inflammatory cytokines (yellow arrows) and increased OPG/RANKL ratio (red arrows) under stimulation by the combination of spike protein, anti-spike protein antibody S1 and endothelin-1 (SAE). Three biological replicates for each group.
Extended Data Fig. 9 Endothelin receptor antagonism restored spike protein-induced alteration of RANKL/OPG balance and bone microstructure.
a, Schematic design for the cellular experiment to study the effect of endothelin receptor blockers on chondrocyte catabolism. b, Relative mRNA expression of MMP1 and p16INK4a in C28/I2 after RBD stimulation with endothelin receptor blockers pre-treatment. n = 3. Two-way ANOVA with uncorrected Fisher’s LSD. c, Relative mRNA expression of OPG, RANKL and OPG/RANKL ratio in HUVEC upon stimulation of ET-1 and 10 µg/ml SP for 24 h. One-way ANOVA with Tukey’s multiple comparison. d, Relative mRNA ratio of OPG, RANKL and RANKL/OPG in C28/I2 upon stimulation by SP and ET-1, with the treatment effect of endothelin receptor blockers. Two-tailed unpaired t-test. Three independent biological repeats for c and d. e, Quantification based on micro-CT reconstruction of the proximal epiphysis of tibias. Vehicle (n = 4); SP RBD (n = 4); Low Mac (n = 4); High Mac (n = 5). One-tailed non-parametric Mann-Whitney test. The data in panels b-e are mean ± S.E.M.
Supplementary information
Supplementary Information
Supplementary Table 2.
Supplementary Table 1
Semi-quantification of SARS-CoV-2 viral components. a, Grading of the presence of SARS-CoV-2 nucleocapsid protein (NP) in bone marrow cavity in subchondral bone region in tibias of hamsters based on integrated optical density (IOD) in per mille (‰). IODs of all sections were divided into three categories from ‘−’, indicating the lowest abundance, to ‘++’, indicating the highest abundance. b, Table showing the number of samples in each grade based on IOD. Chi-square test. c, Grading of the presence of SARS-CoV-2 SP in bone marrow cavity in subchondral bone region in tibias of hamsters based on IOD in percentage (%). IODs of all sections were divided into four categories from ‘−’, indicating the lowest abundance, to ‘+++’, indicating the highest abundance. d, Table showing the number of samples in each grade based on IOD. Mock (n = 5); 4 d.p.i. PBS (n = 5); 4 d.p.i. Mac (n = 4); 30 d.p.i. PBS (n = 5) and 30 d.p.i. Mac (n = 5). Chi-square test.
Source data
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data + unprocessed western blots.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 6
Statistical source data.
Source Data Extended Data Figures
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Au, M.T., Ni, J., Tang, K. et al. Blockade of endothelin receptors mitigates SARS-CoV-2-induced osteoarthritis. Nat Microbiol 9, 2538–2552 (2024). https://doi.org/10.1038/s41564-024-01802-x
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41564-024-01802-x