Abstract
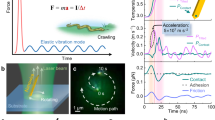
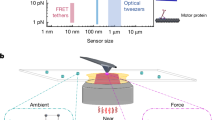
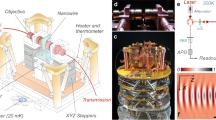
Precise force measurement is critical to probe biological events and physics processes, spanning from molecular motor’s motion to the Casimir effect, as well as the detection of gravitational waves. Yet, despite extensive technological developments, the three-dimensional nanoscale measurement of weak forces in aqueous solutions still faces major challenges. Techniques that rely on optically trapped nanoprobes are of significant potential but are beset with limitations, including probe heating induced by high trapping power, undetectable scattering signals and localization errors. Here we report the measurement of the long-distance interaction force in aqueous solutions with a minimum detected force value of 108.2 ± 510.0 attonewton. To achieve this, we develop a super-resolved photonic force microscope based on optically trapped lanthanide-doped nanoparticles coupled with nanoscale three-dimensional tracking-based force sensing. The tracking method leverages neural-network-empowered super-resolution localization, where the position of the force probe is extracted from the optical-astigmatism-modified point spread function. We achieve a force sensitivity down to 1.8 fN Hz–1/2, which approaches the nanoscale thermal limit. We experimentally measure electrophoresis forces acting on single nanoparticles as well as the surface-induced interaction force on a single nanoparticle. This work opens the avenue of nanoscale thermally limited force sensing and offers new opportunities for detecting sub-femtonewton forces over long distances and biomechanical forces at the single-molecule level.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The data that support the findings of this study are available within the Article and its Supplementary Information. Other relevant data are available from the corresponding authors upon reasonable request. Source data are provided with this paper.
Code availability
All the custom codes are available from the corresponding authors upon reasonable request.
Change history
15 August 2024
A Correction to this paper has been published: https://doi.org/10.1038/s41566-024-01518-8
References
Ting, L. H. et al. Contractile forces in platelet aggregates under microfluidic shear gradients reflect platelet inhibition and bleeding risk. Nat. Commun. 10, 1204 (2019).
Hultin, S. et al. AmotL2 links VE-cadherin to contractile actin fibres necessary for aortic lumen expansion. Nat. Commun. 5, 3743 (2014).
Blakely, B. L. et al. A DNA-based molecular probe for optically reporting cellular traction forces. Nat. Methods 11, 1229–1232 (2014).
Yamaguchi, N. et al. Rear traction forces drive adherent tissue migration in vivo. Nat. Cell Biol. 24, 194–204 (2022).
Brockman, J. M. et al. Live-cell super-resolved PAINT imaging of piconewton cellular traction forces. Nat. Methods 17, 1018–1024 (2020).
Meijering, A. E. C. et al. Nonlinear mechanics of human mitotic chromosomes. Nature 605, 545–550 (2022).
Stowe, T. D. et al. Attonewton force detection using ultrathin silicon cantilevers. Appl. Phys. Express 71, 288–290 (1997).
Bian, K. et al. Scanning probe microscopy. Nat. Rev. Methods Prim. 1, 36 (2021).
Chen, Y. et al. An integrin αIIbβ3 intermediate affinity state mediates biomechanical platelet aggregation. Nat. Mater. 18, 760–769 (2019).
Ju, L. et al. Compression force sensing regulates integrin αIIbβ3 adhesive function on diabetic platelets. Nat. Commun. 9, 1087 (2018).
Stabley, D. R., Jurchenko, C., Marshall, S. S. & Salaita, K. S. Visualizing mechanical tension across membrane receptors with a fluorescent sensor. Nat. Methods 9, 64–67 (2012).
Brockman, J. M. et al. Mapping the 3D orientation of piconewton integrin traction forces. Nat. Methods 15, 115–118 (2018).
Alonso-Caballero, A., Tapia-rojo, R., Badilla, C. L. & Fernandez, J. M. Magnetic tweezers meets AFM: ultra-stable protein dynamics across the force spectrum. Preprint at bioRxiv https://doi.org/10.1101/2021.01.04.425265 (2021).
Florin, E. L., Pralle, A., Stelzer, E. H. K. & Hörber, J. K. H. Photonic force microscope calibration by thermal noise analysis. Appl. Phys. A 66, S75–S78 (1998).
Lester, M., Arias-González, J. R. & Nieto-Vesperinas, M. Fundamentals and model of photonic-force microscopy. Opt. Lett. 26, 707–709 (2001).
Neuman, K. C. & Nagy, A. Single-molecule force spectroscopy: optical tweezers, magnetic tweezers and atomic force microscopy. Nat. Methods 5, 491–505 (2008).
Gieseler, J. et al. Optical tweezers—from calibration to applications: a tutorial. Adv. Opt. Photon. 13, 74–241 (2021).
Maragò, O. M., Jones, P. H., Gucciardi, P. G., Volpe, G. & Ferrari, A. C. Optical trapping and manipulation of nanostructures. Nat. Nanotechnol. 8, 807–819 (2013).
Grier, D. G. A revolution in optical manipulation. Nature 424, 810–816 (2003).
Thompson, R. E., Larson, D. R. & Webb, W. W. Precise nanometer localization analysis for individual fluorescent probes. Biophys. J. 82, 2775–2783 (2002).
Liu, L., Kheifets, S., Ginis, V. & Capasso, F. Subfemtonewton force spectroscopy at the thermal limit in liquids. Phys. Rev. Lett. 116, 228001 (2016).
Czerwinski, F., Richardson, A. C. & Oddershede, L. B. Quantifying noise in optical tweezers by Allan variance. Opt. Express 17, 13255–13269 (2009).
Schnoering, G., Rosales-Cabara, Y., Wendehenne, H., Canaguier-Durand, A. & Genet, C. Thermally limited force microscopy on optically trapped single metallic nanoparticles. Phys. Rev. Appl. 11, 034023 (2019).
Zensen, C., Villadsen, N., Winterer, F., Keiding, S. R. & Lohmüller, T. Pushing nanoparticles with light—a femtonewton resolved measurement of optical scattering forces. APL Photonics 1, 026102 (2016).
Seol, Y., Carpenter, A. E. & Perkins, T. T. Gold nanoparticles: enhanced optical trapping and sensitivity coupled with significant heating. Opt. Lett. 31, 2429–2431 (2006).
Ding, L. et al. Optical nonlinearity enabled super‐resolved multiplexing microscopy. Adv. Mater. 36, 2308844 (2023).
Shan, X. et al. Optical tweezers beyond refractive index mismatch using highly doped upconversion nanoparticles. Nat. Nanotechnol. 16, 531–537 (2021).
Ding, L. et al. Lanthanide ion resonance‐driven Rayleigh scattering of nanoparticles for dual‐modality interferometric scattering microscopy. Adv. Sci. 9, 2203354 (2022).
Chen, C. et al. Exploiting dynamic nonlinearity in upconversion nanoparticles for super-resolution imaging. Nano Lett. 22, 7136–7143 (2022).
Liu, B. et al. Multiplexed structured illumination super-resolution imaging with lifetime-engineered upconversion nanoparticles. Nanoscale Adv. 4, 30–38 (2022).
Liang, Y. et al. Migrating photon avalanche in different emitters at the nanoscale enables 46th-order optical nonlinearity. Nat. Nanotechnol. 17, 524–530 (2022).
Pu, R. et al. Super-resolution microscopy enabled by high-efficiency surface-migration emission depletion. Nat. Commun. 13, 6636 (2022).
Wang, F. et al. Microscopic inspection and tracking of single upconversion nanoparticles in living cells. Light Sci. Appl. 7, 18006–18007 (2018).
Bates, M., Huang, B., Dempsey, G. T. & Zhuang, X. Multicolor super-resolution imaging with photo-switchable fluorescent probes. Science 317, 1749–1753 (2007).
Lelek, M. et al. Single-molecule localization microscopy. Nat. Rev. Methods Prim. 1, 39 (2021).
Sigal, Y. M., Zhou, R. & Zhuang, X. Visualizing and discovering cellular structures with super-resolution microscopy. Science 361, 880–887 (2018).
Liebel, M. et al. 3D tracking of extracellular vesicles by holographic fluorescence imaging. Sci. Adv. 6, eabc2508 (2020).
Huang, B., Wang, W., Bates, M. & Zhuang, X. Three-dimensional super-resolution imaging by stochastic optical reconstruction microscopy. Science 319, 810–813 (2008).
Crocker, J. C. & Grier, D. G. Methods of digital video microscopy for colloidal studies. J. Colloid Interface Sci. 179, 298–310 (1996).
Rodríguez-Sevilla, P. et al. Optical forces at the nanoscale: size and electrostatic effects. Nano Lett. 18, 602–609 (2018).
Loeb, A. L., Wiersema, P. H. & Overbeek, J. T. G. The Electrical Double Layer around a Spherical Colloid Particle (MIT Press, 1961).
Hunter, R. J. Zeta Potential in Colloid Science: Principles and Applications (Academic Press, 1981).
Haag, A.-L., Nagai, Y., Lennox, R. & Grütter, P. Characterization of a gold coated cantilever surface for biosensing applications. EPJ Tech. Instrum. 2, 1–12 (2015).
Park, S. & Hamad-Schifferli, K. Evaluation of hydrodynamic size and zeta-potential of surface-modified Au nanoparticle-DNA conjugates via Ferguson analysis. J. Phys. Chem. C 112, 7611–7616 (2008).
Nehme, E. et al. DeepSTORM3D: dense 3D localization microscopy and PSF design by deep learning. Nat. Methods 17, 734–740 (2020).
Friedrich, L. & Rohrbach, A. Surface imaging beyond the diffraction limit with optically trapped spheres. Nat. Nanotechnol. 10, 1064–1069 (2015).
Rodríguez-Sevilla, P. et al. Thermal scanning at the cellular level by an optically trapped upconverting fluorescent particle. Adv. Mater. 28, 2421–2426 (2016).
Pant, A., Xia, X., Davis, E. J. & Pauzauskie, P. J. Solid-state laser refrigeration of a composite semiconductor Yb:YLiF4 optomechanical resonator. Nat. Commun. 11, 3235 (2020).
Rodríguez-Sevilla, P., Arita, Y., Liu, X., Jaque, D. & Dholakia, K. The temperature of an optically trapped, rotating microparticle. ACS Photonics 5, 3772–3778 (2018).
Helgadottir, S., Argun, A. & Volpe, G. Digital microscopy enhanced by deep learning. Optica 6, 506–513 (2019).
Nuzzo, R. G., Dubois, L. H. & Allara, D. L. Fundamental studies of microscopic wetting on organic surfaces. 1. Formation and structural characterization of a self-consistent series of polyfunctional organic monolayers. J. Am. Chem. Soc. 112, 558–569 (1990).
Acknowledgements
We thank the experimental assistance from M. Maddahfar, J. Liao and X. He; equipment support from C. Jagadish and the Analysis & Testing Center in Beihang University; and DNN assistance from P. Nie. We acknowledge financial support from the National Natural Science Foundation of China (U23A20481, 62275010 and 52073006), the Beijing Natural Science Foundation (1232027) and the fellowship of China Postdoctoral Science Foundation (2022TQ0020).
Author information
Authors and Affiliations
Contributions
F.W. conceived and supervised the project. L.D., X.S. and F.W. constructed the optical setup. F.W. and S.S.J.W. built the theoretical simulation and analytical model. X.S. and P.N. built the deep-learning model. S.W. and J.S. synthesized the nanoparticles. L.D. and H.Z. built the analysis of the net charge of the nanoparticles. L.D., X.S. and D.W. performed all the experiments. L.D., X.S. and F.W. analysed the results, prepared the figures and wrote the manuscript in consultation with all authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Photonics thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Sections 1–8, Equations (1)–(31), Tables 1–6 and Figs. 1–12.
Source data
Source Data Fig. 1
Data for drawing Fig. 1.
Source Data Fig. 2
Data for drawing Fig. 2.
Source Data Fig. 3
Data for drawing Fig. 3.
Source Data Fig. 4
Data for drawing Fig. 4.
Source Data Fig. 5
Data for drawing Fig. 5.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shan, X., Ding, L., Wang, D. et al. Sub-femtonewton force sensing in solution by super-resolved photonic force microscopy. Nat. Photon. 18, 913–921 (2024). https://doi.org/10.1038/s41566-024-01462-7
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41566-024-01462-7
This article is cited by
-
Infrared nanosensors of piconewton to micronewton forces
Nature (2025)
-
Achievement of a vacuum-levitated metal mechanical oscillator with ultra-low damping rate at room temperature
Communications Physics (2025)
-
A simulation design of cantilever beam type Microforce detection sensor based on photoelectric position sensitive detector (PSD)
Journal of Optics (2025)
-
A material change for ultra-high precision force sensing
Light: Science & Applications (2024)