Abstract
The operating lifetime under real-world climates is a critical metric to evaluate the commercial potential of any photovoltaic technology. Organic solar cells (OSCs) have experienced rapid breakthroughs in performance over the past decade owing to advances in device and materials engineering, including interfaces, electron acceptors, and donors. However, the intrinsic photodegradation of polymer donors remains poorly understood, and a path to stable OSCs is yet to be demonstrated under outdoor testing conditions. Herein we elucidate the side-chain-induced degradation mechanism in polymer donors and present an outdoor stability database covering 15 representative non-fullerene-based OSCs, supported by in-lab photostability and thermostability analysis. By understanding the performance losses induced by several photoactive layers and interfaces, we demonstrate that encapsulated non-fullerene-based OSCs can retain 91% of the initial efficiency after seven months of operation under hot and sunny Saudi Arabian climates. These findings reveal encouraging prospects of non-fullerene-based OSCs for outdoor applications.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
We declare that the data supporting the findings of this study are available within this paper and its Supplementary Information. Alternative formats for raw data files may be requested from the corresponding author on reasonable request. The raw data for the NMR spectra (Extended Data Fig. 3) and the outdoor stability database (Extended Data Fig. 8) are available in the data files provided with this paper. Source Data are provided with this paper.
References
Li, Y. X., Huang, X. J., Sheriff, H. K. M. & Forrest, S. R. Semitransparent organic photovoltaics for building-integrated photovoltaic applications. Nat. Rev. Mater. 8, 186–201 (2023).
Almora, O. et al. Device performance of emerging photovoltaic materials (version 3). Adv. Energy Mater. 13, 2203313 (2023).
Karki, A., Gillett, A. J., Friend, R. H. & Nguyen, T. Q. The path to 20% power conversion efficiencies in nonfullerene acceptor organic solar cells. Adv. Energy Mater. 11, 2003441 (2021).
Jiang, Y. et al. Non-fullerene acceptor with asymmetric structure and phenyl-substituted alkyl side chain for 20.2% efficiency organic solar cells. Nat. Energy 9, 975–986 (2024).
Burlingame, Q., Ball, M. & Loo, Y. L. It’s time to focus on organic solar cell stability. Nat. Energy 5, 947–949 (2020).
Shoaee, S. et al. What we have learnt from PM6:Y6. Adv. Mater. 36, e2302005 (2024).
Luke, J., Yang, E. J., Labanti, C., Park, S. Y. & Kim, J. S. Key molecular perspectives for high stability in organic photovoltaics. Nat. Rev. Mater. 8, 839–852 (2023).
Liu, T. R. et al. Photochemical decomposition of Y-series non-fullerene acceptors is responsible for degradation of high-efficiency organic solar cells. Adv. Energy Mater. 13, 2300046 (2023).
Luke, J. et al. Strong intermolecular interactions induced by high quadrupole moments enable excellent photostability of non-fullerene acceptors for organic photovoltaics. Adv. Energy Mater. 12, 2201267 (2022).
Che, Y., Niazi, M. R., Izquierdo, R. & Perepichka, D. F. Mechanism of the photodegradation of A–D–A acceptors for organic photovoltaics. Angew. Chem. Int. Ed. 60, 24833–24837 (2021).
Xu, H. et al. Dissecting the structure-stability relationship of Y-series electron acceptors for real-world solar cell applications. Joule 7, 2135–2151 (2023).
Du, X. Y. et al. Efficient polymer solar cells based on non-fullerene acceptors with potential device lifetime approaching 10 years. Joule 3, 215–226 (2019).
An, C. & Hou, J. Benzo[1,2-b:4,5-b′]dithiophene-based conjugated polymers for highly efficient organic photovoltaics. Acc. Mater. Res. 3, 540–551 (2022).
Ghasemi, M. et al. A molecular interaction-diffusion framework for predicting organic solar cell stability. Nat. Mater. 20, 525–532 (2021).
Yang, W. Y. et al. Balancing the efficiency, stability, and cost potential for organic solar cells via a new figure of merit. Joule 5, 1209–1230 (2021).
Weitz, P. et al. Revealing photodegradation pathways of organic solar cells by spectrally resolved accelerated lifetime analysis. Adv. Energy Mater. 13, 2202564 (2022).
Kim, S. et al. New insights into the photodegradation mechanism of the PTB7-Th film: photooxidation of π-conjugated backbone upon sunlight illumination. J. Phys. Chem. C 124, 2762–2770 (2020).
Perthué, A. et al. Influence of traces of oxidized polymer on the performances of bulk heterojunction solar cells. Mater. Chem. Front. 3, 1632–1641 (2019).
Dominguez, I. F., Topham, P. D., Bussiere, P. O., Begue, D. & Rivaton, A. Unravelling the photodegradation mechanisms of a low bandgap polymer by combining experimental and modeling approaches. J. Phys. Chem. C 119, 2166–2176 (2015).
Martynov, I. V., Inasaridze, L. N. & Troshin, P. A. Resist or oxidize: identifying molecular structure–photostability relationships for conjugated polymers used in organic solar cells. ChemSusChem 15, e202101336 (2022).
Tournebize, A. et al. Impact of UV–visible light on the morphological and photochemical behavior of a low-bandgap poly(2,7-carbazole) derivative for use in high-performance solar cells. Adv. Energy Mater. 3, 478–487 (2013).
Wang, Y. W. et al. The critical role of the donor polymer in the stability of high-performance non-fullerene acceptor organic solar cells. Joule 7, 810–829 (2023).
Hintz, H. et al. Photodegradation of P3HT—a systematic study of environmental factors. Chem. Mater. 23, 145–154 (2011).
Han, J., Xu, H., Paleti, S. H. K., Sharma, A. & Baran, D. Understanding photochemical degradation mechanisms in photoactive layer materials for organic solar cells. Chem. Soc. Rev. 53, 7426–7454 (2024).
Azmi, R. et al. Damp heat-stable perovskite solar cells with tailored-dimensionality 2D/3D heterojunctions. Science 376, 73–77 (2022).
Shen, D. E. et al. Enhancement of photostability through side chain tuning in dioxythiophene-based conjugated polymers. Chem. Mater. 34, 1041–1051 (2022).
Tournebize, A. et al. Is there a photostable conjugated polymer for efficient solar cells? Polym. Degrad. Stab. 112, 175–184 (2015).
Silva, H. S. et al. Designing intrinsically photostable low band gap polymers: a smart tool combining EPR spectroscopy and DFT calculations. J. Mater. Chem. A 4, 15647–15654 (2016).
Tournebize, A. et al. Side chain structure and dispersity impact the photostability of low band gap polymers. Polym. Degrad. Stab. 146, 155–160 (2017).
Yamilova, O. R. et al. What is killing organic photovoltaics: light-induced crosslinking as a general degradation pathway of organic conjugated molecules. Adv. Energy Mater. 10, 1903163 (2020).
Xu, H. et al. Progress in the stability of small molecule acceptor-based organic solar cells. Adv. Mater. 37, e2407119 (2024).
Zhang, Y., Samuel, I. D. W., Wang, T. & Lidzey, D. G. Current status of outdoor lifetime testing of organic photovoltaics. Adv. Sci. 5, 1800434 (2018).
Greenbank, W. et al. Degradation behavior of scalable nonfullerene organic solar cells assessed by outdoor and indoor ISOS stability protocols. Energy Technol. 8, 2000295 (2020).
Rodríguez-Martínez, X. et al. Laminated organic photovoltaic modules for agrivoltaics and beyond: an outdoor stability study of all-polymer and polymer:small molecule blends. Adv. Funct. Mater. 33, 2213220 (2022).
Tang, H. et al. Rationale for highly efficient and outdoor-stable terpolymer solar cells. Energy Environ. Sci. 16, 2056–2067 (2023).
Khenkin, M. V. et al. Consensus statement for stability assessment and reporting for perovskite photovoltaics based on ISOS procedures. Nat. Energy 5, 35–49 (2020).
Reese, M. O. et al. Consensus stability testing protocols for organic photovoltaic materials and devices. Sol. Energy Mater. Sol. Cells 95, 1253–1267 (2011).
Sheriff, H. K. M., Li, Y., Arneson, C. E. & Forrest, S. R. Reliability of colorfast semitransparent organic photovoltaics. Device 2, 100369 (2024).
Yuan, J. et al. Single-junction organic solar cell with over 15% efficiency using fused-ring acceptor with electron-deficient core. Joule 3, 1140–1151 (2019).
Vollbrecht, J. et al. Quantifying the nongeminate recombination dynamics in nonfullerene bulk heterojunction organic solar cells. Adv. Energy Mater. 9, 1901438 (2019).
Xu, X. et al. Interface-enhanced organic solar cells with extrapolated T80 lifetimes of over 20 years. Sci. Bull. 65, 208–216 (2020).
Burlingame, Q. et al. Intrinsically stable organic solar cells under high-intensity illumination. Nature 573, 394–397 (2019).
Li, Y. X. et al. Lifetime over 10,000 hours for organic solar cells with Ir/IrO electron-transporting layer. Nat. Commun. 14, 1241 (2023).
Wopke, C. et al. Traps and transport resistance are the next frontiers for stable non-fullerene acceptor solar cells. Nat. Commun. 13, 3786 (2022).
Cheng, P. & Zhan, X. Stability of organic solar cells: challenges and strategies. Chem. Soc. Rev. 45, 2544–2582 (2016).
Zhao, Y. S. et al. Revealing the photo-degradation mechanism of PM6:Y6 based high-efficiency organic solar cells. J. Mater. Chem. C 9, 13972–13980 (2021).
Brinkmann, K. O. et al. Perovskite–organic tandem solar cells with indium oxide interconnect. Nature 604, 280–286 (2022).
Lu, T. & Chen, F. Bond order analysis based on the Laplacian of electron density in fuzzy overlap space. J. Phys. Chem. A 117, 3100–3108 (2013).
Siddika, S. et al. Molecular interactions that drive morphological and mechanical stabilities in organic solar cells. Joule 7, 1593–1608 (2023).
Gunther, M. et al. Increasing photostability of inverted nonfullerene organic solar cells by using fullerene derivative additives. ACS Appl. Mater. Interfaces 13, 19072–19084 (2021).
Zhang, D. F. et al. Observation of reversible light degradation in organic photovoltaics induced by long-persistent radicals. Energy Environ. Sci. 16, 5970–5981 (2023).
Jiang, X. Y. et al. Operando study of the influence of small molecule acceptors on the morphology induced device degradation of organic solar cells with different degrees of π–π stacking. Energy Environ. Sci. 16, 5970–5981 (2023).
Cha, H. et al. Suppression of recombination losses in polymer:nonfullerene acceptor organic solar cells due to aggregation dependence of acceptor electron affinity. Adv. Energy Mater. 9, 1901254 (2019).
Li, S. et al. Refined molecular microstructure and optimized carrier management of multicomponent organic photovoltaics toward 19.3% certified efficiency. Energy Environ. Sci. 16, 2262–2273 (2023).
Wang, L. et al. Donor–acceptor mutually diluted heterojunctions for layer-by-layer fabrication of high-performance organic solar cells. Nat. Energy 9, 208–218 (2024).
Deng, J. et al. Ferroelectric polymer drives performance enhancement of non-fullerene organic solar cells. Angew. Chem. Int. Ed. 61, e202202177 (2022).
Li, Y. et al. Non-fullerene acceptor organic photovoltaics with intrinsic operational lifetimes over 30 years. Nat. Commun. 12, 5419 (2021).
Zhang, K. et al. 11.2% All-polymer tandem solar cells with simultaneously improved efficiency and stability. Adv. Mater. 30, e1803166 (2018).
Aydin, E. et al. Interplay between temperature and bandgap energies on the outdoor performance of perovskite/silicon tandem solar cells. Nat. Energy 5, 851–859 (2020).
Babics, M. et al. One-year outdoor operation of monolithic perovskite/silicon tandem solar cells. Cell Rep. Phys. Sci. 4, 101280 (2023).
Ma, R. J. et al. Unveiling the morphological and physical mechanism of burn-in loss alleviation by ternary matrix toward stable and efficient all-polymer solar cells. Adv. Mater. 35, 2212275 (2023).
Sun, R. et al. Cost-efficient recycling of organic photovoltaic devices. Joule 8, 2523–2538 (2024).
Petersson, G. A. et al. Gaussian 09 Revision E.01 (Gaussian Inc., 2009).
Lu, T. & Chen, F. W. Multiwfn: a multifunctional wavefunction analyzer. J. Comput. Chem. 33, 580–592 (2012).
Acknowledgements
This publication is based on work supported by the King Abdullah University of Science and Technology (KAUST) Office of Sponsored Research (OSR) under award no. CCF-3079. J.H. expresses gratitude to the Alexander von Humboldt Foundation and the support during his stay in T. B. Marder’s group at Julius-Maximilians-Universität Würzburg. H.X. would like to extend thanks to A. V. Marsh and M. Heeney for providing the training and technical support related to the GPC instrumentation. We would like to thank the KAUST weather team for providing access to weather station data. We acknowledge the use of the KAUST Solar Center and the support from its staff.
Author information
Authors and Affiliations
Contributions
D.B. conceived the idea and directed the project. H.X. and J.H. designed the experiments, fabricated the solar cells and conducted the DFT calculations. M.B. and S.D.W. conducted the encapsulation of outdoor solar cells and outdoor stability measurements (Fig. 5d). L.H.H., J.B. and J.T. built the outdoor set-up and conducted outdoor stability measurements for PM6, D18 and PCE10-based devices (Fig. 5a). H.C. and H.X. performed the GPC measurements. Y.L., D.R.V. and H.X. conducted the Raman spectra measurements. D.R.V. conducted the GIWAXS and GISAXS measurements. M.S. and J.M. performed the GISAXS fitting analysis. L.Z. conducted the HR-TEM measurements. Y.Z. performed the AFM measurements and assisted in the fabrication of OFETs. F.L., S.D.W. and D.B. supervised the project and contributed to the manuscript. The manuscript was written by H.X. and J.H. and edited by all of the co-authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Photonics thanks Alexander Gillett and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Investigation of the contribution of BHJ layers and interfaces to the photodegradation of PM6:Y6-based devices.
(a) Scheme of the device fabrication and photo-aging conditions (plasma lamp with a spectrum close to AM1.5 G, N2 atmosphere) for understanding the role of interface stability. (b) J-V curves of the fresh and 200-hour aged-PM6:Y6-based devices. (c) TPC lifetime and (d) fitted n values (VOC versus Plight) for the fresh and 200-hour aged-PM6:Y6-based devices. (e) PV parameters for the fresh and 200-hour aged devices. Herein, both half-cell aged-PM6:Y6- and PCE10:Y6-based devices were examined to demonstrate further the role of BHJ and interface stability. It is also noteworthy, as reported in the literature57, that photochemical degradation of polymer donors could also be a source that induces chemical changes at interfaces between BHJ and transport layers, contributing to device degradation. The error bars represent the s.d. of independent measurements: n = 8 for PM6:Y6 and n = 6 for PCE10:Y6. Initial PCEs for the fresh-PM6:Y6-based devices: 14.9(14.8 ± 0.1)% and the fresh PCE10:Y6-based devices: 8.9(8.7 ± 0.2)%. The increased TPC lifetimes, decreased PCEs, changes in the fitted n values (VOC versus Plight)40, and VOC changes between aged-PEDOT:PSS:BHJ-based devices and aged full-cells demonstrate that the PCE losses come from both the degradation of the BHJ layer and interface. The detailed PV parameters are provided in Supplementary Table 3.
Extended Data Fig. 2 Investigation of the contribution of polymer donor PM6 and acceptor Y6 to the photodegradation of PM6:Y6 devices.
(a) Scheme of the fabrication of aged-PM6:fresh-Y6 and fresh-PM6:aged-Y6 devices (with fresh interlayers). Photo-aging condition: plasma lamp with a spectrum close to AM1.5 G, N2 atmosphere. (b) J-V curves of the aged-PM6:fresh-Y6 and fresh-PM6:aged-Y6 devices. Photovoltaic parameters are summarized in Supplementary Table 3. (c–e) The exciton dissociation and charge collection efficiency, TPC and TPV lifetime, α and n values for the 200-hour aged devices. We also subjected PCE10 to 200-hour photo-aging and subsequently fabricated the devices using fresh-Y6 and fresh interlayers, and Supplementary Fig. 12c shows that the devices based on aged-PCE10:fresh-Y6 still demonstrate stable PCEs compared to the fresh devices. To further elucidate the photodegradation effects of PM6 and Y6, we extended the photo-aging process from 200 hours to 500 hours. Notably, after 500 hours of extended aging, insoluble by-products formed when dissolving PM6 in CHCl3 during device fabrication, accompanied by an increase in solution viscosity. Although mixing aged-PM6 with fresh-Y6 is different from the one with in-situ photodegradation in the blends (due to the formation of insoluble photodegraded products and the remixing process of PM6:Y6), herein, we can observe the existence of photodegradation of PM6, which agrees with the reported work46,47.
Extended Data Fig. 3 NMR spectra of the fresh, 500-hour aged, and 1000-hour aged polymer thin films.
a, NMR spectra from the fresh and aged PM6 films. b, NMR spectra from the fresh and aged D18 films. c, NMR spectra from the fresh and aged PCE10 films. The NMR spectra of polymer donors were recorded in CDCl3 with a concentration of 1 mg/mL (Supplementary Figs. 52–60). In addition to the proven side-chain-induced degradation for classic polymer donors in the literature21,26,27,28,29,31, the appearance of new peaks at ~1.21 and ~1.18 ppm in Extended Data Fig. 3 for all three aged polymer donors also offers chemical evidence for the side-chain-induced photodegradation pathway. It is worth mentioning that insoluble solids were formed in CDCl3 solution for the aged D18 sample. These results agree with the NMR spectra in the literature and the side-chain degradation for dioxythiophene-based conjugated polymers reported by Reynolds et al.26. Source data for all the NMR spectra are provided with this paper.
Extended Data Fig. 4 Raman spectra and GPC curves of the polymer donors.
(a) Raman spectra and (b) GPC curves of the polymer thin films before and after photo-aging. Discussion on Raman and GPC changes arising from side-chain cross-linking, see Supplementary Note 4. The simulated Raman spectra of polymer donors and their degradation modes are presented in Supplementary Figs. 17–29. The Raman spectra for 500-hour aged polymer films are presented in Supplementary Fig. 35. The LBO values and chemical structures of the model polymers for DFT calculations are presented in Supplementary Figs. 33. PCE10 with BDT-TT linkage is more photostable than PTQ10 in the absence of O2, see discussion in Supplementary Fig. 39.
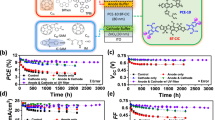
Extended Data Fig. 5 Optimization of interfaces for outdoor stability measurements.
(a) Thermal stability of PM6:Y6 blends with the PDINO and PNDIT-F3N interfaces. The error bars represent the s.d. of independent measurements. Initial PCEs: PDINO-based devices (65 °C): 14.3(14.1 ± 0.1)%, n = 6. Stability data for PDINO-based devices (RT, storage stability) and PNDIT-F3N-based devices (65 °C) is extracted from Supplementary Fig. 32 and Fig. 5b. (b) Photostability of PM6:Y6, D18:Y6, and PCE10:Y6 blends with the PNDIT-F3N interface. Initial PCEs: 15.0%, 15.1%, 9.3%; (c) PCEs, (d) TPC lifetimes, and (e) fitted n values (VOC versus Plight) for the PM6:Y6-based devices with different photo-aging conditions. Error bars in Extended Data Fig. 5c represent the s.d. of the mean, with the centres indicating the mean values. Data are presented as mean values +/− s.d., with the maximum values shown in parentheses. Initial PCEs for the PNDIT-F3N-based devices: 15.9(15.4 ± 0.3)%, n = 8; Full-cell stability data in Extended Data Fig. 5c is extracted from Fig. 1c (PDINO-based devices) and Extended Data Fig. 5b (PNDIT-F3N-based devices). TPC lifetimes and n values (VOC versus Plight) for PDINO-based devices are extracted from Extended Data Fig. 1. Herein, PDINO is more photostable than PNDIT-F3N, fitting for investigating the BHJ photostability in devices. PNDIT-F3N is thermally stable and can suppressed the thermal diffusion effect within/across the interlayers under heat stress, fitting for the outdoor stability testing58. The results also show that the PCE losses come from both the BHJ layer and interface degradation.
Extended Data Fig. 6 Irradiance and temperature effect on outdoor performance of the PM6-, D18-, and PCE10-based devices.
a, Temperature-dependent J-V curves of PM6:Y6. b, Temperature-dependent J-V curves of D18:Y6. c, Temperature-dependent J-V curves of PCE10:Y6. d, Efficiency evolutions of the devices on 16th September 2022 at different irradiance. e, Efficiency evolutions of the devices on 16th September 2022 at different time. Due to the spectra difference (AM 1.5 G and solar irradiance at KAUST) and temperature effect59, the calculated PCEs from outdoor data (PCE*) are different from the PCEs under standard AM 1.5 G condition measured in-lab (details in Supplementary Fig. 49 and Table 8). In this study, we calculated PCEs based on the highest power output values during PM 12:00-12:30.
Extended Data Fig. 7 Photo-, thermal, and outdoor stability of the PM6:PCE13:PY-IT-based ternary devices.
(a) PCE13 with fragile S-containing side-chains (LBO values in Supplementary Fig. 50). (b) Photostability of the PM6:PCE13:PY-IT-based ternary devices. Initial PCEs for the devices: PM6:PY-IT (15.4%); PCE13:PY-IT (11.3%); PM6:PCE13:PY-IT (0.95:0.05:1, 15.9%); PM6:PCE13:PY-IT (0.9:0.1:1, 15.7%). The photostability test was conducted under open-circuit conditions at 27 °C, using AM 1.5 G 100 mW cm−2 illumination in an N2 atmosphere. Notably, different from the direct energetic trap states caused by the tiny oxidized fullerenes in blends24, the introduction of small amount of guest polymer donor with unstable side-chains had a negligible impact on the photo- and outdoor stability of ternary OSCs. When transitioning to the comparable binary systems, it becomes evident that PM6-based devices is more photostable than the PCE13-based devices. (c) Thermal stability of the PM6:PCE13:PY-IT-based ternary devices. Initial photovoltaic parameters are listed in Supplementary Table 9. The error bars represent the s.d. of independent measurements (n = 6), and the centres represent the average values. (d) outdoor stability of the PM6:PCE13:PY-IT-based ternary devices. Initial PCEs for the encapsulated devices: PM6:PY-IT (15.1/14.4/15.0%); PCE13:PY-IT (7.7/7.6/7.4%); PM6: PCE13:PY-IT (0.95:0.05:1, 12.8/13.7/13.5%); PM6:PCE13:PY-IT (0.9:0.1:1, 13.4/14.2/14.0%).
Extended Data Fig. 8 Summary of the outdoor stability testing located in the KAUST testing site, Saudi Arabia.
Poutput is the power output of the devices under outdoor conditions. The highest Poutput during the first and last day were utilized to compare the outdoor stability, and the devices in the same group were aged under identical real-world climates. The whiskers represent the maximum and minimum values, and the box edges indicate the 75th and 25th percentiles. The center lines in the box-plot chart indicate the mean values. In group 2, the first 4-week outdoor stability database for PM6:N3, PM6:BTP-BO-4Cl, and PM6:BTP-eC9 blends, and the first 60-day outdoor stability database for PM6:Y12, PM6:Y6, and PM6:Y7 blends are available from the previous work11. All the outdoor stability database is available in the source data files provided with this paper.
Supplementary information
Source data
Source Data Extended Data Fig. 3
Unprocessed NMR Source Data.
Source Data Extended Data Fig. 8
Raw data for the outdoor stability database.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xu, H., Han, J., Babics, M. et al. Elucidating the photodegradation pathways of polymer donors for organic solar cells with seven months of outdoor operational stability. Nat. Photon. 19, 415–425 (2025). https://doi.org/10.1038/s41566-025-01644-x
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41566-025-01644-x
This article is cited by
-
Organic solar cells: beyond 20%
Science China Materials (2025)