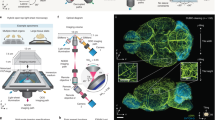
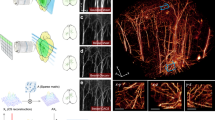
Abstract
Imaging large cleared tissues requires scaling the throughput of imaging techniques. Light sheet microscopy is a promising technique for high-throughput imaging; however, its reliance on conventional microscope objectives limits the optimization of the trade-off between spatial resolution and field of view. Here we introduce curved light sheet microscope to perform optical sectioning with curved light sheets. This concept addresses the long-standing field curvature problem and lowers the barriers in designing high-throughput objectives. Leveraging a customized objective, the curved light sheet microscope achieves diffraction-limited resolution of 1.0 μm laterally and 2.5 μm axially, with uniform contrast over a field of view of more than 1 × 1 cm2. Our technique is also compatible with various tissue clearing techniques. We demonstrate that imaging an entire intact cleared mouse brain at a voxel size of 0.625 × 0.625 × 1.25 μm3 can be completed in less than 3 h, without the need for image tiling. We share a full optical description of the objective and report imaging of neuronal and vascular networks, as well as tracing of brain-wide long-distance axonal projections in intact mouse brains.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Design data of the objective and curved light sheet generation module—along with the associated imaging parameters—are provided in Supplementary Information. Due to their large size, imaging datasets acquired with the curved light sheet microscope are not available in a public repository and are available from the corresponding author upon reasonable request.
Code availability
The imaging datasets were acquired using vendor-provided software for stage scanning (Kinesis, Thorlabs) and TDI camera control (CamExpert, Teledyne). The code for the PSF analysis is available from the corresponding author on reasonable request.
References
Dodt, H.-U. et al. Ultramicroscopy: three-dimensional visualization of neuronal networks in the whole mouse brain. Nat. Methods 4, 331–336 (2007).
Power, R. M. & Huisken, J. A guide to light-sheet fluorescence microscopy for multiscale imaging. Nat. Methods 14, 360–373 (2017).
Ueda, H. R. et al. Whole-brain profiling of cells and circuits in mammals by tissue clearing and light-sheet microscopy. Neuron 106, 369–387 (2020).
Weiss, K. R. et al. Tutorial: practical considerations for tissue clearing and imaging. Nat. Protoc. 16, 2732–2748 (2021).
Tomer, R. et al. Advanced CLARITY for rapid and high-resolution imaging of intact tissues. Nat. Protoc. 9, 1682–1697 (2014).
Tomer, R. et al. SPED light sheet microscopy: fast mapping of biological system structure and function. Cell 163, 1796–1806 (2015).
Voigt, F. F. et al. The mesoSPIM initiative: open-source light-sheet microscopes for imaging cleared tissue. Nat. Methods 16, 1105–1108 (2019).
Vladimirov, N. et al. Benchtop mesoSPIM: a next-generation open-source light-sheet microscope for cleared samples. Nat. Commun. 15, 2679 (2024).
Glaser, A. K. et al. Multi-immersion open-top light-sheet microscope for high throughput imaging of cleared tissues. Nat. Commun. 10, 2781 (2019).
Glaser, A. K. et al. Light-sheet microscopy for slide-free non-destructive pathology of large clinical specimens. Nat. Biomed. Eng. 1, 0084 (2017).
Glaser, A. K. et al. A hybrid open-top light-sheet microscope for versatile multi-scale imaging of cleared tissues. Nat. Methods 19, 613–619 (2022).
Xu, F. et al. High-throughput mapping of a whole rhesus monkey brain at micrometer resolution. Nat. Biotechnol. 39, 1521–1528 (2021).
Prince, M. N. H. et al. Signal improved ultra-fast light-sheet microscope for large tissue imaging. Commun. Eng. 3, 59 (2024).
Glaser, A. et al. Expansion-assisted selective plane illumination microscopy for nanoscale imaging of centimeter-scale tissues. eLife 12, RP91979 (2023).
Chakraborty, T. et al. Light-sheet microscopy of cleared tissues with isotropic, subcellular resolution. Nat. Methods 16, 1109–1113 (2019).
Chen, Y. et al. A versatile tiling light sheet microscope for imaging of cleared tissues. Cell Rep. 33, 108349 (2020).
Nie, J. et al. Fast, 3D isotropic imaging of whole mouse brain using multiangle-resolved subvoxel SPIM. Adv. Sci. 7, 1901891 (2020).
Fang, C. et al. Minutes-timescale 3D isotropic imaging of entire organs at subcellular resolution by content-aware compressed-sensing light-sheet microscopy. Nat. Commun. 12, 107 (2021).
Zhang, Z. et al. Multi-scale light-sheet fluorescence microscopy for fast whole brain imaging. Front. Neuroanat. 15, 732464 (2021).
Richardson, D. S. & Lichtman, J. W. Clarifying tissue clearing. Cell 162, 246–257 (2015).
Ueda, H. R. et al. Tissue clearing and its applications in neuroscience. Nat. Rev. Neurosci. 21, 61–79 (2020).
Richardson, D. S. et al. Tissue clearing. Nat. Rev. Methods Primers 1, 84 (2021).
Pan, C. et al. Shrinkage-mediated imaging of entire organs and organisms using uDISCO. Nat. Methods 13, 859–867 (2016).
Tainaka, K. et al. Whole-body imaging with single-cell resolution by tissue decolorization. Cell 159, 911–924 (2014).
Cai, R. et al. Panoptic imaging of transparent mice reveals whole-body neuronal projections and skull–meninges connections. Nat. Neurosci. 22, 317–327 (2019).
Jing, D. et al. Tissue clearing of both hard and soft tissue organs with the PEGASOS method. Cell Res. 28, 803–818 (2018).
Zhao, S. et al. Cellular and molecular probing of intact human organs. Cell 180, 796–812 (2020).
Zhang, Y. & Gross, H. Systematic design of microscope objectives. Part I: system review and analysis. Adv. Opt. Technol. 8, 313–347 (2019).
Park, J. et al. Review of bio-optical imaging systems with a high space-bandwidth product. Adv. Photon. 3, 044001 (2021).
McConnell, G. et al. A novel optical microscope for imaging large embryos and tissue volumes with sub-cellular resolution throughout. eLife 5, e18659 (2016).
Fan, J. et al. Video-rate imaging of biological dynamics at centimetre scale and micrometre resolution. Nat. Photon. 13, 809–816 (2019).
Voigt, F. F. et al. Reflective multi-immersion microscope objectives inspired by the Schmidt telescope. Nat. Biotechnol. 42, 65–71 (2024).
Nicholas, J. et al. A large field of view two-photon mesoscope with subcellular resolution for in vivo imaging. eLife 5, e14472 (2016).
Stirman, J. et al. Wide field-of-view, multi-region, two-photon imaging of neuronal activity in the mammalian brain. Nat. Biotechnol. 34, 857–862 (2016).
Yu, C. H. et al. The Cousa objective: a long-working distance air objective for multiphoton imaging in vivo. Nat. Methods 21, 132–141 (2024).
Ichimur, T. et al. Volumetric trans-scale imaging of massive quantity of heterogeneous cell populations in centimeter-wide tissue and embryo. eLife 13, RP93633 (2024).
Hoyer, C. ISO 19012-1:2013: Microscopes — Designation of Microscope Objectives. Part 1: Flatness of Field/Plan (International Organization for Standardization (ISO), 2013); www.iso.org/standard/61652.html
Schacht, P., Johnson, S. B. & Santi, P. A. Implementation of a continuous scanning procedure and a line scan camera for thin-sheet laser imaging microscopy. Biomed. Opt. Express 1, 598–609 (2010).
Gong, H. et al. Continuously tracing brain-wide long-distance axonal projections in mice at a one-micron voxel resolution. NeuroImage 74, 87–98 (2013).
Bélanger, P. A. & Rioux, M. Ring pattern of a lens-axicon doublet illuminated by a Gaussian beam. Appl. Opt. 17, 1080–1088 (1978).
Dean, K. M. et al. Deconvolution-free subcellular imaging with axially swept light sheet microscopy. Biophys. J. 108, 2807–2815 (2015).
Renier, N. et al. iDISCO: a simple, rapid method to immunolabel large tissue samples for volume imaging. Cell 159, 896–910 (2014).
Osten, P. & Margrie, T. Mapping brain circuitry with a light microscope. Nat. Methods 10, 515–523 (2013).
Wang, M. et al. Brain-wide projection reconstruction of single functionally defined neurons. Nat. Commun. 13, 1531 (2022).
Lin, H. M. et al. Reconstruction of intratelencephalic neurons in the mouse secondary motor cortex reveals the diverse projection patterns of single neurons. Front. Neuroanat. 12, 86 (2018).
Winnubst, J. et al. Reconstruction of 1,000 projection neurons reveals new cell types and organization of long-range connectivity in the mouse brain. Cell 179, 268–281 (2019).
Hellwig, B., Schüz, A. & Aertsen, A. Synapses on axon collaterals of pyramidal cells are spaced at random intervals: a Golgi study in the mouse cerebral cortex. Biol. Cybern. 71, 1–12 (1994).
Michael, N. et al. A platform for brain-wide imaging and reconstruction of individual neurons. eLife 5, e10566 (2016).
Gao, W. et al. Recent advances in curved image sensor arrays for bioinspired vision system. Nano Today 42, 101366 (2022).
Brady, D. et al. Multiscale gigapixel photography. Nature 486, 386–389 (2012).
Yi, Y. et al. Mapping of individual sensory nerve axons from digits to spinal cord with the transparent embedding solvent system. Cell Res. 34, 124–139 (2024).
Lowery, R. L. & Majewska, A. K. Intracranial injection of adeno-associated viral vectors. J. Vis. Exp. https://doi.org/10.3791/2140 (2010).
Renier, N. et al. Mapping of brain activity by automated volume analysis of immediate early genes. Cell 165, 1789–1802 (2016).
Tyson, A. L. et al. Accurate determination of marker location within whole-brain microscopy images. Sci. Rep. 12, 867 (2022).
Schindelin, J. et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682 (2012).
Acknowledgements
This work was supported by the CAMS Innovation Fund for Medical Sciences (2024-I2M-3-024 to J. Wu), the start-up funds from CIBR (to J. Wu), and the Beijing Municipal Science & Technology Commission (Z220009 to J. Wu). We thank the CIBR LARC staff for animal care and the CIBR Imaging Core, Instrumentation Core, Computing and Data Science Core for their support.
Author information
Authors and Affiliations
Contributions
J. Wu conceived the project. J. Wu and H.Z. supervised the research. J. Wu and L.T. designed the microscope. L.T. constructed the microscope, and collected and analysed the data. J. Wang, J.D., J.S., X.-j.C., R.S., P.C., R.G., W.-p.G., W.S. and H.Z. prepared the samples. Q.S. and F.X. traced the neurons. J. Wu and L.T. wrote the paper with inputs from all authors.
Corresponding author
Ethics declarations
Competing interests
J. Wu and L.T. are inventors on a patent application related to this work filed by CIBR. The other authors declare no competing interests.
Peer review
Peer review information
Nature Photonics thanks Tonmoy Chakraborty, Kevin Dean and Adam Glaser for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–19, Table 1 and Notes 1–4.
Supplementary Video 1
Whole-brain imaging of a PEGASOS-cleared Thy1-eGFP mouse brain.
Supplementary Video 2
Whole-brain vascular network imaging of a PEGASOS-cleared mouse brain (Tie2-Cre::Ai47).
Supplementary Video 3
Whole-brain vascular network imaging of a PEGASOS-cleared mouse brain (Pitx2-Cre::Ai47).
Supplementary Video 4
Imaging of a propidium-iodide-labelled mouse brain cleared by iDISCO.
Supplementary Video 5
Imaging of a propidium-iodide-labelled mouse kidney cleared by iDISCO.
Supplementary Video 6
Imaging of a propidium-iodide-labelled mouse brain cleared by a commercial hydrophilic tissue clearing kit.
Supplementary Video 7
Imaging of a mouse kidney (Tie2-Cre::Ai47) cleared by CUBIC.
Supplementary Video 8
Imaging of sparsely labelled neurons in the motor cortex of a PEGASOS-cleared mouse brain (AAV2/9-hsyn-Cre and AAV2/9-EF1 α-DIO-mScarlet injection).
Supplementary Video 9
Two-colour imaging of a PEGASOS-cleared intact mouse brain with sparse labelling of neurons co-expressing eGFP and mScarlet in the primary motor cortex.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tang, L., Wang, J., Ding, J. et al. Curved light sheet microscopy for centimetre-scale cleared tissue imaging. Nat. Photon. 19, 577–584 (2025). https://doi.org/10.1038/s41566-025-01659-4
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41566-025-01659-4