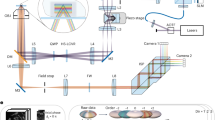
Abstract
Structured illumination microscopy (SIM) is a powerful tool for live-cell super-resolution imaging. Conventional two-dimensional (2D)-SIM uses one-dimensional stripe patterns and rotates them at three angles to achieve uniform resolution. Here, to alleviate photobleaching and improve the temporal resolution of 2D-SIM, we develop triangle-beam interference SIM (3I-SIM), which generates a 2D lattice pattern based on radially polarized beam interference. The radial polarization enhances the signal-to-noise ratio of the high-frequency components. Compared with conventional 2D-SIM, 3I-SIM reduces photobleaching and improves the temporal resolution to 242 Hz. Benefiting from unidirectional phase shift, 3I-SIM provides threefold higher rolling frame rate than conventional 2D-SIM to visualize fast biological dynamics. We further developed 3I-Net, a deep neural network with a co-supervised training scheme, to enhance the performance of 3I-SIM under an extremely low signal intensity. Its higher sensitivity enables the consecutive acquisition of over 100,000 time points at a spatial resolution of 100 nm. We continuously monitor the fine morphological changes in neuronal growth cones for up to 13 h, as well as the transient signals from actin filaments regulating endoplasmic reticulum dynamics. We believe 3I-SIM will offer a suitable platform to study complex and rapid biological processes with high data throughput.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The fluorescence data demonstrating the efficacy of the 3I-SIM analytical reconstruction algorithm are available via Figshare at https://figshare.com/articles/dataset/3I-SIM_dataset/26334118. The DL training dataset of the four typical organelles for 3I-SIM reconstruction is available via Zenodo at https://zenodo.org/records/14969641 (ref. 51). Other data are available from the corresponding authors upon reasonable request.
Code availability
The code of the analytical reconstruction algorithm, user-interactive reconstruction software, DL 3I-Net model and pretrained models on the four typical subcellular structures are available via GitHub at https://github.com/YiweiHou/3I-SIMReconstruction.
References
Hell, S. W. Far-field optical nanoscopy. Science 316, 1153–1158 (2007).
Huang, B., Bates, M. & Zhuang, X. Super-resolution fluorescence microscopy. Annu. Rev. Biochem. 78, 993–1016 (2009).
Gustafsson, M. G. L. Surpassing the lateral resolution limit by a factor of two using structured illumination microscopy. J. Microsc. 198, 82–87 (2000).
Guo, Y. et al. Visualizing intracellular organelle and cytoskeletal interactions at nanoscale resolution on millisecond timescales. Cell 175, 1430–1442.e1417 (2018).
Huang, X. et al. Fast, long-term, super-resolution imaging with Hessian structured illumination microscopy. Nat. Biotechnol. 36, 451–459 (2018).
Demmerle, J. et al. Strategic and practical guidelines for successful structured illumination microscopy. Nat. Protoc. 12, 988–1010 (2017).
Müller, M., Mönkemöller, V., Hennig, S., Hübner, W. & Huser, T. Open-source image reconstruction of super-resolution structured illumination microscopy data in ImageJ. Nat. Commun. 7, 10980 (2016).
Young, L. J., Ströhl, F. & Kaminski, C. F. A guide to structured illumination TIRF microscopy at high speed with multiple colors. J. Vis. Exp. e53988 (2016).
Booth, M. J. Adaptive optical microscopy: the ongoing quest for a perfect image. Light Sci. Appl. 3, e165 (2014).
Zhanghao, K. et al. Super-resolution imaging of fluorescent dipoles via polarized structured illumination microscopy. Nat. Commun. 10, 4694 (2019).
Chen, X. et al. Superresolution structured illumination microscopy reconstruction algorithms: a review. Light Sci. Appl. 12, 172 (2023).
Xu, X. et al. Ultra-high spatio-temporal resolution imaging with parallel acquisition-readout structured illumination microscopy (PAR-SIM). Light Sci. Appl. 13, 125 (2024).
Gustafsson, M. G. L. Nonlinear structured-illumination microscopy: Wide-field fluorescence imaging with theoretically unlimited resolution. Proc. Natl Acad. Sci. USA 102, 13081–13086 (2005).
Temma, K. et al. Selective-plane-activation structured illumination microscopy. Nat. Methods 21, 889–896 (2024).
Gustafsson, M. G. L. et al. Three-dimensional resolution doubling in wide-field fluorescence microscopy by structured illumination. Biophys. J. 94, 4957–4970 (2008).
Li, X. et al. Three-dimensional structured illumination microscopy with enhanced axial resolution. Nat. Biotechnol. 41, 1307–1319 (2023).
Lin, R., Kipreos, E. T., Zhu, J., Khang, C. H. & Kner, P. Subcellular three-dimensional imaging deep through multicellular thick samples by structured illumination microscopy and adaptive optics. Nat. Commun. 12, 3148 (2021).
Cao, R. et al. Open-3DSIM: an open-source three-dimensional structured illumination microscopy reconstruction platform. Nat. Methods 20, 1183–1186 (2023).
Helle, Ø. I. et al. Structured illumination microscopy using a photonic chip. Nat. Photon. 14, 431–438 (2020).
Wen, G. et al. High-fidelity structured illumination microscopy by point-spread-function engineering. Light Sci. Appl. 10, 70 (2021).
Smith, C. S. et al. Structured illumination microscopy with noise-controlled image reconstructions. Nat. Methods 18, 821–828 (2021).
Wang, Z. et al. High-speed image reconstruction for optically sectioned, super-resolution structured illumination microscopy. Adv. Photon. 4, 026003 (2022).
Hou, Y. et al. Multi-resolution analysis enables fidelity-ensured deconvolution for fluorescence microscopy. eLight 4, 14 (2024).
Zhao, W. et al. Sparse deconvolution improves the resolution of live-cell super-resolution fluorescence microscopy. Nat. Biotechnol. 40, 606–617 (2022).
Chen, J. et al. Three-dimensional residual channel attention networks denoise and sharpen fluorescence microscopy image volumes. Nat. Methods 18, 678–687 (2021).
Qiao, C. et al. Evaluation and development of deep neural networks for image super-resolution in optical microscopy. Nat. Methods 18, 194–202 (2021).
Betzig, E. Excitation strategies for optical lattice microscopy. Opt. Express 13, 3021–3036 (2005).
Schropp, M. & Uhl, R. Two-dimensional structured illumination microscopy. J. Microsc. 256, 23–36 (2014).
Chen, B.-C. et al. Lattice light-sheet microscopy: imaging molecules to embryos at high spatiotemporal resolution. Science 346, 1257998 (2014).
Gong, H., Guo, W. & Neil, M. A. A. GPU-accelerated real-time reconstruction in Python of three-dimensional datasets from structured illumination microscopy with hexagonal patterns. Philos. Trans. A Math. Phys. Eng. Sci. 379, 20200162 (2021).
Ingerman, E. A., London, R. A., Heintzmann, R. & Gustafsson, M. G. L. Signal, noise and resolution in linear and nonlinear structured-illumination microscopy. J. Microsc. 273, 3–25 (2019).
Dean, K. M. et al. Analysis of red-fluorescent proteins provides insight into dark-state conversion and photodegradation. Biophys. J. 101, 961–969 (2011).
Nixon-Abell, J. et al. Increased spatiotemporal resolution reveals highly dynamic dense tubular matrices in the peripheral ER. Science 354, aaf3928 (2016).
Luzio, J. P., Pryor, P. R. & Bright, N. A. Lysosomes: fusion and function. Nat. Rev. Mol. Cell Biol. 8, 622–632 (2007).
Lu, M. et al. The structure and global distribution of the endoplasmic reticulum network are actively regulated by lysosomes. Sci. Adv. 6, eabc7209 (2020).
Olzmann, J. A. & Carvalho, P. Dynamics and functions of lipid droplets. Nat. Rev. Mol. Cell Biol. 20, 137–155 (2019).
Mathiowetz, A. J. & Olzmann, J. A. Lipid droplets and cellular lipid flux. Nat. Cell Biol. 26, 331–345 (2024).
Valm, A. M. et al. Applying systems-level spectral imaging and analysis to reveal the organelle interactome. Nature 546, 162–167 (2017).
Zhang, M. et al. Cytosolic delivery of cell-impermeable fluorescent probes by mixtures of cell-penetrating peptides for multicolor long-term live-cell nanoscopy. Cell Rep. Phys. Sci. 4, 101674 (2023).
He, K., Zhang, X., Ren, S. & Sun, J. Deep residual learning for image recognition. In IEEE Conference on Computer Vision and Pattern Recognition (CVPR) 770–778 (IEEE, 2016).
Zhang, Y. et al. Image super-resolution using very deep residual channel attention networks. In European Conference on Computer Vision (ECCV) 286–301 (Springer, 2018).
Jin, L. et al. Deep learning enables structured illumination microscopy with low light levels and enhanced speed. Nat. Commun. 11, 1934 (2020).
Lowery, L. A. & Vactor, D. V. The trip of the tip: understanding the growth cone machinery. Nat. Rev. Mol. Cell Biol. 10, 332–343 (2009).
Wilson, C., Rozés-Salvador, V. & Cáceres, A. Protocol for evaluating neuronal polarity in murine models. STAR Protoc. 1, 100114 (2020).
Schelski, M. & Bradke, F. Microtubule retrograde flow retains neuronal polarization in a fluctuating state. Sci. Adv. 8, eabo2336 (2022).
Perkins, H. T. & Allan, V. Intertwined and finely balanced: endoplasmic reticulum morphology, dynamics, function, and diseases. Cells 10, 2341 (2021).
Schiavon, C. R. et al. Actin chromobody imaging reveals sub-organellar actin dynamics. Nat. Methods 17, 917–921 (2020).
Simon, C. et al. Actin dynamics drive cell-like membrane deformation. Nat. Phys. 15, 602–609 (2019).
Allard, A. et al. Actin modulates shape and mechanics of tubular membranes. Sci. Adv. 6, eaaz3050 (2020).
Mangeat, T. et al. Super-resolved live-cell imaging using random illumination microscopy. Cell Rep. Methods 1, 100009 (2021).
Hou, Y. 3I-SIM deep learning reconstruction dataset. Zenodo https://doi.org/10.5281/zenodo.14969641 (2025).
Acknowledgements
P.X. acknowledges funding support from the National Key R&D Program of China (2022YFC3401100) and the National Natural Science Foundation of China (62025501, 31971376 and 92150301). M.L. acknowledges the National Natural Science Foundation of China (62335008 and 62405010). We thank the National Center for Protein Sciences at Peking University in Beijing, China, for assistance with Oxford Instruments Imaris software. We thank the Optical Imaging Facility at the Chinese Institute for Brain Research in Beijing, China, for assistance with ZEISS Elyra 7.
Author information
Authors and Affiliations
Contributions
P.X., M.L. and Y.F. conceived the 3I-SIM imaging system. P.X. and M.L. supervised this project. Y.F. designed the optical path and built the 3I-SIM system. Y.H. and X.C. developed the analytical 3I-SIM reconstruction framework and codes. Y.H. developed the DL reconstruction framework and codes. Y.F. performed all the imaging experiments on biological structures. Q.L. helped deduce the polarization engineering. J.L. helped in preparing the hippocampal neurons. Q.G. and P.Z. helped in preparing the AC–ER sample and aided relevant discussions. D.K. and B.J. helped with the data analysis. Y.F. and Y.H. composed all the figures and videos. Y.F., Y.H., M.L. and P.X. wrote the manuscript with input from all authors. All authors are involved in the discussions of the results.
Corresponding authors
Ethics declarations
Competing interests
P.X., Y.F., Y.H., M.L. and Q.X. have filed a Chinese patent application (CN119310725A) on the presented framework. Also, P.X., Y.H., Y.F. and M.L. have filed a Chinese patent application (CN119477724A). The other authors declare no competing interests.
Peer review
Peer review information
Nature Photonics thanks Hui Li and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–21, Notes 1–4 and Tables 1–3.
Supplementary Video 1
Long-term imaging of actin filaments in a HUVEC cell for over an hour, continuously recording 5,900 time points at a temporal resolution of 1 Hz.
Supplementary Video 2
Continuous recording of the dynamic motion of the ER network at 14,000 time points with an ultrahigh spatiotemporal resolution of 1,697 Hz.
Supplementary Video 3
Role of ER during continuous movement and mutual contact with LEs or Lysos.
Supplementary Video 4
Dynamic interactions of microtubule and ER networks.
Supplementary Video 5
Visualizing the dynamic movement of LDs.
Supplementary Video 6
Long-term observation of hippocampal neurons.
Supplementary Video 7
Visualization of ER-associated actin dynamics at millisecond scales.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fu, Y., Hou, Y., Liang, Q. et al. Triangle-beam interference structured illumination microscopy. Nat. Photon. 19, 1122–1131 (2025). https://doi.org/10.1038/s41566-025-01730-0
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41566-025-01730-0