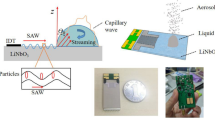
Abstract
Droplet microfluidics, a versatile technique for the precise manipulation of discrete droplets, has revolutionized biological and chemical research. So far, the successful implementation of droplet microfluidics necessitates the choice of non-wetting surfaces with minimal pinning forces, which hinders its broader adoptions in clinical applications. Here we report acousto-dewetting, a liquid dewetting principle that enables the three-dimensional, remotely controllable and precise operation of droplets on surfaces of any wettability, including superhydrophilic surfaces. This principle originates from the intricate interplay between acoustic streaming and droplet dynamics due to the extreme confinement of ultrasound within droplets, with an enhancement in pressure gradient of three orders of magnitude compared with traditional ultrasound-based approaches. We show that on superhydrophilic surfaces, acousto-dewetting achieves a contact line moving velocity that is two orders of magnitude higher than the previous limit and eliminates the undesired viscous film stemming from viscous dissipations. We developed a droplet microfluidics approach that achieves versatile droplet manipulation in various extreme scenarios associated with superhydrophilic surfaces, and applied it to an in vivo clinical setting for the rapid and safe removal of thrombus as well as drug delivery.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Source data are provided with this paper. All other data that support the plots within this paper and other findings of this study are available from the corresponding authors upon reasonable request.
References
Gach, P. C., Iwai, K., Kim, P. W., Hillson, N. J. & Singh, A. K. Droplet microfluidics for synthetic biology. Lab Chip 17, 3388–3400 (2017).
Lignos, I. et al. Synthesis of cesium lead halide perovskite nanocrystals in a droplet-based microfluidic platform: fast parametric space mapping. Nano Lett. 16, 1869–1877 (2016).
Yuan, Z. et al. Ultrasonic tweezer for multifunctional droplet manipulation. Sci. Adv. 9, eadg2352 (2023).
Chen, H. et al. Continuous directional water transport on the peristome surface of Nepenthes alata. Nature 532, 85–89 (2016).
Sun, Q. et al. Surface charge printing for programmed droplet transport. Nat. Mater. 18, 936–941 (2019).
Li, A. et al. Programmable droplet manipulation by a magnetic-actuated robot. Sci. Adv. 6, eaay5808 (2020).
Velev, O. D., Prevo, B. G. & Bhatt, K. H. On-chip manipulation of free droplets. Nature 426, 515–516 (2003).
Tang, X., Li, W. & Wang, L. Furcated droplet motility on crystalline surfaces. Nat. Nanotechnol. 16, 1106–1112 (2021).
Jiang, J. et al. Directional pumping of water and oil microdroplets on slippery surface. Proc. Natl Acad. Sci. USA 116, 2482–2487 (2019).
Kwon, G. et al. Visible light guided manipulation of liquid wettability on photoresponsive surfaces. Nat. Commun. 8, 14968 (2017).
Ding, X. et al. On-chip manipulation of single microparticles, cells, and organisms using surface acoustic waves. Proc. Natl Acad. Sci. USA 109, 11105–11109 (2012).
Ding, X. et al. Surface acoustic wave microfluidics. Lab Chip 13, 3626–3649 (2013).
Yang, S. et al. Harmonic acoustics for dynamic and selective particle manipulation. Nat. Mater. 21, 540–546 (2022).
Marzo, A. et al. Holographic acoustic elements for manipulation of levitated objects. Nat. Commun. 6, 8661 (2015).
Lo, W.-C., Fan, C.-H., Ho, Y.-J., Lin, C.-W. & Yeh, C.-K. Tornado-inspired acoustic vortex tweezer for trapping and manipulating microbubbles. Proc. Natl Acad. Sci. USA 118, e2023188118 (2021).
Marzo, A. & Drinkwater, B. W. Holographic acoustic tweezers. Proc. Natl Acad. Sci. USA 116, 84–89 (2019).
Gökçe, O., Castonguay, S., Temiz, Y., Gervais, T. & Delamarche, E. Self-coalescing flows in microfluidics for pulse-shaped delivery of reagents. Nature 574, 228–232 (2019).
Kaminski, T. S., Scheler, O. & Garstecki, P. Droplet microfluidics for microbiology: techniques, applications and challenges. Lab Chip 16, 2168–2187 (2016).
Gopinathan, K. A., Mishra, A., Mutlu, B. R., Edd, J. F. & Toner, M. A microfluidic transistor for automatic control of liquids. Nature 622, 735–741 (2023).
Wehner, M. et al. An integrated design and fabrication strategy for entirely soft, autonomous robots. Nature 536, 451–455 (2016).
Lv, P., Xue, Y., Shi, Y., Lin, H. & Duan, H. Metastable states and wetting transition of submerged superhydrophobic structures. Phys. Rev. Lett. 112, 196101 (2014).
Roach, P., Farrar, D. & Perry, C. C. Interpretation of protein adsorption: surface-induced conformational changes. J. Am. Chem. Soc. 127, 8168–8173 (2005).
Zhang, Y. & Nguyen, N.-T. Magnetic digital microfluidics—a review. Lab Chip 17, 994–1008 (2017).
Jiang, S. et al. Multifunctional Janus microplates arrays actuated by magnetic fields for water/light switches and bio‐inspired assimilatory coloration. Adv. Mater. 31, 1807507 (2019).
Wang, F. et al. Light-induced charged slippery surfaces. Sci. Adv. 8, eabp9369 (2022).
Ichimura, K., Oh, S.-K. & Nakagawa, M. Light-driven motion of liquids on a photoresponsive surface. Science 288, 1624–1626 (2000).
Wang, J. et al. Programmable wettability on photocontrolled graphene film. Sci. Adv. 4, eaat7392 (2018).
Xu, W. et al. Triboelectric wetting for continuous droplet transport. Sci. Adv. 8, eade2085 (2022).
Jin, Y. et al. Electrostatic tweezer for droplet manipulation. Proc. Natl Acad. Sci. USA 119, e2105459119 (2022).
Pollack, M. G., Shenderov, A. D. & Fair, R. B. Electrowetting-based actuation of droplets for integrated microfluidics. Lab Chip 2, 96–101 (2002).
Lamanna, J. et al. Digital microfluidic isolation of single cells for -Omics. Nat. Commun. 11, 5632 (2020).
Urbanski, J. P., Thies, W., Rhodes, C., Amarasinghe, S. & Thorsen, T. Digital microfluidics using soft lithography. Lab Chip 6, 96–104 (2006).
Zhang, P., Bachman, H., Ozcelik, A. & Huang, T. J. Acoustic microfluidics. Annu. Rev. Anal. Chem. 13, 17–43 (2020).
Eggers, J. Hydrodynamic theory of forced dewetting. Phys. Rev. Lett. 93, 094502 (2004).
De Gennes, P.-G., Brochard-Wyart, F. & Quéré, D. Gouttes, Bulles, Perles Et Ondes Vol. 159 (Belin Paris, 2005).
Shiri, S., Sinha, S., Baumgartner, D. A. & Cira, N. J. Thermal Marangoni flow impacts the shape of single component volatile droplets on thin, completely wetting substrates. Phys. Rev. Lett. 127, 024502 (2021).
Daniel, S., Chaudhury, M. K. & Chen, J. C. Fast drop movements resulting from the phase change on a gradient surface. Science 291, 633–636 (2001).
Liu, H.-C. et al. Wearable bioadhesive ultrasound shear wave elastography. Sci. Adv. 10, eadk8426 (2024).
Wang, C. & Zhao, X. See how your body works in real time—wearable ultrasound is on its way. Nature 630, 817–819 (2024).
Furmidge, C. Studies at phase interfaces. I. The sliding of liquid drops on solid surfaces and a theory for spray retention. J. Colloid. Sci. 17, 309–324 (1962).
Gao, N. et al. How drops start sliding over solid surfaces. Nat. Phys. 14, 191–196 (2018).
Mitchell, M. J. et al. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 20, 101–124 (2021).
Tohmyoh, H. Polymer acoustic matching layer for broadband ultrasonic applications. J. Acoust. Soc. Am. 120, 31–34 (2006).
Acknowledgements
S.L. acknowledges financial support from the National Natural Science Foundation of China under grant no. 62303321. Z.W. acknowledges financial support from the National Natural Science Foundation of China (nos. T2293694 and 52333015), Research Grants Council of Hong Kong (nos. SRFS2223-1S01, N_PolyU5172/24 and 15237824), the National Key Research and Development Program of China (no. 2023YFE0209900) and the Innovation and Technology Commission of Hong Kong (no. MHP/025/23).
Author information
Authors and Affiliations
Contributions
S.L. and Z.W. conceived the research. P.S. and S.L. designed the experiments. S.L., P.S. and M.W. prepared the samples. S.L., M.W., Y. Jia and J.L. developed the acoustic device. S.L., P.S., M.W., Y. Jiang, J.L., Y. Jia and Y.Y. performed the experiments. S.L. and Z.S. conducted the hydrodynamic simulations. All authors analysed the data. P.S., S.L. and Z.W. wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Physics thanks Ruediger Berger, Sascha Hilgenfeldt and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Substrate wettability used in existing droplet microfluidics.
Substrates with hydrophobicity, even super-hydrophobicity, are preferential to the reported droplet microfluidics. So far, only the electro-dewetting based droplet microfluidics is based on hydrophilic surface, but it is limited by the addition of surfactant to liquids and specific substrates.
Extended Data Fig. 2 Setup of ADW droplet microfluidics.
a, The integrated transducer has four functional layers, including a 50×50 electrode array, a piezoelectric ceramic, a ground electrode, and an acoustic impedance matching layer. b, Schematics illustrating the layout of ADW droplet microfluidics. The integrated transducer is bonded to the driving board through anisotropic conductive film (ACF); the solid substrate (petri dish), over which the droplet sits, is placed on the water at a distance of L away from the acoustic device. Not drawn to scale, for clarity. c, Setup of ADW droplet microfluidics. The acoustic device is placed at the bottom of the water tank atop the driving board; the driving board is controlled by wave modulation unit to output 2500-channel 2.32 MHz electrical square waves, whose phases are separately modulated; two cameras are orthogonally mounted to view the droplet; the top camera is used to localized the droplet in XY-plane for acoustic beam focusing; the lateral camera is used to measure the droplet contact angle; the system is fixed on a vibration-isolation plate.
Extended Data Fig. 3 Fabrication process of the acoustic device.
a, step I-II, Spin coat photoresist AZ5214 on the back side of naked \(1{\rm{mm}}\) thick PZT. Step III, Use ultraviolet lithography to photoetch electrode array pattern onto AZ5214 and develop. Step IV-V, Sputter 10 nm titanium and 100 nm Nickle onto PZT in sequence. Immersing the PZT in acetone for 5 mins under ultrasonic treatment to remove redundant metal and photoresist, resulting in the formation of the electrode array. Step VI, Sputter 20 nm titanium, 100 nm Nickle and 20 nm titanium on the top side of PZT in sequence. Step VII, Spin coat SU-8 on the top electrode and ensure the matching layer thickness is 310 μm. Finally, the acoustic device is coated by 5 μm thick Parylene for waterproofing. Not drawn to scale. b-c, Simulation results of the acoustic energy transmission coefficient \({{\rm{T}}}_{1}\) from acoustic device to propagation medium (water) versus SU-8 matching layer thickness and acoustic energy transmission coefficient \({{\rm{T}}}_{2}\) from propagation medium (water) to droplet versus substrate thickness, respectively. The overall acoustic energy transmission ratio from acoustic device to droplet is \({\rm{T}}={{\rm{T}}}_{1}\times {{\rm{T}}}_{2}\).
Extended Data Fig. 4 Streaming flow characterization.
a, Schematic showing the experimental setup of PIV characterization. b-c, Simulation (b) and PIV (c) results showing the streaming flow inside droplet.
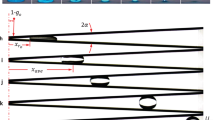
Extended Data Fig. 5 Plot of the angle of meniscus against time t, nondimensionalized by τ.
The error band corresponds to the standard deviation of the measurements.
Extended Data Fig. 6 Experiments of time scale mismatch and Fs characterization.
a, Timescale mismatch results in ultrasound escape from droplet. b, Experiment setup under which \({F}_{s}\) dominates the kinetics of a solid particle to verify the magnitude of \({F}_{s}\). c, High-speed imaging of the kinetics of a solid particle (PS), using which we can calculate the force magnitude of \({F}_{s}\).
Extended Data Fig. 7 Acousto-dewetting and acousto-dewetting based droplet manipulation on a hydrophilic surface.
The surface has an apparent contact angle of 16° and contact angle hysteresis of 44.1° (advancing angle of 50° and receding angle of 5.9°).
Extended Data Fig. 8 Rehabilitation of rats after treated by ADW droplet microfluidics.
a-b, Recovery state of rats in control group (a) and treated group (b). Scale bar, 1 cm. c-d, Recovery state of blood flows for (c) control group and (d) ADW droplet microfluidics treated group. Scale bar, 2 mm.
Extended Data Fig. 9 Biosafety of ADW droplet microfluidics and Assessment of the side effect of ADW droplet microfluidics for thrombus removal.
a, H&E staining of the organ tissues (kidney, heart, liver and stomach) after exposure to 2.75 MPa (at a driving voltage of 10 Vpeak-to-peak) ultrasound for 5 min, compared with the control group. b, H&E staining of the metabolic organ liver after thrombus removal by using ADW droplet microfluidics. c, Drug plasma concentration at the thrombus (n = 4) treated by intravenous injection (IV) and ADW droplet microfluidics. Statistical analysis was performed by two-sided t test; ns, not significant; *P < 0.05; **P ≤ 0.01; ***P ≤ 0.001; ****P ≤ 0.0001. Scale bar, 200 μm.
Extended Data Fig. 10 Anti-bacterial drug transport in a rat stomach by using ADW microfluidics.
a, A droplet dissolved with anti-bacterial drugs is controlled to move in a rat stomach. Scale bar, 3 mm. b, Selective growth of H. pylori bacteria from stomach of treated (left) group and control (right, untreated) group at 1:10000 dilution. Scale bar, 1 cm. c, Quantitative analysis of the bacterial in the stomach of treatment (left) group and control (right) group (n = 4). Statistical analysis was performed by two-sided t test; ns, not significant; *P < 0.05; **P ≤ 0.01; ***P ≤ 0.001; ****P ≤ 0.0001.
Supplementary information
Supplementary Information
Supplementary Notes 1–5, captions for Supplementary Videos 1–6, Methods and References.
Supplementary Video 1
Overview of ADW droplet microfluidics. The surface used in demonstrating acousto-dewetting is a plasma-treated PS plate with an apparent contact angle of 60° and contact angle hysteresis of 16° (advancing angle of 68° and receding angle of 52°).
Supplementary Video 2
Kinetics of acousto-dewetting on a superhydrophilic surface.
Supplementary Video 3
Acousto-dewetting-based droplet manipulation in contrast to an external-force-based method. For the latter, gravity is in the same direction as the movement of liquid and for the former, gravity is perpendicular to the substrate.
Supplementary Video 4
Acousto-dewetting-based droplet manipulation in various scenarios. The substrates used are plasma-treated PS plates with an apparent contact angle of 60° and contact angle hysteresis of 16° (advancing angle of 68° and receding angle of 52°). Droplet splitting is demonstrated on a superhydrophilic surface (similar to that mentioned in Fig. 2). This is achieved by simultaneously generating two ultrasound beams moving in opposite directions by using our integrated transducer.
Supplementary Video 5
Application of ADW droplet microfluidics for thrombus removal in vivo.
Supplementary Video 6
Application of ADW droplet microfluidics for drug delivery in vivo.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 5
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, S., Sun, P., Wang, M. et al. Acousto-dewetting enables droplet microfluidics on superhydrophilic surfaces. Nat. Phys. 21, 808–816 (2025). https://doi.org/10.1038/s41567-025-02844-6
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41567-025-02844-6
This article is cited by
-
Droplets beamed up
Nature Physics (2025)