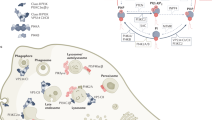
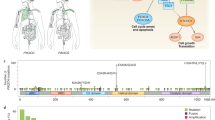
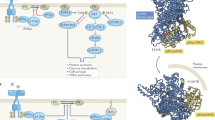
Abstract
Phosphoinositide kinases, extending beyond the well-known phosphoinositide 3-kinase (PI3K), are key players in the dynamic and site-specific phosphorylation of lipid phosphoinositides. Unlike PI3Ks, phosphatidylinositol 4-kinases (PI4Ks) and phosphatidylinositol phosphate kinases (PIPKs) do not usually exhibit mutational alterations, but mostly show altered expression in tumours, orchestrating a broad spectrum of signalling, metabolic and immune processes, all of which are crucial in the pathogenesis of cancer. Dysregulation of PI4Ks and PIPKs has been associated with various malignancies, which has sparked considerable interest towards their therapeutic targeting. In this Review we summarize the current understanding of the lesser-studied phosphoinositide kinase families, PI4K and PIPK, focusing on their functions and relevance in cancer. In addition, we provide an overview of ongoing efforts driving the preclinical and clinical development of phosphoinositide kinase-targeting molecules.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Morrow, A. A. et al. The lipid kinase PI4KIIIβ is highly expressed in breast tumors and activates Akt in cooperation with Rab11a. Mol. Cancer Res. 12, 1492–1508 (2014).
Kamalesh, K. et al. Phosphatidylinositol 5-phosphate 4-kinase regulates early endosomal dynamics during clathrin-mediated endocytosis. J. Cell Sci. 130, 2119–2133 (2017).
Sharma, S., Mathre, S., Ramya, V., Shinde, D. & Raghu, P. Phosphatidylinositol 5 phosphate 4-kinase regulates plasma-membrane PIP(3) turnover and insulin signaling. Cell Rep. 27, 1979–1990.e1977 (2019).
Shin, Y. J. et al. PIP4K2A as a negative regulator of PI3K in PTEN-deficient glioblastoma. J. Exp. Med. 216, 1120–1134 (2019).
Behari, J. et al. Conserved RNA binding activity of phosphatidyl inositol 5-phosphate 4-kinase (PIP4K2A). Front. Mol. Biosci. 8, 631281 (2021).
Li, C., Yoon, B., Stefani, G. & Slack, F. J. Lipid kinase PIP5K1A regulates let-7 microRNA biogenesis through interacting with nuclear export protein XPO5. Nucleic Acids Res. 51, 9849–9862 (2023).
Chao, W.-T., Daquinag, A. C., Ashcroft, F. & Kunz, J. Type I PIPK-α regulates directed cell migration by modulating Rac1 plasma membrane targeting and activation. J. Cell Biol. 190, 247–262 (2010).
Lacalle, R. A. et al. Type I phosphatidylinositol 4-phosphate 5-kinase controls neutrophil polarity and directional movement. J. Cell Biol. 179, 1539–1553 (2007).
Wang, D. G. et al. PIP4Ks suppress insulin signaling through a catalytic-independent mechanism. Cell Rep. 27, 1991–2001.e1995 (2019).
Vanhaesebroeck, B., Perry, M. W. D., Brown, J. R., André, F. & Okkenhaug, K. PI3K inhibitors are finally coming of age. Nat. Rev. Drug Discov. 20, 741–769 (2021).
Hoxhaj, G. & Manning, B. D. The PI3K–AKT network at the interface of oncogenic signalling and cancer metabolism. Nat. Rev. Cancer 20, 74–88 (2020).
Fruman, D. A. & Rommel, C. PI3K and cancer: lessons, challenges and opportunities. Nat. Rev. Drug Discov. 13, 140–156 (2014).
Janku, F., Yap, T. A. & Meric-Bernstam, F. Targeting the PI3K pathway in cancer: are we making headway? Nat. Rev. Clin. Oncol. 15, 273–291 (2018).
Vasan, N. & Cantley, L. C. At a crossroads: how to translate the roles of PI3K in oncogenic and metabolic signalling into improvements in cancer therapy. Nat. Rev. Clin. Oncol. 19, 471–485 (2022).
Engelman, J. A. Targeting PI3K signalling in cancer: opportunities, challenges and limitations. Nat. Rev. Cancer 9, 550–562 (2009).
Boura, E. & Nencka, R. Phosphatidylinositol 4-kinases: function, structure, and inhibition. Exp. Cell Res. 337, 136–145 (2015).
Dornan, G. L., McPhail, J. A. & Burke, J. E. Type III phosphatidylinositol 4 kinases: structure, function, regulation, signalling and involvement in disease. Biochem. Soc. Trans. 44, 260–266 (2016).
Whitman, M., Downes, C. P., Keeler, M., Keller, T. & Cantley, L. Type I phosphatidylinositol kinase makes a novel inositol phospholipid, phosphatidylinositol-3-phosphate. Nature 332, 644–646 (1988).
Hutter, C. & Zenklusen, J. C. The Cancer Genome Atlas: creating lasting value beyond its data. Cell 173, 283–285 (2018).
Huang, X., Cao, Y., Bao, P., Zhu, B. & Cheng, Z. High expression of PI4K2A predicted poor prognosis of colon adenocarcinoma (COAD) and correlated with immunity. Cancer Med. 12, 837–851 (2023).
Waugh, M. G. Amplification of chromosome 1q genes encoding the phosphoinositide signalling enzymes PI4KB, AKT3, PIP5K1A and PI3KC2B in breast cancer. J. Cancer 5, 790–796 (2014).
Pataer, A. et al. Therapeutic targeting of the PI4K2A/PKR lysosome network is critical for misfolded protein clearance and survival in cancer cells. Oncogene 39, 801–813 (2020).
Ilboudo, A. et al. Overexpression of phosphatidylinositol 4-kinase type IIIα is associated with undifferentiated status and poor prognosis of human hepatocellular carcinoma. BMC Cancer 14, 7 (2014).
Jiang, X. et al. Targeting PI4KA sensitizes refractory leukemia to chemotherapy by modulating the ERK/AMPK/OXPHOS axis. Theranostics 12, 6972–6988 (2022).
Semenas, J. et al. The role of PI3K/AKT-related PIP5K1α and the discovery of its selective inhibitor for treatment of advanced prostate cancer. Proc. Natl Acad. Sci. USA 111, E3689–E3698 (2014).
Jiang, L. et al. Rupatadine inhibits colorectal cancer cell proliferation through the PIP5K1A/Akt/CDK2 pathway. Biomed. Pharmacother. 176, 116826 (2024).
Sun, Y. et al. Type I gamma phosphatidylinositol phosphate kinase modulates invasion and proliferation and its expression correlates with poor prognosis in breast cancer. Breast Cancer Res. 12, R6 (2010).
Sarwar, M. et al. The role of PIP5K1α/pAKT and targeted inhibition of growth of subtypes of breast cancer using PIP5K1α inhibitor. Oncogene 38, 375–389 (2019).
Peng, W. et al. Type Iγ phosphatidylinositol phosphate kinase promotes tumor growth by facilitating Warburg effect in colorectal cancer. eBioMedicine 44, 375–386 (2019).
Li, H. et al. Smurf1 regulates lung cancer cell growth and migration through interaction with and ubiquitination of PIPKIγ. Oncogene 36, 5668–5680 (2017).
Lima, K. et al. PIP4K2A and PIP4K2C transcript levels are associated with cytogenetic risk and survival outcomes in acute myeloid leukemia. Cancer Genet. 233-234, 56–66 (2019).
Zhang, S. et al. Regulatory network and prognostic effect investigation of PIP4K2A in leukemia and solid cancers. Front. Genet. https://doi.org/10.3389/fgene.2018.00721 (2019).
Wang, J. et al. Hsa_circ_0007099 and PIP4K2A coexpressed in diffuse large B-cell lymphoma with clinical significance. Genes Dis. 11, 101056 (2024).
Walsh, K. M. et al. Novel childhood ALL susceptibility locus BMI1–PIP4K2A is specifically associated with the hyperdiploid subtype. Blood 121, 4808–4809 (2013).
Xu, H. et al. Novel susceptibility variants at 10p12.31–12.2 for childhood acute lymphoblastic leukemia in ethnically diverse populations. J. Natl Cancer Inst. 105, 733–742 (2013).
Liao, F. et al. Association between PIP4K2A polymorphisms and acute lymphoblastic leukemia susceptibility. Medicine 95, e3542 (2016).
Migliorini, G. et al. Variation at 10p12.2 and 10p14 influences risk of childhood B-cell acute lymphoblastic leukemia and phenotype. Blood 122, 3298–3307 (2013).
Keune, W. J. et al. Low PIP4K2B expression in human breast tumors correlates with reduced patient survival: a role for PIP4K2B in the regulation of E-cadherin expression. Cancer Res. 73, 6913–6925 (2013).
Li, J. et al. PI4KIIα is a novel regulator of tumor growth by its action on angiogenesis and HIF-1α regulation. Oncogene 29, 2550–2559 (2010).
Bura, A., Čabrijan, S., Đurić, I., Bruketa, T. & Jurak Begonja, A. A plethora of functions condensed into tiny phospholipids: the story of PI4P and PI(4,5)P(2). Cells https://doi.org/10.3390/cells1210141 (2023).
Li, G. et al. Research progress on phosphatidylinositol 4-kinase inhibitors. Biochem. Pharmacol. 220, 115993 (2024).
Balla, A. & Balla, T. Phosphatidylinositol 4-kinases: old enzymes with emerging functions. Trends Cell Biol. 16, 351–361 (2006).
Mandal, K. Review of PIP2 in cellular signaling, functions and diseases. Int. J. Mol. Sci. https://doi.org/10.3390/ijms21218342 (2020).
Mayer, I. A. & Arteaga, C. L. The PI3K/AKT pathway as a target for cancer treatment. Annu. Rev. Med. 67, 11–28 (2016).
Burke, J. E. Structural basis for regulation of phosphoinositide kinases and their involvement in human disease. Mol. Cell 71, 653–673 (2018).
Tan, J. & Brill, J. A. Cinderella story: PI4P goes from precursor to key signaling molecule. Crit. Rev. Biochem. Mol. Biol. 49, 33–58 (2014).
de Rubio, R. G. et al. Phosphatidylinositol 4-phosphate is a major source of GPCR-stimulated phosphoinositide production. Sci. Signal. https://doi.org/10.1126/scisignal.aan1210 (2018).
Delage, E., Puyaubert, J., Zachowski, A. & Ruelland, E. Signal transduction pathways involving phosphatidylinositol 4-phosphate and phosphatidylinositol 4,5-bisphosphate: convergences and divergences among eukaryotic kingdoms. Prog. Lipid Res. 52, 1–14 (2013).
Waugh, M. G. The Great Escape: how phosphatidylinositol 4-kinases and PI4P promote vesicle exit from the Golgi (and drive cancer). Biochem. J. 476, 2321–2346 (2019).
Clayton, E. L., Minogue, S. & Waugh, M. G. Mammalian phosphatidylinositol 4-kinases as modulators of membrane trafficking and lipid signaling networks. Prog. Lipid Res. 52, 294–304 (2013).
Kuna, R. S. & Field, S. J. GOLPH3: a Golgi phosphatidylinositol(4)phosphate effector that directs vesicle trafficking and drives cancer. J. Lipid Res. 60, 269–275 (2019).
Barlow-Busch, I., Shaw, A. L. & Burke, J. E. PI4KA and PIKfyve: essential phosphoinositide signaling enzymes involved in myriad human diseases. Curr. Opin. Cell Biol. 83, 102207 (2023).
Mesmin, B. et al. A four-step cycle driven by PI(4)P hydrolysis directs sterol/PI(4)P exchange by the ER–Golgi tether OSBP. Cell 155, 830–843 (2013).
Hanada, K. et al. Molecular machinery for non-vesicular trafficking of ceramide. Nature 426, 803–809 (2003).
Posor, Y., Jang, W. & Haucke, V. Phosphoinositides as membrane organizers. Nat. Rev. Mol. Cell Biol. 23, 797–816 (2022).
Barylko, B. et al. A novel family of phosphatidylinositol 4-kinases conserved from yeast to humans. J. Biol. Chem. 276, 7705–7708 (2001).
Jung, G. et al. Stabilization of phosphatidylinositol 4-kinase type IIβ by interaction with Hsp90. J. Biol. Chem. 286, 12775–12784 (2011).
Banerji, S. et al. Oxysterol binding protein-dependent activation of sphingomyelin synthesis in the golgi apparatus requires phosphatidylinositol 4-kinase IIα. Mol. Biol. Cell 21, 4141–4150 (2010).
Wang, H. et al. GABARAPs regulate PI4P-dependent autophagosome:lysosome fusion. Proc. Natl Acad. Sci. USA 112, 7015–7020 (2015).
Alli-Balogun, G. O. et al. Phosphatidylinositol 4-kinase IIβ negatively regulates invadopodia formation and suppresses an invasive cellular phenotype. Mol. Biol. Cell 27, 4033–4042 (2016).
Li, J. et al. Dual inhibition of EGFR at protein and activity level via combinatorial blocking of PI4KIIα as anti-tumor strategy. Protein Cell 5, 457–468 (2014).
Minogue, S. et al. Phosphatidylinositol 4-kinase is required for endosomal trafficking and degradation of the EGF receptor. J. Cell Sci. 119, 571–581 (2006).
Isaji, T. et al. A complex between phosphatidylinositol 4-kinase IIα and integrin α3β1 is required for N-glycan sialylation in cancer cells. J. Biol. Chem. 294, 4425–4436 (2019).
Tan, J. X. & Finkel, T. A phosphoinositide signalling pathway mediates rapid lysosomal repair. Nature 609, 815–821 (2022).
Hämälistö, S. & Jäättelä, M. Lysosomes in cancer-living on the edge (of the cell). Curr. Opin. Cell Biol. 39, 69–76 (2016).
Ebner, M. et al. Nutrient-regulated control of lysosome function by signaling lipid conversion. Cell 186, 5328–5346.e5326 (2023).
Chen, Y., Barylko, B., Eichorst, J. P., Mueller, J. D. & Albanesi, J. P. Identification of the GABARAP binding determinant in PI4K2A. Biosci. Rep. https://doi.org/10.1042/bsr20240200 (2024).
Albanesi, J., Wang, H., Sun, H. Q., Levine, B. & Yin, H. GABARAP-mediated targeting of PI4K2A/PI4KIIα to autophagosomes regulates PtdIns4P-dependent autophagosome–lysosome fusion. Autophagy 11, 2127–2129 (2015).
Baba, T., Toth, D. J., Sengupta, N., Kim, Y. J. & Balla, T. Phosphatidylinositol 4,5-bisphosphate controls Rab7 and PLEKHM1 membrane cycling during autophagosome–lysosome fusion. EMBO J. 38, e100312 (2019).
Liu, H. et al. PtdIns4P exchange at endoplasmic reticulum–autolysosome contacts is essential for autophagy and neuronal homeostasis. Autophagy 19, 2682–2701 (2023).
Craige, B., Salazar, G. & Faundez, V. Phosphatidylinositol-4-kinase type II alpha contains an AP-3-sorting motif and a kinase domain that are both required for endosome traffic. Mol. Biol. Cell 19, 1415–1426 (2008).
Goul, C. S. & Zoncu, R. PITTching in for lysosome repair. Dev. Cell 57, 2347–2349 (2022).
Chauhan, N. & Patro, B. S. Emerging roles of lysosome homeostasis (repair, lysophagy and biogenesis) in cancer progression and therapy. Cancer Lett. 584, 216599 (2024).
Tan, X. et al. EMT-activated secretory and endocytic vesicular trafficking programs underlie a vulnerability to PI4K2A antagonism in lung cancer. J. Clin. Invest. https://doi.org/10.1172/jci165863 (2023).
Mazzocca, A., Liotta, F. & Carloni, V. Tetraspanin CD81-regulated cell motility plays a critical role in intrahepatic metastasis of hepatocellular carcinoma. Gastroenterology 135, 244–256.e241 (2008).
Ketel, K. et al. A phosphoinositide conversion mechanism for exit from endosomes. Nature 529, 408–412 (2016).
Liu, D. A. et al. A phosphoinositide switch mediates exocyst recruitment to multivesicular endosomes for exosome secretion. Nat. Commun. 14, 6883 (2023).
Bojjireddy, N. et al. Pharmacological and genetic targeting of the PI4KA enzyme reveals its important role in maintaining plasma membrane phosphatidylinositol 4-phosphate and phosphatidylinositol 4,5-bisphosphate levels. J. Biol. Chem. 289, 6120–6132 (2014).
Hammond, G. R. et al. PI4P and PI(4,5)P2 are essential but independent lipid determinants of membrane identity. Science 337, 727–730 (2012).
D’Angelo, G., Vicinanza, M., Di Campli, A. & De Matteis, M. A. The multiple roles of PtdIns(4)P — not just the precursor of PtdIns(4,5)P2. J. Cell Sci. 121, 1955–1963 (2008).
Rahajeng, J. et al. Efficient Golgi forward trafficking requires GOLPH3-driven, PI4P-dependent membrane curvature. Dev. Cell 50, 573–585.e575 (2019).
Tan, X. et al. PI4KIIIβ is a therapeutic target in chromosome 1q-amplified lung adenocarcinoma. Sci. Transl. Med. https://doi.org/10.1126/scitranslmed.aax3772 (2020).
Shi, L. et al. Addiction to Golgi-resident PI4P synthesis in chromosome 1q21.3-amplified lung adenocarcinoma cells. Proc. Natl Acad. Sci. USA https://doi.org/10.1073/pnas.2023537118 (2021).
Berger, K. L., Kelly, S. M., Jordan, T. X., Tartell, M. A. & Randall, G. Hepatitis C virus stimulates the phosphatidylinositol 4-kinase IIIα-dependent phosphatidylinositol 4-phosphate production that is essential for its replication. J. Virol. 85, 8870–8883 (2011).
Kwon, J. et al. Targeting phosphatidylinositol 4-kinase IIIα for radiosensitization: a potential model of drug repositioning using an anti-hepatitis C viral agent. Int. J. Radiat. Oncol. Biol. Phys. 96, 867–876 (2016).
Park, Y., Park, J. M., Kim, D. H., Kwon, J. & Kim, I. A. Inhibition of PI4K IIIα radiosensitizes in human tumor xenograft and immune-competent syngeneic murine tumor model. Oncotarget 8, 110392–110405 (2017).
Kattan, W. E. et al. Components of the phosphatidylserine endoplasmic reticulum to plasma membrane transport mechanism as targets for KRAS inhibition in pancreatic cancer. Proc. Natl Acad. Sci. USA https://doi.org/10.1073/pnas.2114126118 (2021).
Chung, J. et al. PI4P/phosphatidylserine countertransport at ORP5- and ORP8-mediated ER-plasma membrane contacts. Science 349, 428–432 (2015).
Adhikari, H. et al. Oncogenic KRAS is dependent upon an EFR3A–PI4KA signaling axis for potent tumorigenic activity. Nat. Commun. 12, 5248 (2021).
Gajardo, T. et al. Actin dynamics regulation by TTC7A/PI4KIIIα limits DNA damage and cell death under confinement. J. Allergy Clin. Immunol. 152, 949–960 (2023).
Kakar, R., Ghosh, C. & Sun, Y. Phosphoinositide signaling in immune cell migration. Biomolecules https://doi.org/10.3390/biom13121705 (2023).
Ren, C. et al. Leukocyte cytoskeleton polarization is initiated by plasma membrane curvature from cell attachment. Dev. Cell 49, 206–219.e207 (2019).
Govindarajan, B. et al. Adaptor proteins mediate CXCR4 and PI4KA crosstalk in prostate cancer cells and the significance of PI4KA in bone tumor growth. Sci. Rep. 13, 20634 (2023).
Sbrissa, D. et al. A novel cross-talk between CXCR4 and PI4KIIIα in prostate cancer cells. Oncogene 38, 332–344 (2019).
Curtis, C. et al. The genomic and transcriptomic architecture of 2,000 breast tumours reveals novel subgroups. Nature 486, 346–352 (2012).
Mesquita, B. et al. Frequent copy number gains at 1q21 and 1q32 are associated with overexpression of the ETS transcription factors ETV3 and ELF3 in breast cancer irrespective of molecular subtypes. Breast Cancer Res. Treat. 138, 37–45 (2013).
de Graaf, P. et al. Phosphatidylinositol 4-kinaseβ is critical for functional association of rab11 with the Golgi complex. Mol. Biol. Cell 15, 2038–2047 (2004).
Tokuda, E. et al. Phosphatidylinositol 4-phosphate in the Golgi apparatus regulates cell–cell adhesion and invasive cell migration in human breast cancer. Cancer Res. 74, 3054–3066 (2014).
Sechi, S., Frappaolo, A., Karimpour-Ghahnavieh, A., Piergentili, R. & Giansanti, M. G. Oncogenic roles of GOLPH3 in the physiopathology of cancer. Int. J. Mol. Sci. https://doi.org/10.3390/ijms21030933 (2020).
Rong, Y. et al. Clathrin and phosphatidylinositol-4,5-bisphosphate regulate autophagic lysosome reformation. Nat. Cell Biol. 14, 924–934 (2012).
Sridhar, S. et al. The lipid kinase PI4KIIIβ preserves lysosomal identity. EMBO J. 32, 324–339 (2013).
Judith, D. et al. ATG9A shapes the forming autophagosome through Arfaptin 2 and phosphatidylinositol 4-kinase IIIβ. J. Cell Biol. 218, 1634–1652 (2019).
Luteijn, R. D. et al. The activation of the adaptor protein STING depends on its interactions with the phospholipid PI4P. Sci. Signal. 17, eade3643 (2024).
Wang, R., Hussain, A., Guo, Q. & Ma, M. cGAS–STING at the crossroads in cancer therapy. Crit. Rev. Oncol. Hematol. 193, 104194 (2024).
Llorente, A., Arora, G. K., Grenier, S. F. & Emerling, B. M. PIP kinases: a versatile family that demands further therapeutic attention. Adv. Biol. Regul. 87, 100939 (2023).
Rameh, L. E., Tolias, K. F., Duckworth, B. C. & Cantley, L. C. A new pathway for synthesis of phosphatidylinositol-4,5-bisphosphate. Nature 390, 192–196 (1997).
van den Bout, I. & Divecha, N. PIP5K-driven PtdIns(4,5)P2 synthesis: regulation and cellular functions. J. Cell Sci. 122, 3837–3850 (2009).
Rameh, L. E. & Blind, R. D. 25 Years of PI5P. Front. Cell Dev. Biol. 11, 1272911 (2023).
Bulley, S. J., Clarke, J. H., Droubi, A., Giudici, M.-L. & Irvine, R. F. Exploring phosphatidylinositol 5-phosphate 4-kinase function. Adv. Biol. Regul. 57, 193–202 (2015).
Zhang, X. et al. Phosphatidylinositol-4-phosphate 5-kinase isozymes catalyze the synthesis of 3-phosphate-containing phosphatidylinositol signaling molecules. J. Biol. Chem. 272, 17756–17761 (1997).
Halstead, J. R. et al. A novel pathway of cellular phosphatidylinositol(3,4,5)-trisphosphate synthesis is regulated by oxidative stress. Curr. Biol. 11, 386–395 (2001).
Sbrissa, D., Ikonomov, O. C., Deeb, R. & Shisheva, A. Phosphatidylinositol 5-phosphate biosynthesis is linked to PIKfyve and is involved in osmotic response pathway in mammalian cells. J. Biol. Chem. 277, 47276–47284 (2002).
Kunz, J. et al. The activation loop of phosphatidylinositol phosphate kinases determines signaling specificity. Mol. Cell 5, 1–11 (2000).
Kunz, J., Fuelling, A., Kolbe, L. & Anderson, R. A. Stereo-specific substrate recognition by phosphatidylinositol phosphate kinases is swapped by changing a single amino acid residue. J. Biol. Chem. 277, 5611–5619 (2002).
Liu, A., Sui, D., Wu, D. & Hu, J. The activation loop of PIP5K functions as a membrane sensor essential for lipid substrate processing. Sci. Adv. 2, e1600925 (2016).
Wills, R. C. & Hammond, G. R. V. PI(4,5)P2: signaling the plasma membrane. Biochem. J. 479, 2311–2325 (2022).
Hansen, S. B. Lipid agonism: the PIP2 paradigm of ligand-gated ion channels. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 1851, 620–628 (2015).
Kwiatkowska, K. One lipid, multiple functions: how various pools of PI(4,5)P2 are created in the plasma membrane. Cell. Mol. Life Sci. 67, 3927–3946 (2010).
Thapa, N., Choi, S., Tan, X., Wise, T. & Anderson, R. A. Phosphatidylinositol phosphate 5-kinase Iγ and phosphoinositide 3-kinase/Akt signaling couple to promote oncogenic growth. J. Biol. Chem. 290, 18843–18854 (2015).
Cao, S. et al. Silencing of type Iγ phosphatidylinositol phosphate kinase suppresses ovarian cancer cell proliferation, migration and invasion. Oncol. Rep. 38, 253–262 (2017).
Tran, M. H. et al. NEDD4-induced degradative ubiquitination of phosphatidylinositol 4-phosphate 5-kinase α and its implication in breast cancer cell proliferation. J. Cell. Mol. Med. 22, 4117–4129 (2018).
Choi, S. et al. Agonist-stimulated phosphatidylinositol-3,4,5-trisphosphate generation by scaffolded phosphoinositide kinases. Nat. Cell Biol. 18, 1324–1335 (2016).
Zhang, H. et al. LRRC8A as a central mediator promotes colon cancer metastasis by regulating PIP5K1B/PIP2 pathway. Biochim. Biophys. Acta Mol. Basis Dis. 1870, 167066 (2024).
Larsson, P. et al. The functional interlink between AR and MMP9/VEGF signaling axis is mediated through PIP5K1α/pAKT in prostate cancer. Int. J. Cancer 146, 1686–1699 (2020).
Larsson, P. F. et al. FcγRIIIa receptor interacts with androgen receptor and PIP5K1α to promote growth and metastasis of prostate cancer. Mol. Oncol. 16, 2496–2517 (2022).
Wang, T. et al. PIP5K1α is required for promoting tumor progression in castration-resistant prostate cancer. Front. Cell Dev. Biol. 10, 798590 (2022).
Semenas, J. et al. Targeted inhibition of ERα signaling and PIP5K1α/Akt pathways in castration-resistant prostate cancer. Mol. Oncol. 15, 968–986 (2021).
Adhikari, H. & Counter, C. M. Interrogating the protein interactomes of RAS isoforms identifies PIP5K1A as a KRAS-specific vulnerability. Nat. Commun. 9, 3646 (2018).
Choi, S., Chen, M., Cryns, V. L. & Anderson, R. A. A nuclear phosphoinositide kinase complex regulates p53. Nat. Cell Biol. 21, 462–475 (2019).
Chen, M. et al. A p53–phosphoinositide signalosome regulates nuclear AKT activation. Nat. Cell Biol. 24, 1099–1113 (2022).
Balla, A., Tuymetova, G., Barshishat, M., Geiszt, M. & Balla, T. Characterization of type II phosphatidylinositol 4-kinase isoforms reveals association of the enzymes with endosomal vesicular compartments. J. Biol. Chem. 277, 20041–20050 (2002).
Bairstow, S. F. et al. Type Iγ661 phosphatidylinositol phosphate kinase directly interacts with AP2 and regulates endocytosis. J. Biol. Chem. 281, 20632–20642 (2006).
Coppolino, M. G. et al. Inhibition of phosphatidylinositol-4-phosphate 5-kinase Iα impairs localized actin remodeling and suppresses phagocytosis. J. Biol. Chem. 277, 43849–43857 (2002).
Mao, Y. S. et al. Essential and unique roles of PIP5K-γ and -α in Fcγ receptor-mediated phagocytosis. J. Cell Biol. 184, 281–296 (2009).
Szymańska, E., Korzeniowski, M., Raynal, P., Sobota, A. & Kwiatkowska, K. Contribution of PIP-5 kinase Iα to raft-based FcγRIIA signaling. Exp. Cell Res. 315, 981–995 (2009).
Xu, Q. et al. Phosphatidylinositol phosphate kinase PIPKIγ and phosphatase INPP5E coordinate initiation of ciliogenesis. Nat. Commun. 7, 10777 (2016).
Li, L. et al. Cdk5-mediated phosphorylation regulates phosphatidylinositol 4-phosphate 5-kinase type I γ 90 activity and cell invasion. FASEB J. 33, 631–642 (2019).
Jafari, N. et al. p70S6K1 (S6K1)-mediated phosphorylation regulates phosphatidylinositol 4-phosphate 5-kinase type I γ degradation and cell invasion. J. Biol. Chem. 291, 25729–25741 (2016).
Peng, J. M., Lin, S. H., Yu, M. C. & Hsieh, S. Y. CLIC1 recruits PIP5K1A/C to induce cell-matrix adhesions for tumor metastasis. J. Clin. Invest. https://doi.org/10.1172/jci133525 (2021).
Sun, Y., Ling, K., Wagoner, M. P. & Anderson, R. A. Type Iγ phosphatidylinositol phosphate kinase is required for EGF-stimulated directional cell migration. J. Cell Biol. 178, 297–308 (2007).
Ni, W. et al. SUMOylation is required for PIPK1γ-driven keratinocyte migration and growth. FEBS J. 286, 4709–4720 (2019).
Durand, N. et al. Protein kinase D1 regulates focal adhesion dynamics and cell adhesion through phosphatidylinositol-4-phosphate 5-kinase type-l γ. Sci. Rep. 6, 35963 (2016).
Li, X. et al. Ubiquitylation of phosphatidylinositol 4-phosphate 5-kinase type I γ by HECTD1 regulates focal adhesion dynamics and cell migration. J. Cell Sci. 126, 2617–2628 (2013).
Wu, Z. et al. PIPKIγ regulates focal adhesion dynamics and colon cancer cell invasion. PLoS ONE 6, e24775 (2011).
Thapa, N., Tan, X., Choi, S., Wise, T. & Anderson, R. A. PIPKIγ and talin couple phosphoinositide and adhesion signaling to control the epithelial to mesenchymal transition. Oncogene 36, 899–911 (2017).
Thapa, N. et al. Phosphoinositide signaling regulates the exocyst complex and polarized integrin trafficking in directionally migrating cells. Dev. Cell 22, 116–130 (2012).
Ling, K. et al. Type Iγ phosphatidylinositol phosphate kinase modulates adherens junction and E-cadherin trafficking via a direct interaction with mu 1B adaptin. J. Cell Biol. 176, 343–353 (2007).
Sun, Y., Hedman, A. C., Tan, X., Schill, N. J. & Anderson, R. A. Endosomal type Iγ PIP 5-kinase controls EGF receptor lysosomal sorting. Dev. Cell 25, 144–155 (2013).
Chen, C. et al. EGFR-induced phosphorylation of type Iγ phosphatidylinositol phosphate kinase promotes pancreatic cancer progression. Oncotarget 8, 42621–42637 (2017).
Chen, C. et al. Targeting type Iγ phosphatidylinositol phosphate kinase inhibits breast cancer metastasis. Oncogene 34, 4635–4646 (2015).
Sarwar, M. et al. Targeted suppression of AR-V7 using PIP5K1α inhibitor overcomes enzalutamide resistance in prostate cancer cells. Oncotarget 7, 63065–63081 (2016).
Kawase, A. et al. Decrease in multidrug resistance-associated protein 2 activities by knockdown of phosphatidylinositol 4-phosphate 5-kinase in hepatocytes and cancer cells. J. Pharm. Pharm. Sci. 22, 576–584 (2019).
Iorns, E., Lord, C. J. & Ashworth, A. Parallel RNAi and compound screens identify the PDK1 pathway as a target for tamoxifen sensitization. Biochem. J. 417, 361–370 (2009).
Sun, Y. et al. CircPIP5K1A serves as a competitive endogenous RNA contributing to ovarian cancer progression via regulation of miR-661/IGFBP5 signaling. J. Cell Biochem. 120, 19406–19414 (2019).
Feng, N. et al. Circ_PIP5K1A regulates cisplatin resistance and malignant progression in non-small cell lung cancer cells and xenograft murine model via depending on miR-493-5p/ROCK1 axis. Respir. Res. 22, 248 (2021).
Zheng, K. et al. CircRNA PIP5K1A promotes the progression of glioma through upregulation of the TCF12/PI3K/AKT pathway by sponging miR-515-5p. Cancer Cell Int. 21, 27 (2021).
Zhang, Q. et al. Circular RNA PIP5K1A promotes colon cancer development through inhibiting miR-1273a. World J. Gastroenterol. 25, 5300–5309 (2019).
Binmadi, N. O., Proia, P., Zhou, H., Yang, Y.-H. & Basile, J. R. Rho-mediated activation of PI(4)P5K and lipid second messengers is necessary for promotion of angiogenesis by semaphorin 4D. Angiogenesis 14, 309–319 (2011).
Xue, J. et al. PIPKIγ regulates CCL2 expression in colorectal cancer by activating AKT–STAT3 signaling. J. Immunol. Res. 2019, 3690561 (2019).
Gawden-Bone, C. M. et al. PIP5 kinases regulate membrane phosphoinositide and actin composition for targeted granule secretion by cytotoxic lymphocytes. Immunity 49, 427–437.e424 (2018).
Xue, J. et al. Type Iγ phosphatidylinositol phosphate kinase regulates PD-L1 expression by activating NF-κB. Oncotarget 8, 42414–42427 (2017).
Ghosh, C. et al. Type I gamma phosphatidylinositol phosphate 5-kinase i5 controls cell sensitivity to interferon. Dev. Cell 59, 1028–1042.e1025 (2024).
Lokuta, M. A. et al. Type Iγ PIP kinase is a novel uropod component that regulates rear retraction during neutrophil chemotaxis. Mol. Biol. Cell 18, 5069–5080 (2007).
Bultsma, Y., Keune, W.-J. & Divecha, N. PIP4Kβ interacts with and modulates nuclear localization of the high-activity PtdIns5P-4-kinase isoform PIP4Kα. Biochem. J. 430, 223–235 (2010).
Clarke, J. H. & Irvine, R. F. Evolutionarily conserved structural changes in phosphatidylinositol 5-phosphate 4-kinase (PI5P4K) isoforms are responsible for differences in enzyme activity and localization. Biochem. J. 454, 49–57 (2013).
Sumita, K. et al. The lipid kinase PI5P4Kβ is an intracellular GTP sensor for metabolism and tumorigenesis. Mol. Cell 61, 187–198 (2016).
Takeuchi, K. et al. The GTP responsiveness of PI5P4Kβ evolved from a compromised trade-off between activity and specificity. Structure 30, 886–899.e884 (2022).
Wills, R. C. et al. A novel homeostatic mechanism tunes PI(4,5)P2-dependent signaling at the plasma membrane. J. Cell Sci. https://doi.org/10.1242/jcs.261494 (2023).
Llorente, A., Loughran, R. M. & Emerling, B. M. Targeting phosphoinositide signaling in cancer: relevant techniques to study lipids and novel avenues for therapeutic intervention. Front. Cell Dev. Biol. 11, 1297355 (2023).
Bulley, S. J. et al. In B cells, phosphatidylinositol 5-phosphate 4-kinase–α synthesizes PI(4,5)P2 to impact mTORC2 and Akt signaling. Proc. Natl Acad. Sci. USA 113, 10571–10576 (2016).
Gupta, A. et al. Phosphatidylinositol 5-phosphate 4-kinase (PIP4K) regulates TOR signaling and cell growth during Drosophila development. Proc. Natl Acad. Sci. USA 110, 5963–5968 (2013).
Lamia, K. A. et al. Increased insulin sensitivity and reduced adiposity in phosphatidylinositol 5-phosphate 4-kinase β−/− mice. Mol. Cell Biol. 24, 5080–5087 (2004).
Carricaburu, V. et al. The phosphatidylinositol (PI)-5-phosphate 4-kinase type II enzyme controls insulin signaling by regulating PI-3,4,5-trisphosphate degradation. Proc. Natl Acad. Sci. USA 100, 9867–9872 (2003).
Lundquist, M. R. et al. Phosphatidylinositol-5-phosphate 4-kinases regulate cellular lipid metabolism by facilitating autophagy. Mol. Cell 70, 531–544.e539 (2018).
Mackey, A. M., Sarkes, D. A., Bettencourt, I., Asara, J. M. & Rameh, L. E. PIP4kγ is a substrate for mTORC1 that maintains basal mTORC1 signaling during starvation. Sci. Signal. 7, ra104 (2014).
Jude, J. G. et al. A targeted knockdown screen of genes coding for phosphoinositide modulators identifies PIP4K2A as required for acute myeloid leukemia cell proliferation and survival. Oncogene 34, 1253–1262 (2015).
Lima, K. et al. The PIP4K2 inhibitor THZ-P1-2 exhibits antileukemia activity by disruption of mitochondrial homeostasis and autophagy. Blood Cancer J. 12, 151 (2022).
Lima, K. et al. Potency and efficacy of pharmacological PIP4K2 inhibitors in acute lymphoblastic leukemia. Eur. J. Pharmacol. 977, 176723 (2024).
Lima, K. et al. Pharmacological inhibition of PIP4K2 potentiates venetoclax-induced apoptosis in acute myeloid leukemia. Int. J. Mol. Sci. 24, 16899 (2023).
Triscott, J. et al. PI5P4Kα supports prostate cancer metabolism and exposes a survival vulnerability during androgen receptor inhibition. Sci. Adv. 9, eade8641 (2023).
Triscott, J. et al. Loss of PI5P4Kα Slows the Progression of a Pten Mutant Basal Cell Model of Prostate Cancer. Mol. Cancer Res. 23, 33–45 (2025).
Rogava, M. et al. Loss of Pip4k2c confers liver-metastatic organotropism through insulin-dependent PI3K-AKT pathway activation. Nat. Cancer https://doi.org/10.1038/s43018-023-00704-x (2024).
Nie, D. et al. Metabolic enzyme SLC27A5 regulates PIP4K2A pre-mRNA splicing as a noncanonical mechanism to suppress hepatocellular carcinoma metastasis. Adv. Sci. 11, e2305374 (2024).
Jones, D. R. et al. Nuclear PtdIns5P as a transducer of stress signaling: an in vivo role for PIP4Kβ. Mol. Cell 23, 685–695 (2006).
Jones, D. R., Foulger, R., Keune, W. J., Bultsma, Y. & Divecha, N. PtdIns5P is an oxidative stress-induced second messenger that regulates PKB activation. FASEB J. 27, 1644–1656 (2013).
Bua, D. J., Martin, G. M., Binda, O. & Gozani, O. Nuclear phosphatidylinositol-5-phosphate regulates ING2 stability at discrete chromatin targets in response to DNA damage. Sci. Rep. 3, 2137 (2013).
Keune, W. J. et al. Regulation of phosphatidylinositol-5-phosphate signaling by Pin1 determines sensitivity to oxidative stress. Sci. Signal. 5, ra86 (2012).
Gelato, K. A. et al. Accessibility of different histone H3-binding domains of UHRF1 is allosterically regulated by phosphatidylinositol 5-phosphate. Mol. Cell 54, 905–919 (2014).
Stijf-Bultsma, Y. et al. The basal transcription complex component TAF3 transduces changes in nuclear phosphoinositides into transcriptional output. Mol. Cell 58, 453–467 (2015).
Emerling, B. M. et al. Depletion of a putatively druggable class of phosphatidylinositol kinases inhibits growth of p53-null tumors. Cell 155, 844–857 (2013).
Palamiuc, L. et al. Hippo and PI5P4K signaling intersect to control the transcriptional activation of YAP. Sci. Signal. 17, eado6266 (2024).
Luoh, S. W., Venkatesan, N. & Tripathi, R. Overexpression of the amplified Pip4k2β gene from 17q11–12 in breast cancer cells confers proliferation advantage. Oncogene 23, 1354–1363 (2004).
Terkelsen, T. et al. High-throughput proteomics of breast cancer interstitial fluid: identification of tumor subtype-specific serologically relevant biomarkers. Mol. Oncol. 15, 429–461 (2021).
Hu, A. et al. PIP4K2A regulates intracellular cholesterol transport through modulating PI(4,5)P2 homeostasis. J. Lipid Res. 59, 507–514 (2018).
Loughran, R. M. et al. Noncanonical PI(4,5)P2 coordinates lysosome positioning through cholesterol trafficking. Preprint at bioRxiv https://doi.org/10.1101/2025.01.02.629779 (2025).
Ravi, A. et al. PI5P4Ks drive metabolic homeostasis through peroxisome-mitochondria interplay. Dev. Cell 56, 1661–1676 e1610 (2021).
Zheng, L. & Conner, S. D. PI5P4Kγ functions in DTX1-mediated Notch signaling. Proc. Natl Acad. Sci. USA 115, E1983–e1990 (2018).
Shi, Q. et al. Notch signaling pathway in cancer: from mechanistic insights to targeted therapies. Signal. Transduct. Target. Ther. 9, 128 (2024).
Shim, H. et al. Deletion of the gene Pip4k2c, a novel phosphatidylinositol kinase, results in hyperactivation of the immune system. Proc. Natl Acad. Sci. USA 113, 7596–7601 (2016).
Poli, A. et al. PIP4Ks impact on PI3K, FOXP3, and UHRF1 signaling and modulate human regulatory T cell proliferation and immunosuppressive activity. Proc. Natl Acad. Sci. USA 118, e2010053118 (2021).
Lees, J. A., Li, P., Kumar, N., Weisman, L. S. & Reinisch, K. M. Insights into lysosomal PI(3,5)P(2) homeostasis from a structural-biochemical analysis of the PIKfyve lipid kinase complex. Mol. Cell 80, 736–743.e734 (2020).
Shisheva, A. PIKfyve: partners, significance, debates and paradoxes. Cell Biol. Int. 32, 591–604 (2008).
Hayakawa, A. et al. Structural basis for endosomal targeting by FYVE domains. J. Biol. Chem. 279, 5958–5966 (2004).
Sbrissa, D., Ikonomov, O. C. & Shisheva, A. PIKfyve lipid kinase is a protein kinase: downregulation of 5′-phosphoinositide product formation by autophosphorylation. Biochemistry 39, 15980–15989 (2000).
Rivero-Ríos, P. & Weisman, L. S. Roles of PIKfyve in multiple cellular pathways. Curr. Opin. Cell Biol. 76, 102086 (2022).
Ikonomov, O. C. et al. The phosphoinositide kinase PIKfyve is vital in early embryonic development: preimplantation lethality of PIKfyve−/− embryos but normality of PIKfyve+/− mice*. J. Biol. Chem. 286, 13404–13413 (2011).
Takasuga, S. et al. Critical roles of type III phosphatidylinositol phosphate kinase in murine embryonic visceral endoderm and adult intestine. Proc. Natl Acad. Sci. USA 110, 1726–1731 (2013).
Zolov, S. N. et al. In vivo, Pikfyve generates PI(3,5)P2, which serves as both a signaling lipid and the major precursor for PI5P. Proc. Natl Acad. Sci. USA 109, 17472–17477 (2012).
Ikonomov, O. C., Sbrissa, D. & Shisheva, A. Mammalian cell morphology and endocytic membrane homeostasis require enzymatically active phosphoinositide 5-kinase PIKfyve. J. Biol. Chem. 276, 26141–26147 (2001).
Bissig, C., Hurbain, I., Raposo, G. & van Niel, G. PIKfyve activity regulates reformation of terminal storage lysosomes from endolysosomes. Traffic 18, 747–757 (2017).
Choy, C. H. et al. Lysosome enlargement during inhibition of the lipid kinase PIKfyve proceeds through lysosome coalescence. J. Cell Sci. https://doi.org/10.1242/jcs.213587 (2018).
Yordanov, T. E. et al. Biogenesis of lysosome-related organelles complex-1 (BORC) regulates late endosomal/lysosomal size through PIKfyve-dependent phosphatidylinositol-3,5-bisphosphate. Traffic 20, 674–696 (2019).
Ikonomov, O. C., Sbrissa, D. & Shisheva, A. Localized PtdIns 3,5-P2 synthesis to regulate early endosome dynamics and fusion. Am. J. Physiol. Cell Physiol. 291, C393–C404 (2006).
Sbrissa, D. et al. Core protein machinery for mammalian phosphatidylinositol 3,5-bisphosphate synthesis and turnover that regulates the progression of endosomal transport. Novel Sac phosphatase joins the ArPIKfyve–PIKfyve complex. J. Biol. Chem. 282, 23878–23891 (2007).
Krishna, S. et al. PIKfyve regulates vacuole maturation and nutrient recovery following engulfment. Dev. Cell 38, 536–547 (2016).
Jefferies, H. B. J. et al. A selective PIKfyve inhibitor blocks PtdIns(3,5)P2 production and disrupts endomembrane transport and retroviral budding. EMBO Rep. 9, 164–170-170 (2008).
de Lartigue, J. et al. PIKfyve regulation of endosome-linked pathways. Traffic 10, 883–893 (2009).
Kerr, M. C. et al. Inhibition of the PtdIns(5) kinase PIKfyve disrupts intracellular replication of Salmonella. EMBO J. 29, 1331–1347 (2010).
Klein, A. D., Petruzzi, K. L., Lee, C. & Overholtzer, M. Stress-induced microautophagy is coordinated with lysosome biogenesis and regulated by PIKfyve. Mol. Biol. Cell 35, ar70 (2024).
Ikonomov, O. C., Sbrissa, D. & Shisheva, A. Small molecule PIKfyve inhibitors as cancer therapeutics: translational promises and limitations. Toxicol. Appl. Pharmacol. 383, 114771 (2019).
Gayle, S. et al. Identification of apilimod as a first-in-class PIKfyve kinase inhibitor for treatment of B-cell non-Hodgkin lymphoma. Blood 129, 1768–1778 (2017).
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/show/NCT02594384 (2015).
Hou, J. Z. et al. Inhibition of PIKfyve using YM201636 suppresses the growth of liver cancer via the induction of autophagy. Oncol. Rep. 41, 1971–1979 (2019).
Sharma, G. et al. A family of PIKFYVE inhibitors with therapeutic potential against autophagy-dependent cancer cells disrupt multiple events in lysosome homeostasis. Autophagy 15, 1694–1718 (2019).
Ikonomov, O. C. et al. Kinesin adapter JLP links PIKfyve to microtubule-based endosome-to-trans-Golgi network traffic of furin. J. Biol. Chem. 284, 3750–3761 (2009).
Rutherford, A. C. et al. The mammalian phosphatidylinositol 3-phosphate 5-kinase (PIKfyve) regulates endosome-to-TGN retrograde transport. J. Cell Sci. 119, 3944–3957 (2006).
Giridharan, S. S. P. et al. Lipid kinases VPS34 and PIKfyve coordinate a phosphoinositide cascade to regulate retriever-mediated recycling on endosomes. eLife 11, e69709 (2022).
Stallaert, W. et al. Contact inhibitory Eph signaling suppresses EGF-promoted cell migration by decoupling EGFR activity from vesicular recycling. Sci. Signal. https://doi.org/10.1126/scisignal.aat0114 (2018).
McCartney, A. J. et al. Activity-dependent PI(3,5)P2 synthesis controls AMPA receptor trafficking during synaptic depression. Proc. Natl Acad. Sci. USA 111, E4896–E4905 (2014).
Kim, J. et al. The phosphoinositide kinase PIKfyve mediates epidermal growth factor receptor trafficking to the nucleus. Cancer Res. 67, 9229–9237 (2007).
Er, E. E., Mendoza, M. C., Mackey, A. M., Rameh, L. E. & Blenis, J. AKT facilitates EGFR trafficking and degradation by phosphorylating and activating PIKfyve. Sci. Signal. 6, ra45 (2013).
Ferrarone, J. R. et al. Genome-wide CRISPR screens in spheroid culture reveal that the tumor suppressor LKB1 inhibits growth via the PIKFYVE lipid kinase. Proc. Natl Acad. Sci. USA 121, e2403685121 (2024).
Tsuruta, F., Green, E. M., Rousset, M. & Dolmetsch, R. E. PIKfyve regulates CaV1.2 degradation and prevents excitotoxic cell death. J. Cell Biol. 187, 279–294 (2009).
She, J. et al. Structural insights into the voltage and phospholipid activation of the mammalian TPC1 channel. Nature 556, 130–134 (2018).
She, J. et al. Structural mechanisms of phospholipid activation of the human TPC2 channel. eLife 8, e45222 (2019).
Dong, X. P. et al. PI(3,5)P(2) controls membrane trafficking by direct activation of mucolipin Ca2+ release channels in the endolysosome. Nat. Commun. 1, 38 (2010).
Sopjani, M. et al. Regulation of the Ca2+ channel TRPV6 by the kinases SGK1, PKB/Akt, and PIKfyve. J. Membr. Biol. 233, 35–41 (2010).
Shen, J. et al. Deficiency of MIP/MTMR14 phosphatase induces a muscle disorder by disrupting Ca2+ homeostasis. Nat. Cell Biol. 11, 769–776 (2009).
Touchberry, C. D. et al. Phosphatidylinositol 3,5-bisphosphate (PI(3,5)P2) potentiates cardiac contractility via activation of the ryanodine receptor. J. Biol. Chem. 285, 40312–40321 (2010).
Seebohm, G. et al. Regulation of endocytic recycling of KCNQ1/KCNE1 potassium channels. Circ. Res. 100, 686–692 (2007).
Gehring, E. M. et al. PIKfyve upregulates CFTR activity. Biochem. Biophys. Res. Commun. 390, 952–957 (2009).
Klaus, F. et al. PIKfyve-dependent regulation of the Cl- channel ClC-2. Biochem. Biophys. Res. Commun. 381, 407–411 (2009).
Saffi, G. T. et al. INPP4B promotes PDAC aggressiveness via PIKfyve and TRPML-1-mediated lysosomal exocytosis. J. Cell Biol. https://doi.org/10.1083/jcb.202401012 (2024).
Oppelt, A. et al. PIKfyve, MTMR3 and their product PtdIns5P regulate cancer cell migration and invasion through activation of Rac1. Biochem. J. 461, 383–390 (2014).
Dupuis-Coronas, S. et al. The nucleophosmin-anaplastic lymphoma kinase oncogene interacts, activates, and uses the kinase PIKfyve to increase invasiveness*. J. Biol. Chem. 286, 32105–32114 (2011).
Ikonomov, O. C., Sbrissa, D., Mlak, K. & Shisheva, A. Requirement for PIKfyve enzymatic activity in acute and long-term insulin cellular effects. Endocrinology 143, 4742–4754 (2002).
Shisheva, A., Rusin, B., Ikonomov, O. C., DeMarco, C. & Sbrissa, D. Localization and insulin-regulated relocation of phosphoinositide 5-kinase PIKfyve in 3T3-L1 adipocytes*. J. Biol. Chem. 276, 11859–11869 (2001).
Ikonomov, O. C., Sbrissa, D., Delvecchio, K., A. Rillema, J. & Shisheva, A. Unexpected severe consequences of Pikfyve deletion by aP2- or Aq-promoter-driven Cre expression for glucose homeostasis and mammary gland development. Physiol. Rep. 4, e12812 (2016).
Bao, Y. et al. Targeting the lipid kinase PIKfyve upregulates surface expression of MHC class I to augment cancer immunotherapy. Proc. Natl Acad. Sci. USA 120, e2314416120 (2023).
Qiao, Y. et al. Autophagy inhibition by targeting PIKfyve potentiates response to immune checkpoint blockade in prostate cancer. Nat. Cancer 2, 978–993 (2021).
Jiang, J. et al. TRIM68, PIKFYVE, and DYNLL2: the possible novel autophagy- and immunity-associated gene biomarkers for osteosarcoma prognosis. Front. Oncol. 11, 643104 (2021).
Min, S. H. et al. Loss of PIKfyve in platelets causes a lysosomal disease leading to inflammation and thrombosis in mice. Nat. Commun. 5, 4691 (2014).
Cai, X. et al. PIKfyve, a class III PI kinase, is the target of the small molecular IL-12/IL-23 inhibitor apilimod and a player in Toll-like receptor signaling. Chem. Biol. 20, 912–921 (2013).
Cai, X., Xu, Y., Kim, Y.-M., Loureiro, J. & Huang, Q. PIKfyve, a class III lipid kinase, is required for TLR-induced type I IFN production via modulation of ATF3. J. Immunol. 192, 3383–3389 (2014).
Choi, J. E. et al. PIKfyve, expressed by CD11c-positive cells, controls tumor immunity. Nat. Commun. 15, 5487 (2024).
Dayam, R. M. et al. The lipid kinase PIKfyve coordinates the neutrophil immune response through the activation of the Rac GTPase. J. Immunol. 199, 2096–2105 (2017).
Kim, G. H. E., Dayam, R. M., Prashar, A., Terebiznik, M. & Botelho, R. J. PIKfyve inhibition interferes with phagosome and endosome maturation in macrophages. Traffic 15, 1143–1163 (2014).
Baranov, M. V. et al. The phosphoinositide kinase PIKfyve promotes cathepsin-S-mediated major histocompatibility complex class II antigen presentation. iScience 11, 160–177 (2019).
Li, J. et al. PI-273, a substrate-competitive, specific small-molecule inhibitor of PI4KIIα, inhibits the growth of breast cancer cells. Cancer Res. 77, 6253–6266 (2017).
Sinha, R. K., Patel, R. Y., Bojjireddy, N., Datta, A. & Subrahmanyam, G. Epigallocatechin gallate (EGCG) inhibits type II phosphatidylinositol 4-kinases: a key component in pathways of phosphoinositide turnover. Arch. Biochem. Biophys. 516, 45–51 (2011).
Srivastava, R. et al. Resveratrol inhibits type II phosphatidylinositol 4-kinase: a key component in pathways of phosphoinositide turn over. Biochem. Pharmacol. 70, 1048–1055 (2005).
Bojjireddy, N., Sinha, R. K. & Subrahmanyam, G. Piperine inhibits type II phosphatidylinositol 4-kinases: a key component in phosphoinositides turnover. Mol. Cell Biochem. 393, 9–15 (2014).
Bojjireddy, N., Sinha, R. K., Panda, D. & Subrahmanyam, G. Sanguinarine suppresses IgE induced inflammatory responses through inhibition of type II PtdIns 4-kinase(s). Arch. Biochem. Biophys. 537, 192–197 (2013).
Simons, J. P. et al. Loss of phosphatidylinositol 4-kinase 2α activity causes late onset degeneration of spinal cord axons. Proc. Natl Acad. Sci. USA 106, 11535–11539 (2009).
Lamarche, M. J. et al. Anti-hepatitis C virus activity and toxicity of type III phosphatidylinositol-4-kinase beta inhibitors. Antimicrob. Agents Chemother. 56, 5149–5156 (2012).
van der Schaar, H. M. et al. A novel, broad-spectrum inhibitor of enterovirus replication that targets host cell factor phosphatidylinositol 4-kinase IIIβ. Antimicrob. Agents Chemother. 57, 4971–4981 (2013).
Spickler, C. et al. Phosphatidylinositol 4-kinase III beta is essential for replication of human rhinovirus and its inhibition causes a lethal phenotype in vivo. Antimicrob. Agents Chemother. 57, 3358–3368 (2013).
Burke, J. E., Triscott, J., Emerling, B. M. & Hammond, G. R. V. Beyond PI3Ks: targeting phosphoinositide kinases in disease. Nat. Rev. Drug Discov. 22, 357–386 (2023).
Neumann-Mufweba, A., Kimani, S., Khan, S. F., Chibale, K. & Prince, S. The diaryl-imidazopyridazine anti-plasmodial compound, MMV652103, exhibits anti-breast cancer activity. EXCLI J. 21, 656–679 (2022).
Andrews, D. M. et al. Identification and optimization of a novel series of selective PIP5K inhibitors. Bioorg. Med. Chem. 54, 116557 (2022).
Arora, G. K., Palamiuc, L. & Emerling, B. M. Expanding role of PI5P4Ks in cancer: a promising druggable target. FEBS Lett. 596, 3–16 (2022).
Teng, M. et al. Targeting the dark lipid kinase PIP4K2C with a potent and selective binder and degrader. Angew. Chem. Int. Ed. 62, e202302364 (2023).
US National Library of Medicine. ClinicalTrials.gov https://www.clinicaltrials.gov/study/NCT03456804 (2018).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT04159896 (2019).
Li, C. et al. Discovery of a first-in-class degrader for the lipid kinase PIKfyve. J. Med. Chem. 66, 12432–12445 (2023).
Ji, W. et al. Development of potent and selective degraders of PI5P4Kγ. Eur. J. Med. Chem. 247, 115027 (2023).
Corse, E. et al. Abstract 5574: degradation of PIP4K2C by novel bivalent functional degrader LRK-A induces tumor regression in CRC. Cancer Res. 84, 5574–5574 (2024).
Ijuin, T. Phosphoinositide phosphatases in cancer cell dynamics — beyond PI3K and PTEN. Semin. Cancer Biol. 59, 50–65 (2019).
Mössinger, J. et al. Phosphatidylinositol 4-kinase IIα function at endosomes is regulated by the ubiquitin ligase Itch. EMBO Rep. 13, 1087–1094 (2012).
Wieffer, M. et al. PI4K2β/AP-1-based TGN-endosomal sorting regulates Wnt signaling. Curr. Biol. 23, 2185–2190 (2013).
Wang, Y. J. et al. Phosphatidylinositol 4 phosphate regulates targeting of clathrin adaptor AP-1 complexes to the Golgi. Cell 114, 299–310 (2003).
Nakatsu, F. et al. PtdIns4P synthesis by PI4KIIIα at the plasma membrane and its impact on plasma membrane identity. J. Cell Biol. 199, 1003–1016 (2012).
Baskin, J. M. et al. The leukodystrophy protein FAM126A (hyccin) regulates PtdIns(4)P synthesis at the plasma membrane. Nat. Cell Biol. 18, 132–138 (2016).
Wu, X. et al. Structural insights into assembly and regulation of the plasma membrane phosphatidylinositol 4-kinase complex. Dev. Cell 28, 19–29 (2014).
Burke, J. E. et al. Structures of PI4KIIIβ complexes show simultaneous recruitment of Rab11 and its effectors. Science 344, 1035–1038 (2014).
Klima, M. et al. Structural insights and in vitro reconstitution of membrane targeting and activation of human PI4KB by the ACBD3 protein. Sci. Rep. 6, 23641 (2016).
McPhail, J. A., Ottosen, E. H., Jenkins, M. L. & Burke, J. E. The molecular basis of Aichi Virus 3A protein activation of phosphatidylinositol 4 kinase IIIβ, PI4KB, through ACBD3. Structure 25, 121–131 (2017).
Hausser, A. et al. Protein kinase D regulates vesicular transport by phosphorylating and activating phosphatidylinositol-4 kinase IIIβ at the Golgi complex. Nat. Cell Biol. 7, 880–886 (2005).
Chalupska, D. et al. Structural analysis of phosphatidylinositol 4-kinase IIIβ (PI4KB)–14-3-3 protein complex reveals internal flexibility and explains 14-3-3 mediated protection from degradation in vitro. J. Struct. Biol. 200, 36–44 (2017).
Valente, C. et al. A 14-3-3γ dimer-based scaffold bridges CtBP1-S/BARS to PI(4)KIIIβ to regulate post-Golgi carrier formation. Nat. Cell Biol. 14, 343–354 (2012).
Weernink, P. A. et al. Activation of type I phosphatidylinositol 4-phosphate 5-kinase isoforms by the Rho GTPases, RhoA, Rac1, and Cdc42. J. Biol. Chem. 279, 7840–7849 (2004).
Honda, A. et al. Phosphatidylinositol 4-phosphate 5-kinase α is a downstream effector of the small G protein ARF6 in membrane ruffle formation. Cell 99, 521–532 (1999).
Divecha, N. et al. Type I PIPkinases interact with and are regulated by the retinoblastoma susceptibility gene product-pRB. Curr. Biol. 12, 582–587 (2002).
Pan, W. et al. Wnt3a-mediated formation of phosphatidylinositol 4,5-bisphosphate regulates LRP6 phosphorylation. Science 321, 1350–1353 (2008).
Krauss, M., Kukhtina, V., Pechstein, A. & Haucke, V. Stimulation of phosphatidylinositol kinase type I-mediated phosphatidylinositol (4,5)-bisphosphate synthesis by AP-2μ–cargo complexes. Proc. Natl Acad. Sci. USA 103, 11934–11939 (2006).
Luo, B., Prescott, S. M. & Topham, M. K. Diacylglycerol kinase ζ regulates phosphatidylinositol 4-phosphate 5-kinase Iα by a novel mechanism. Cell Signal. 16, 891–897 (2004).
Divecha, N. et al. Interaction of the type Iα PIPkinase with phospholipase D: a role for the local generation of phosphatidylinositol 4, 5-bisphosphate in the regulation of PLD2 activity. EMBO J. 19, 5440–5449 (2000).
Halstead, J. R. et al. Rac controls PIP5K localisation and PtdIns(4,5)P2 synthesis, which modulates vinculin localisation and neurite dynamics. J. Cell Sci. 123, 3535–3546 (2010).
Saito, K. et al. BTK regulates PtdIns-4,5-P2 synthesis: importance for calcium signaling and PI3K activity. Immunity 19, 669–678 (2003).
Ling, K., Doughman, R. L., Firestone, A. J., Bunce, M. W. & Anderson, R. A. Type Iγ phosphatidylinositol phosphate kinase targets and regulates focal adhesions. Nature 420, 89–93 (2002).
Sun, M., Cai, J., Anderson, R. A. & Sun, Y. Type Iγ phosphatidylinositol phosphate 5-kinase i5 controls the ubiquitination and degradation of the tumor suppressor mitogen-inducible gene 6. J. Biol. Chem. 291, 21461–21473 (2016).
Rossin, A. et al. The Btk-dependent PIP5K1γ lipid kinase activation by Fas counteracts FasL-induced cell death. Apoptosis 22, 1344–1352 (2017).
Nakano-Kobayashi, A. et al. Role of activation of PIP5Kγ661 by AP-2 complex in synaptic vesicle endocytosis. EMBO J. 26, 1105–1116 (2007).
Kahlfeldt, N. et al. Molecular basis for association of PIPKIγ-p90 with clathrin adaptor AP-2*. J. Biol. Chem. 285, 2734–2749 (2010).
Di Paolo, G. et al. Recruitment and regulation of phosphatidylinositol phosphate kinase type 1γ by the FERM domain of talin. Nature 420, 85–89 (2002).
Hinchliffe, K. A., Ciruela, A., Letcher, A. J., Divecha, N. & Irvine, R. F. Regulation of type IIα phosphatidylinositol phosphate kinase localisation by the protein kinase CK2. Curr. Biol. 9, 983–986 (1999).
Ling, K. Tyrosine phosphorylation of type Iγ phosphatidylinositol phosphate kinase by Src regulates an integrin–talin switch. J. Cell Biol. 163, 1339–1349 (2003).
Hinchliffe, K. A. & Irvine, R. F. Regulation of type II PIP kinase by PKD phosphorylation. Cell Signal. 18, 1906–1913 (2006).
Zhang, L., Zhang, H., Agborbesong, E., Zhou, J. X. & Li, X. Phosphorylation of MIF by PIP4K2a is necessary for cilia biogenesis. Cell Death Dis. 14, 795 (2023).
Bunce, M. W., Boronenkov, I. V. & Anderson, R. A. Coordinated activation of the nuclear ubiquitin ligase Cul3-SPOP by the generation of phosphatidylinositol 5-phosphate. J. Biol. Chem. 283, 8678–8686 (2008).
Yang, S. et al. MANF regulates hypothalamic control of food intake and body weight. Nat. Commun. 8, 579 (2017).
Ikonomov, O. C. et al. Active PIKfyve associates with and promotes the membrane attachment of the late endosome-to-trans-Golgi network transport factor Rab9 effector p40. J. Biol. Chem. 278, 50863–50871 (2003).
Berwick, D. C. et al. Protein kinase B phosphorylation of PIKfyve regulates the trafficking of GLUT4 vesicles. J. Cell Sci. 117, 5985–5993 (2004).
Karabiyik, C., Vicinanza, M., Son, S. M. & Rubinsztein, D. C. Glucose starvation induces autophagy via ULK1-mediated activation of PIKfyve in an AMPK-dependent manner. Dev. Cell 56, 1961–1975.e1965 (2021).
Liu, Y. et al. Phosphatidylinositol 3-phosphate 5-kinase (PIKfyve) is an AMPK target participating in contraction-stimulated glucose uptake in skeletal muscle. Biochem. J. 455, 195–206 (2013).
Vaidya, A. & Perry, C. M. Simeprevir: first global approval. Drugs 73, 2093–2106 (2013).
Graham, T. R. & Burd, C. G. Coordination of Golgi functions by phosphatidylinositol 4-kinases. Trends Cell Biol. 21, 113–121 (2011).
McPhail, J. A. & Burke, J. E. Molecular mechanisms of PI4K regulation and their involvement in viral replication. Traffic 24, 131–145 (2023).
Anitei, M. et al. Spatiotemporal control of lipid conversion, actin-based mechanical forces, and curvature sensors during clathrin/AP-1-coated vesicle biogenesis. Cell Rep. 20, 2087–2099 (2017).
Mellman, D. L. et al. A PtdIns4,5P2-regulated nuclear poly(A) polymerase controls expression of select mRNAs. Nature 451, 1013–1017 (2008).
Doughman, R. L., Firestone, A. J., Wojtasiak, M. L., Bunce, M. W. & Anderson, R. A. Membrane ruffling requires coordination between type Iα phosphatidylinositol phosphate kinase and Rac signaling. J. Biol. Chem. 278, 23036–23045 (2003).
Ciruela, A., Hinchliffe, K. A., Divecha, N. & Irvine, R. F. Nuclear targeting of the β isoform of type II phosphatidylinositol phosphate kinase (phosphatidylinositol 5-phosphate 4-kinase) by its α-helix 7. Biochem. J. 346, 587–591 (2000).
Vicinanza, M. et al. PI(5)P regulates autophagosome biogenesis. Mol. Cell 57, 219–234 (2015).
Clarke, J. H., Emson, P. C. & Irvine, R. F. Localization of phosphatidylinositol phosphate kinase IIγ in kidney to a membrane trafficking compartment within specialized cells of the nephron. Am. J. Physiol. Renal Physiol. 295, F1422–F1430 (2008).
Cabezas, A., Pattni, K. & Stenmark, H. Cloning and subcellular localization of a human phosphatidylinositol 3-phosphate 5-kinase, PIKfyve/Fab1. Gene 371, 34–41 (2006).
Hoadley, K. A. et al. Cell-of-origin patterns dominate the molecular classification of 10,000 tumors from 33 types of cancer. Cell 173, 291–304.e296 (2018).
Cerami, E. et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2, 401–404 (2012).
Tyner, J. W. et al. Functional genomic landscape of acute myeloid leukaemia. Nature 562, 526–531 (2018).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014).
Cao, L. et al. Proteogenomic characterization of pancreatic ductal adenocarcinoma. Cell 184, 5031–5052.e5026 (2021).
Ritchie, M. E. et al. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 43, e47 (2015).
Fang, R., Jiang, Q., Jia, X. & Jiang, Z. ARMH3-mediated recruitment of PI4KB directs Golgi-to-endosome trafficking and activation of the antiviral effector STING. Immunity 56, 500–515.e506 (2023).
Wu, S. Z. et al. A single-cell and spatially resolved atlas of human breast cancers. Nat. Genet. 53, 1334–1347 (2021).
Mantovani, A., Allavena, P., Marchesi, F. & Garlanda, C. Macrophages as tools and targets in cancer therapy. Nat. Rev. Drug Discov. 21, 799–820 (2022).
Pan, Y., Yu, Y., Wang, X. & Zhang, T. Tumor-associated macrophages in tumor immunity. Front. Immunol. 11, 583084 (2020).
Hao, Y. et al. Integrated analysis of multimodal single-cell data. Cell 184, 3573–3587.e3529 (2021).
Acknowledgements
We extend our gratitude to the Bioinformatics core at SBPMDI (NCI P30 CA 030199) for the data analysis of Figs. 2 and 3 and Box 1. B.M.E. is supported by the NCI (R01 CA237536), NIGMS (R01 GM143583) and ACS (RSG-20-064-01-TBE). A.L. is supported by the ACS postdoctoral fellowship (PF-24-1318560-01-CDP). The authors are also grateful to the Emerling lab for valuable discussions and suggestions for the manuscript.
Author information
Authors and Affiliations
Contributions
A.L., G.K.A. and B.M.E. wrote, researched data for the article and contributed substantially to discussion of the content. R.M. conducted the bioinformatics analysis for the figures. All authors reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Cancer thanks Richard Anderson, Nullin Divecha, Volker Haucke and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Broad Single Cell portal: https://singlecell.broadinstitute.org/single_cell/study/SCP1039/a-single-cell-and-spatially-resolved-atlas-of-human-breast-cancers#study-download
cBioPortal: https://www.cbioportal.org
LinkedOmics: https://www.linkedomics.org/data_download/CPTAC-PDAC/
The Cancer Genome Atlas Program (TCGA): https://www.cancer.gov/ccg/research/genome-sequencing/tcga
Glossary
- Autophagic flux
-
A measure of the rate of autophagic degradation, from autophagosome formation and maturation to fusion with lysosomes and the subsequent degradation of their contents.
- Ciliogenesis
-
A process by which cilia, microtubule-based organelles involved in cell motility and signalling, are assembled and extended from the basal body on the surface of eukaryotic cells.
- Circular RNAs
-
Single-stranded RNA molecules that form a covalently closed loop that lacks free 5′ and 3′ ends, which act as transcriptional modulators, microRNA and protein sponges, protein decoys and stabilizers, scaffolds for protein complexes and, in some cases, as protein templates.
- Cytosolic DNA-sensing pathway
-
A cellular defence mechanism that detects foreign DNA derived from invading viruses and bacteria or mislocalized self-DNA in the cytoplasm and mediates a protective immune response through signalling pathways such as cGAS–STING.
- Degrader
-
A bifunctional small molecule that binds a target protein and recruits an E3 ubiquitin ligase, facilitating polyubiquitination and subsequent proteasomal degradation of the target protein.
- Endosomal sorting
-
A process by which cargo is trafficked from endosomes to other organelles, such as lysosomes for degradation, the trans-Golgi network for redistribution to other subcellular locations, or the plasma membrane for recycling.
- Immune checkpoint protein
-
A regulatory protein expressed by immune cells that modulates immune responses to prevent excessive inflammatory reactions that could damage healthy cells. Some cancer cells exploit immune checkpoint proteins to evade immune recognition and killing by suppressing antitumour immune responses.
- Immune synapse
-
A specialized contact area between a lymphocyte (T cell, B cell or natural killer cell) and a target cell or antigen-presenting cell that allows efficient signalling, cell–cell communication and directed protein secretion.
- Invadopodia
-
Specialized actin-rich cellular protrusions that drive the proteolytic degradation of the extracellular matrix, allowing cancer cells to invade surrounding tissues.
- Lamellipodia
-
Specialized actin-rich cellular protrusions at the leading edge of motile cells that drive movement through actin filament polymerization.
- Luminal A breast cancer subtype
-
A molecular subtype of breast cancer that is oestrogen and progesterone receptor positive, human epidermal growth factor receptor 2 negative, and is characterized by a low proliferative index and favourable prognosis and lower risk of recurrence compared with other subtypes.
- Macropinocytosis
-
A unique form of endocytosis characterized by the non-selective uptake of extracellular fluid, solute molecules and membrane in large vesicles known as macropinosomes.
- Multivesicular endosomes
-
Intracellular organelles characterized by the presence of intraluminal vesicles formed by invagination of the limiting endosomal membrane that function as an intermediate compartment in the endosome–lysosome pathway.
- Palmitoylation
-
A post-translational modification in which palmitic acid, a 16-carbon saturated fatty acid, is covalently attached to specific residues of proteins, influencing their localization, stability and function.
- Phosphoinositides
-
Membrane-associated lipid signalling molecules derived from the phosphorylation of phosphatidylinositol that serve as docking sites for proteins involved in essential cellular processes such as signal transduction, vesicular trafficking and cytoskeletal organization.
- Secretory pathway
-
A cellular process involving a coordinated molecular machinery within the endoplasmic reticulum and Golgi apparatus responsible for the synthesis, folding, post-translational modification, trafficking and delivery of functional secretory proteins to the cell surface and extracellular space.
- Senescence
-
A stable state of cell cycle arrest in response to cellular stress or damage, characterized by cellular remodelling and hypersecretion, in which cells are unable to proliferate despite optimal growth conditions.
- Spastic paraplegia
-
A neurological disorder characterized by progressive muscle tightness and weakness in the lower limbs.
- Trans-Golgi network
-
Major sorting compartment formed by a tubulovesicular network at the trans face of the Golgi apparatus that directs newly synthesized proteins to their appropriate subcellular destinations.
- Warburg effect
-
A metabolic adaptation in which cancer cells preferentially use aerobic glycolysis over oxidative phosphorylation, leading to increased glucose uptake and lactate production even in the presence of oxygen and functioning mitochondria, to support the nutritional requirements of uncontrolled proliferation.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Llorente, A., Arora, G.K., Murad, R. et al. Phosphoinositide kinases in cancer: from molecular mechanisms to therapeutic opportunities. Nat Rev Cancer 25, 463–487 (2025). https://doi.org/10.1038/s41568-025-00810-1
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41568-025-00810-1