Abstract
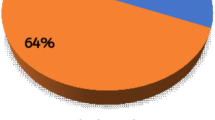
Scabies is one of the most common and highest-burden skin diseases globally. Estimates suggest that >200 million people worldwide have scabies at any one time, with an annual prevalence of 455 million people, with children in impoverished and overcrowded settings being the most affected. Scabies infection is highly contagious and leads to considerable morbidity. Secondary bacterial infections are common and can cause severe health complications, including sepsis or necrotizing soft-tissue infection, renal damage and rheumatic heart disease. There is no vaccine or preventive treatment against scabies and, for the past 30 years, only few broad-spectrum antiparasitic drugs (mainly topical permethrin and oral ivermectin) have been widely available. Treatment failure is common because drugs have short half-lives and do not kill all developmental stages of the scabies parasite. At least two consecutive treatments are needed, which is difficult to achieve in resource-poor and itinerant populations. Another key issue is the lack of a practical, rapid, cheap and accurate diagnostic tool for the timely detection of scabies, which could prevent the cycle of exacerbation and disease persistence in communities. Scabies control will require a multifaceted approach, aided by improved diagnostics and surveillance, new treatments, and increased public awareness.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 1 digital issues and online access to articles
$119.00 per year
only $119.00 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Fischer, K. & Chosidow, O. Scabies (Springer, 2023). This book covers the current status quo in scabies research.
Karimkhani, C. et al. The global burden of scabies: a cross-sectional analysis from the Global Burden of Disease Study 2015. Lancet Infect. Dis. 17, 1247–1254 (2017). Unsurpassed analysis and documentation of the global morbidity and burden of scabies.
Hay, R. J. et al. The global burden of skin disease in 2010: an analysis of the prevalence and impact of skin conditions. J. Invest. Dermatol. 134, 1527–1534 (2014). Important paper providing an analysis of the global burden of skin disease.
World Health Organization. WHO Informal Consultation on a Framework for Scabies Control (WHO, 2019).
Bornstein, S., Mörner, T. & Samuel, W. M. in Parasitic Diseases of Wild Mammals (eds Samuel, W. M., Pybus, M. J. & Kocan, A. A.) 107–119 (Iowa State University Press, 2001).
Kraabøl, M., Gundersen, V., Fangel, K. & Olstad, K. The taxonomy, life cycle and pathology of Sarcoptes scabiei and Notoedres cati (Acarina, Sarcoptidae): a review in a Fennoscandian wildlife perspective. Fauna Nor. 35, 21 (2015).
Roberts, L. J., Huffam, S. E., Walton, S. F. & Currie, B. J. Crusted scabies: clinical and immunological findings in seventy-eight patients and a review of the literature. J. Infect. 50, 375–381 (2005).
Smith, H. J. Transmission of Sarcoptes scabiei in swine by fomites. Can. Vet. J. 27, 252–254 (1986).
Mellanby, K. Transmission of scabies. Br. Med. J. 2, 405–406 (1941).
Bernigaud, C., Fischer, K. & Chosidow, O. The management of scabies in the 21st century: past, advances and potentials. Acta Derm. Venereol. 100, adv00112 (2020).
Slape, D., Russell, R. & McMeniman, E. in Scabies (eds Fischer, K. & Chosidow, O.) 233–268 (Springer, 2023).
Skayem, C. et al. Severe scabies: a French multi-centre study involving 95 patients with crusted and profuse disease and review of the literature. Acta Derm. Venereol. 103, adv00878 (2023).
Niode, N. J. et al. Crusted scabies, a neglected tropical disease: case series and literature review. Infect. Dis. Rep. 14, 479–491 (2022).
Moroni, B., Rossi, L., Bernigaud, C. & Guillot, J. Zoonotic episodes of scabies: a global overview. Pathogens 11, 213 (2022).
Lynar, S., Currie, B. J. & Baird, R. Scabies and mortality. Lancet Infect. Dis. 17, 1234 (2017). This short correspondence discusses the substantial global mortality burden from scabies, in addition to the recognised morbidity.
del Giudice, P., Sainte Marie, D., Gérard, Y., Couppié, P. & Pradinaud, R. Is crusted (Norwegian) scabies a marker of adult T cell leukemia/lymphoma in human T lymphotropic virus type I-seropositive patients? J. Infect. Dis. 176, 1090–1092 (1997).
Scabies — level 3 cause. IHME https://www.healthdata.org/research-analysis/diseases-injuries-risks/factsheets/2021-scabies-level-3-disease (2020).
Romani, L., Steer, A. C., Whitfeld, M. J. & Kaldor, J. M. Prevalence of scabies and impetigo worldwide: a systematic review. Lancet Infect. Dis. 15, 960–967 (2015). This article summarizes population-based studies that reported on the overlapping prevalence of scabies and impetigo.
Zhang, W. et al. Trends in prevalence and incidence of scabies from 1990 to 2017: findings from the Global Burden of Disease study 2017. Emerg. Microbes Infect. 9, 813–816 (2020).
Currie, B. J. & Carapetis, J. R. Skin infections and infestations in Aboriginal communities in northern Australia. Australas. J. Dermatol. 41, 139–143 (2000).
Amoako, Y. A. et al. A scabies outbreak in the North East Region of Ghana: the necessity for prompt intervention. PLoS Negl. Trop. Dis. 14, e0008902 (2020).
Schneider, S., Wu, J., Tizek, L., Ziehfreund, S. & Zink, A. Prevalence of scabies worldwide — an updated systematic literature review in 2022. J. Eur. Acad. Dermatol. Venereol. 37, 1749–1757 (2023).
Enbiale, W. & Ayalew, A. Investigation of a scabies outbreak in drought-affected areas in Ethiopia. Trop. Med. Infect. Dis. 3, 114 (2018).
Delaš Aždajić, M. et al. Increased scabies incidence at the beginning of the 21st century: what do reports from Europe and the world show? Life 12, 1598 (2022).
Donà, M. G. et al. Increasing trend in confirmed scabies cases in the only public dermatological institute of scientific research and care in Italy. Eur. J. Dermatol. 33, 709–710 (2023).
Richardson, N. A. et al. Scabies outbreak management in refugee/migrant camps in Europe 2014-2017: a retrospective qualitative interview study of healthcare staff experiences and perspectives. BMJ Open 13, e075103 (2023).
Abdolrasouli, A., Cousins, C. D., Basu, T. N., Trotman, D. & Hay, R. J. Permethrin-unresponsive scabies in London, UK: a wake-up call. Clin. Exp. Dermatol. 48, 1280–1282 (2023).
Khan, S. S. & Fuller, L. C. Is there a growing global threat of scabies treatment failure? An opportunity to discuss health inequity within UK dermatology. Br. J. Dermatol. 190, 139–140 (2024).
Skin and subcutaneous diseases — level 2 cause. IHME https://www.healthdata.org/research-analysis/diseases-injuries-risks/factsheets/2021-skin-and-subcutaneous-diseases-level-2 (2020).
Lopes, M. J. et al. Perceptions, attitudes and practices towards scabies in communities on the Bijagos Islands, Guinea-Bissau. Trans. R. Soc. Trop. Med. Hyg. 114, 49–56 (2020).
Sara, J., Haji, Y. & Gebretsadik, A. Scabies outbreak investigation and risk factors in East Badewacho District, southern Ethiopia: unmatched case control study. Dermatol. Res. Pract. 2018, 7276938 (2018).
Scabies. CDC https://www.cdc.gov/scabies/prevention/index.html (2024).
Talukder, K. et al. Controlling scabies in madrasahs (Islamic religious schools) in Bangladesh. Public Health 127, 83–91 (2013).
Kouotou, E. A. et al. Burden of human scabies in sub-Saharan African prisons: evidence from the west region of Cameroon. Australas. J. Dermatol. 59, e6–e10 (2018).
Cassell, J. A. et al. Scabies outbreaks in ten care homes for elderly people: a prospective study of clinical features, epidemiology, and treatment outcomes. Lancet Infect. Dis. 18, 894–902 (2018).
Raffi, J., Suresh, R. & Butler, D. C. Review of scabies in the elderly. Dermatol. Ther. 9, 623–630 (2019).
Rahman, M. S., Hasan, A. B. M. N., Jahan, I. & Sharif, A. B. Prevalence of scabies and its associated environmental risk factors among the forcibly displaced Myanmar nationals living in the Cox’s Bazar district of Bangladesh. J. Migr. Health 9, 100220 (2024).
Thornton, J. Scabies in Cox’s Bazar. Lancet 402, 600 (2023).
Engelman, D. et al. Toward the global control of human scabies: introducing the International Alliance for the Control of Scabies. PLoS Negl. Trop. Dis. 7, e2167 (2013). This paper describes a global initiative with the shared objective to foster research, treatment and diagnosis, and to control and, where possible, eliminate infections caused by Sarcoptes scabiei.
Engelman, D. et al. The public health control of scabies: priorities for research and action. Lancet 394, 81–92 (2019).
Hoy, W. E. et al. Post-streptococcal glomerulonephritis is a strong risk factor for chronic kidney disease in later life. Kidney Int. 81, 1026–1032 (2012).
Carapetis, J. R., Steer, A. C., Mulholland, E. K. & Weber, M. The global burden of group A streptococcal diseases. Lancet Infect. Dis. 5, 685–694 (2005).
Rheumatic heart disease — level 3 cause. IHME https://www.healthdata.org/research-analysis/diseases-injuries-risks/factsheets/2021-rheumatic-heart-disease-level-3-disease (2020).
Acute glomerulonephritis — level 3 cause. IHME https://www.healthdata.org/research-analysis/diseases-injuries-risks/factsheets/2021-acute-glomerulonephritis-level-3-disease (2020).
Van Neste, D. Behaviour of Sarcoptes scabiei in its burrow in hyperkeratotic scabies. Dermatologica 171, 343–348 (1985).
Beckham, S. A. et al. Characterization of a serine protease homologous to house dust mite group 3 allergens from the scabies mite Sarcoptes scabiei. J. Biol. Chem. 284, 34413–34422 (2009).
Mahmood, W., Viberg, L. T., Fischer, K., Walton, S. F. & Holt, D. C. An aspartic protease of the scabies mite Sarcoptes scabiei is involved in the digestion of host skin and blood macromolecules. PLoS Negl. Trop. Dis. 7, e2525 (2013).
Fernando, D. D. & Fischer, K. Proteases and pseudoproteases in parasitic arthropods of clinical importance. FEBS J. 287, 4284–4299 (2020).
Korhonen, P. K. et al. High-quality nuclear genome for Sarcoptes scabiei — a critical resource for a neglected parasite. PLoS Negl. Trop. Dis. 14, e0008720 (2020). This paper reports high-quality genome and transcriptome data for S. scabiei.
Fischer, K., Holt, D., Currie, B. & Kemp, D. Scabies: important clinical consequences explained by new molecular studies. Adv. Parasitol. 79, 339–373 (2012).
Holt, D. C., Burgess, S. T., Reynolds, S. L., Mahmood, W. & Fischer, K. Intestinal proteases of free-living and parasitic astigmatid mites. Cell Tissue Res. 351, 339–352 (2013).
Bergstrom, F. C. et al. Scabies mite inactivated serine protease paralogs inhibit the human complement system. J. Immunol. 182, 7809–7817 (2009).
Reynolds, S. L. et al. Scabies mite inactive serine proteases are potent inhibitors of the human complement lectin pathway. PLoS Negl. Trop. Dis. 8, e2872 (2014).
Swe, P. M., Christian, L. D., Lu, H. C., Sriprakash, K. S. & Fischer, K. Complement inhibition by Sarcoptes scabiei protects Streptococcus pyogenes — an in vitro study to unravel the molecular mechanisms behind the poorly understood predilection of S. pyogenes to infect mite-induced skin lesions. PLoS Negl. Trop. Dis. 11, e0005437 (2017).
Swe, P. M. & Fischer, K. A scabies mite serpin interferes with complement-mediated neutrophil functions and promotes staphylococcal growth. PLoS Negl. Trop. Dis. 8, e2928 (2014).
Mika, A. et al. Complement inhibitors from scabies mites promote streptococcal growth — a novel mechanism in infected epidermis? PLoS Negl. Trop. Dis. 6, e1563 (2012).
Terao, Y. et al. Group A streptococcal cysteine protease degrades C3 (C3b) and contributes to evasion of innate immunity. J. Biol. Chem. 283, 6253–6260 (2008).
Fernie-King, B. A. et al. Streptococcal inhibitor of complement (SIC) inhibits the membrane attack complex by preventing uptake of C567 onto cell membranes. Immunology 103, 390–398 (2001).
Langley, R. et al. The staphylococcal superantigen-like protein 7 binds IgA and complement C5 and inhibits IgA-FcαRI binding and serum killing of bacteria. J. Immunol. 174, 2926–2933 (2005).
Ramsland, P. A. et al. Structural basis for evasion of IgA immunity by Staphylococcus aureus revealed in the complex of SSL7 with Fc of human IgA1. Proc. Natl Acad. Sci. USA 104, 15051–15056 (2007).
Hallstrom, T., Jarva, H., Riesbeck, K. & Blom, A. M. Interaction with C4b-binding protein contributes to nontypeable Haemophilus influenzae serum resistance. J. Immunol. 178, 6359–6366 (2007).
Hallstrom, T. et al. Haemophilus influenzae interacts with the human complement inhibitor factor H. J. Immunol. 181, 537–545 (2008).
Rooijakkers, S. H. & van Strijp, J. A. Bacterial complement evasion. Mol. Immunol. 44, 23–32 (2007).
Akesson, P., Sjoholm, A. G. & Bjorck, L. Protein SIC, a novel extracellular protein of Streptococcus pyogenes interfering with complement function. J. Biol. Chem. 271, 1081–1088 (1996).
Bisno, A. L., Brito, M. O. & Collins, C. M. Molecular basis of group A streptococcal virulence. Lancet Infect. Dis. 3, 191–200 (2003).
Laarman, A. J. et al. Staphylococcus aureus metalloprotease aureolysin cleaves complement C3 to mediate immune evasion. J. Immunol. 186, 6445–6453 (2011).
Laarman, A., Milder, F., van Strijp, J. & Rooijakkers, S. Complement inhibition by gram-positive pathogens: molecular mechanisms and therapeutic implications. J. Mol. Med. 88, 115–120 (2010).
Rooijakkers, S. H. et al. Early expression of SCIN and CHIPS drives instant immune evasion by Staphylococcus aureus. Cell Microbiol. 8, 1282–1293 (2006).
Ermert, D. et al. Virulence of group A streptococci is enhanced by human complement inhibitors. PLoS Pathog. 11, e1005043 (2015).
Foster, T. J. Immune evasion by staphylococci. Nat. Rev. Microbiol. 3, 948–958 (2005).
Hasan, T., Krause, V. L., James, C. & Currie, B. J. Crusted scabies; a 2-year prospective study from the Northern Territory of Australia. PLoS Negl. Trop. Dis. 14, e0008994 (2020).
Bhat, S. A. et al. Early immune suppression leads to uncontrolled mite proliferation and potent host inflammatory responses in a porcine model of crusted versus ordinary scabies. PLoS Negl. Trop. Dis. 14, e0008601 (2020).
Elwood, H., Berry, R. S., Gardner, J. M. & Shalin, S. C. Superficial fibrin thrombi… and other findings: a review of the histopathology of human scabietic infections. J. Cutan. Pathol. 42, 346–352 (2015).
Fernando, D. D. et al. A unique group of scabies mite pseudoproteases promotes cutaneous blood coagulation and delays plasmin-induced fibrinolysis. PLoS Negl. Trop. Dis. 15, e0008997 (2021).
Fernando, D. D. et al. Phylogenetic relationships, stage-specific expression and localisation of a unique family of inactive cysteine proteases in Sarcoptes scabiei. Parasit. Vectors 11, 301 (2018).
Arlian, L. G., Morgan, M. S. & Paul, C. C. Evidence that scabies mites (Acari: Sarcoptidae) influence production of interleukin-10 and the function of T-regulatory cells (Tr1) in humans. J. Med. Entomol. 43, 283–287 (2006).
Morgan, M. S. & Arlian, L. G. Response of human skin equivalents to Sarcoptes scabiei. J. Med. Entomol. 47, 877–883 (2010).
Elder, B. L., Arlian, L. G. & Morgan, M. S. Sarcoptes scabiei (Acari: Sarcoptidae) mite extract modulates expression of cytokines and adhesion molecules by human dermal microvascular endothelial cells. J. Med. Entomol. 43, 910–915 (2006).
Arlian, L. G., Fall, N. & Morgan, M. S. In vivo evidence that Sarcoptes scabiei (Acari: Sarcoptidae) is the source of molecules that modulate splenic gene expression. J. Med. Entomol. 44, 1054–1063 (2007).
Bergamin, G., Hudson, J., Currie, B. J. & Mounsey, K. E. A systematic review of immunosuppressive risk factors and comorbidities associated with the development of crusted scabies. Int. J. Infect. Dis. 143, 107036 (2024).
Mounsey, K. et al. A tractable experimental model for study of human and animal scabies. PLoS Negl. Trop. Dis. 4, e756 (2010). This paper describes the pig scabies model, which has enabled the development of comprehensive molecular databases.
Currie, B. J., Maguire, G. P. & Wood, Y. K. Ivermectin and crusted (Norwegian) scabies. Med. J. Aust. 163, 559–560 (1995).
Mounsey, K. E. et al. Prospective study in a porcine model of Sarcoptes scabiei indicates the association of Th2 and Th17 pathways with the clinical severity of scabies. PLoS Negl. Trop. Dis. 9, e0003498 (2015).
Skerratt, L. F. Cellular response in the dermis of common wombats (Vombatus ursinus) infected with Sarcoptes scabiei var. wombati. J. Wildl. Dis. 39, 193–202 (2003).
Walton, S. F., Beroukas, D., Roberts-Thomson, P. & Currie, B. J. New insights into disease pathogenesis in crusted (Norwegian) scabies: the skin immune response in crusted scabies. Br. J. Dermatol. 158, 1247–1255 (2008).
Amer, M., Mostafa, F. F., Nasr, A. N. & el-Harras, M. The role of mast cells in treatment of scabies. Int. J. Dermatol. 34, 186–189 (1995).
Nimmervoll, H. et al. Pathology of sarcoptic mange in red foxes (Vulpes vulpes): macroscopic and histologic characterization of three disease stages. J. Wildl. Dis. 49, 91–102 (2013).
Shehwana, H. et al. Transcriptome analysis of host inflammatory responses to the ectoparasitic mite Sarcoptes scabiei var. hominis. Front. Immunol. 12, 778840 (2021).
Bhat, S. A., Mounsey, K. E., Liu, X. & Walton, S. F. Host immune responses to the itch mite, Sarcoptes scabiei, in humans. Parasit. Vectors 10, 385 (2017).
Liu, X. et al. Crusted scabies is associated with increased IL-17 secretion by skin T cells. Parasite Immunol. 36, 594–604 (2014).
Walton, S. F. et al. Increased allergic immune response to Sarcoptes scabiei antigens in crusted versus ordinary scabies. Clin. Vaccin. Immunol. 17, 1428–1438 (2010).
Rodriguez-Lago, L. & Borrego, L. Norwegian scabies in an atopic patient under dupilumab treatment. Dermatitis 33, e54–e55 (2022).
Nwufoh, O. C., Sadiq, N. A., Adediran, O. A., Jarikre, T. A. & Emikpe, B. O. Sequential histopathological changes and cytokine expressions in dogs naturally infested with Sarcoptes scabiei mites. Acta Parasitol. 65, 452–461 (2020).
Rampton, M. et al. Antibody responses to Sarcoptes scabiei apolipoprotein in a porcine model: relevance to immunodiagnosis of recent infection. PLoS ONE 8, e65354 (2013).
Boralevi, F. et al. Clinical phenotype of scabies by age. Pediatrics 133, e910–e916 (2014).
Sanders, K. M. et al. Non-histaminergic itch mediators elevated in the skin of a porcine model of scabies and of human scabies patients. J. Invest. Dermatol. 139, 971–973 (2019).
Estrada-Chávez, G., Estrada, R., Engelman, D., Molina, J. & Chávez-López, G. Cushing syndrome due to inappropriate corticosteroid topical treatment of undiagnosed scabies. Trop. Med. Infect. Dis. 3, 82 (2018).
Marlière, V., Roul, S., Labrèze, C. & Taïeb, A. Crusted (Norwegian) scabies induced by use of topical corticosteroids and treated successfully with ivermectin. J. Pediatr. 135, 122–124 (1999).
Hashimoto, T., Satoh, T. & Yokozeki, H. Pruritus in ordinary scabies: IL-31 from macrophages induced by overexpression of thymic stromal lymphopoietin and periostin. Allergy 74, 1727–1737 (2019).
Bowen, A. C., Tong, S. Y. C., Chatfield, M. D. & Carapetis, J. R. The microbiology of impetigo in Indigenous children: associations between Streptococcus pyogenes, Staphylococcus aureus, scabies, and nasal carriage. BMC Infect. Dis. 14, 727 (2014).
Thean, L. J. et al. Prevention of bacterial complications of scabies using mass drug administration: a population-based, before-after trial in Fiji, 2018–2020. Lancet Reg. Health West. Pac. 22, 100433 (2022).
Bernigaud, C. et al. First description of the composition and the functional capabilities of the skin microbial community accompanying severe scabies infestation in humans. Microorganisms 9, 907 (2021).
Swe, P. M., Zakrzewski, M., Kelly, A., Krause, L. & Fischer, K. Scabies mites alter the skin microbiome and promote growth of opportunistic pathogens in a porcine model. PLoS Negl. Trop. Dis. 8, e2897 (2014). This paper describes in vivo experimental evidence in a longitudinal preclinical study to demonstrate that scabies mites drastically alter the healthy skin microbiota and promote the growth of pathogens.
McDonald, M. I. et al. Low rates of streptococcal pharyngitis and high rates of pyoderma in Australian aboriginal communities where acute rheumatic fever is hyperendemic. Clin. Infect. Dis. 43, 683–689 (2006).
Williamson, D. A. et al. M-protein analysis of Streptococcus pyogenes isolates associated with acute rheumatic fever in New Zealand. J. Clin. Microbiol. 53, 3618–3620 (2015).
Wyber, R., Bowen, A. C., Ralph, A. P. & Peiris, D. Primary prevention of acute rheumatic fever. Aust. J. Gen. Pract. 50, 265–269 (2021).
Francis, J. R. et al. A cluster of acute rheumatic fever cases among Aboriginal Australians in a remote community with high baseline incidence. Aust. N. Z. J. Public Health 43, 288–293 (2019).
Steer, A. C. et al. High burden of impetigo and scabies in a tropical country. PLoS Negl. Trop. Dis. 3, e467 (2009).
Lin, S., Farber, J. & Lado, L. A case report of crusted scabies with methicillin-resistant Staphylococcus aureus bacteremia. J. Am. Geriatr. Soc. 57, 1713–1714 (2009).
Mulholland, E. K. et al. Etiology of serious infections in young Gambian infants. Pediatr. Infect. Dis. 18, S35–S41 (1999).
Tong, S. Y., Varrone, L., Chatfield, M. D., Beaman, M. & Giffard, P. M. Progressive increase in community-associated methicillin-resistant Staphylococcus aureus in Indigenous populations in northern Australia from 1993 to 2012. Epidemiol. Infect. 143, 1519–1523 (2015).
Engelman, D. et al. The 2020 International Alliance for the Control of Scabies consensus criteria for the diagnosis of scabies. Br. J. Dermatol. 183, 808–820 (2020).
Lavery, M. J., Stull, C., Kinney, M. O. & Yosipovitch, G. Nocturnal pruritus: the battle for a peaceful night’s sleep. Int. J. Mol. Sci. 17, 425 (2016).
Patel, T., Ishiuji, Y. & Yosipovitch, G. Nocturnal itch: why do we itch at night. Acta Derm. Venereol. 87, 295–298 (2007).
Podder, I., Mondal, H. & Kroumpouzos, G. Nocturnal pruritus and sleep disturbance associated with dermatologic disorders in adult patients. Int. J. Womens Dermatol. 7, 403–410 (2021).
Chosidow, O. Scabies. N. Engl. J. Med. 354, 1718–1727 (2006).
Mason, D. S. et al. The prevalence of scabies and impetigo in the Solomon Islands: a population-based survey. PLoS Negl. Trop. Dis. 10, e0004803 (2016).
Hengge, U. R., Currie, B. J., Jager, G., Lupi, O. & Schwartz, R. A. Scabies: a ubiquitous neglected skin disease. Lancet Infect. Dis. 6, 769–779 (2006).
Ostlere, L. S., Harris, D. & Rustin, M. H. Scabies associated with a bullous pemphigoid-like eruption. Br. J. Dermatol. 128, 217–219 (1993).
Grodner, C. et al. Crusted scabies in children in France: a series of 20 cases. Eur. J. Pediatr. 181, 1167–1174 (2022).
Thompson, M. J., Engelman, D., Gholam, K., Fuller, L. C. & Steer, A. C. Systematic review of the diagnosis of scabies in therapeutic trials. Clin. Exp. Dermatol. 42, 481–487 (2017).
Brenaut, E., Garlantezec, R., Talour, K. & Misery, L. Itch characteristics in five dermatoses: non-atopic eczema, atopic dermatitis, urticaria, psoriasis and scabies. Acta Derm. Venereol. 93, 573–574 (2013).
Sunderkötter, C., Wohlrab, J. & Hamm, H. Scabies: epidemiology, diagnosis, and treatment. Dtsch Arztebl Int. 118, 695–704 (2021).
Osti, M. H. et al. The diagnosis of scabies by non-expert examiners: a study of diagnostic accuracy. PLoS Negl. Trop. Dis. 13, e0007635 (2019).
Yari, N., Malone, C. H. & Rivas, A. Misdiagnosed crusted scabies in an AIDS patient leads to hyperinfestation. Cutis 99, 202–204 (2017).
Fonseca, V., Price, H. N., Jeffries, M., Alder, S. L. & Hansen, R. C. Crusted scabies misdiagnosed as erythrodermic psoriasis in a 3-year-old girl with Down syndrome. Pediatr. Dermatol. 31, 753–754 (2014).
de Beer, G., Miller, M. A., Tremblay, L. & Monette, J. An outbreak of scabies in a long-term care facility: the role of misdiagnosis and the costs associated with control. Infect. Control. Hosp. Epidemiol. 27, 517–518 (2006).
Trettin, B., Amstrup Lassen, J., Andersen, F. & Agerskov, H. The journey of having scabies — a qualitative study. J. Nurs. Educ. Pract. https://doi.org/10.5430/jnep.v9n2p1 (2019).
Siddig, E. E. & Hay, R. Laboratory-based diagnosis of scabies: a review of the current status. Trans. R. Soc. Trop. Med. Hyg. 116, 4–9 (2022).
Marks, M. et al. Diagnostics to support the control of scabies-development of two target product profiles. PLoS Negl. Trop. Dis. 16, e0010556 (2022).
Shin, K. et al. Clinical characteristics of pruritus in scabies. Indian J. Dermatol. Venereol. Leprol. 83, 492–492 (2017).
Engelman, D., Fuller, L. C., Steer, A. C. & International Alliance for the Control of Scabies Delphi Panel. Consensus criteria for the diagnosis of scabies: a Delphi study of international experts. PLoS Negl. Trop. Dis. 12, e0006549 (2018).
Walker, S. L. et al. A community-based validation of the International Alliance for the Control of Scabies Consensus Criteria by expert and non-expert examiners in Liberia. PLoS Negl. Trop. Dis. 14, e0008717 (2020).
Leung, V. & Miller, M. Detection of scabies: a systematic review of diagnostic methods. Can. J. Infect. Dis. Med. Microbiol. 22, 698494 (2011).
Micali, G., Lacarrubba, F., Verzì, A. E., Chosidow, O. & Schwartz, R. A. Scabies: advances in noninvasive diagnosis. PLoS Negl. Trop. Dis. 10, e0004691 (2016).
Cinotti, E. et al. Videodermoscopy compared to reflectance confocal microscopy for the diagnosis of scabies. J. Eur. Acad. Dermatol. Venereol. 30, 1573–1577 (2016).
Ruini, C. et al. In vivo imaging of Sarcoptes scabiei infestation using line-field confocal optical coherence tomography. J. Eur. Acad. Dermatol. Venereol. 34, e808–e809 (2020).
Rider, S. D., Morgan, M. S. & Arlian, L. G. Draft genome of the scabies mite. Vectors 8, 585 (2015).
Jayaraj, R. et al. A diagnostic test for scabies: IgE specificity for a recombinant allergen of Sarcoptes scabiei. Diagn. Microbiol. Infect. Dis. 71, 403–407 (2011).
He, R. et al. Molecular characterization and allergenicity potential of triosephosphate isomerase from Sarcoptes scabiei. Vet. Parasitol. 257, 40–47 (2018).
Shen, N. et al. Expression and characterisation of a Sarcoptes scabiei protein tyrosine kinase as a potential antigen for scabies diagnosis. Sci. Rep. 7, 9639 (2017).
He, R. et al. Molecular characterization of calmodulin from Sarcoptes scabiei. Parasitol. Int. 66, 1–6 (2017).
Shen, N. et al. A chitinase-like protein from Sarcoptes scabiei as a candidate anti-mite vaccine that contributes to immune protection in rabbits. Parasit. Vectors 11, 599 (2018).
Chng, L. et al. Molecular diagnosis of scabies using a novel probe-based polymerase chain reaction assay targeting high-copy number repetitive sequences in the Sarcoptes scabiei genome. PLoS Negl. Trop. Dis. 15, e0009149 (2021).
Delaunay, P. et al. Scabies polymerase chain reaction with standardized dry swab sampling: an easy tool for cluster diagnosis of human scabies. Br. J. Dermatol. 182, 197–201 (2020).
Fraser, T. A. et al. A Sarcoptes scabiei specific isothermal amplification assay for detection of this important ectoparasite of wombats and other animals. PeerJ 6, e5291 (2018).
Naz, S., Rizvi, D. A., Javaid, A., Ismail, M. & Chaudhry, F. R. Validation of PCR assay for identification of Sarcoptes scabiei var. hominis. Iran. J. Parasitol. 8, 437–440 (2013).
Bharadwaj, M., Bengtson, M., Golverdingen, M., Waling, L. & Dekker, C. Diagnosing point-of-care diagnostics for neglected tropical diseases. PLoS Negl. Trop. Dis. 15, e0009405 (2021).
Neglected tropical diseases. WHO https://www.who.int/news-room/questions-and-answers/item/neglected-tropical-diseases (WHO, 2023).
World Health Organization. Ending the Neglect to Attain the Sustainable Development Goals: A Road Map for Neglected Tropical Diseases 2021–2030 (WHO, 2021).
Mahé, A. et al. Integration of basic dermatological care into primary health care services in Mali. Bull. World Health Organ. 83, 935–941 (2005).
Estrada, R., Chavez-Lopez, G., Estrada-Chavez, G. & Paredes-Solis, S. Specialized dermatological care for marginalized populations and education at the primary care level: is community dermatology a feasible proposal. Int. J. Dermatol. 51, 1345–1350 (2012).
Micali, G., Lacarrubba, F., Verzi, A. E. & Nasca, M. R. Low-cost equipment for diagnosis and management of endemic scabies outbreaks in underserved populations. Clin. Infect. Dis. 60, 327–329 (2015).
Romani, L. et al. Mass drug administration for scabies control in a population with endemic disease. N. Engl. J. Med. 373, 2305–2313 (2015).
Bernigaud, C. et al. How to eliminate scabies parasites from fomites: a high-throughput ex vivo experimental study. J. Am. Acad. Dermatol. 83, 241–245 (2020). Statistically standardized experimental data validating real-life decontamination modalities for scabies, applicable in settings without electricity, to develop clear and simple directions for scabies outbreaks.
Currie, B. J. Scabies and global control of neglected tropical diseases. N. Engl. J. Med. 373, 2371–2372 (2015).
Scabies. WHO https://www.who.int/news-room/fact-sheets/detail/scabies (2023).
Lake, S. J. et al. Mass drug administration for the control of scabies: a systematic review and meta-analysis. Clin. Infect. Dis. 75, 959–967 (2022).
Fernando, D. D. & Fischer, K. Spinosad topical suspension (0.9%): a new topical treatment for scabies. Expert. Rev. Anti Infect. Ther. 20, 1149–1154 (2022).
Currie, B. J. & McCarthy, J. S. Permethrin and ivermectin for scabies. N. Engl. J. Med. 362, 717–725 (2010).
Bernigaud, C. et al. In vitro ovicidal activity of current and under‐development scabicides: which treatments kill scabies eggs? Br. J. Dermatol. 182, 511–513 (2020). This paper discusses systematic and comprehensive testing of all available drugs in use to treat scabies in humans, confirming that the most used drugs to treat scabies are indeed not ovicidal.
Bernigaud, C. et al. Efficacy and pharmacokinetics evaluation of a single oral dose of afoxolaner against Sarcoptes scabiei in the porcine scabies model for human infestation. Antimicrob. Agents Chemother. 62, e02334-17 (2018).
Nolan, K., Kamrath, J. & Levitt, J. Lindane toxicity: a comprehensive review of the medical literature. Pediatr. Dermatol. 29, 141–146 (2012).
Singh, B., Kaur, J. & Singh, K. Microbial degradation of an organophosphate pesticide, malathion. Crit. Rev. Microbiol. 40, 146–154 (2014).
Strong, M. & Johnstone, P. Interventions for treating scabies. Cochrane Database Syst. Rev. 3, CD000320 (2007).
Rosumeck, S., Nast, A. & Dressler, C. Ivermectin and permethrin for treating scabies. Cochrane Database Syst. Rev. 4, CD012994 (2018).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT02407782 (2015).
Thadanipon, K., Anothaisintawee, T., Rattanasiri, S., Thakkinstian, A. & Attia, J. Efficacy and safety of antiscabietic agents: a systematic review and network meta-analysis of randomized controlled trials. J. Am. Acad. Dermatol. 80, 1435–1444 (2019).
Bernigaud, C., Samarawickrama, G. R., Jones, M. K., Gasser, R. B. & Fischer, K. The challenge of developing a single-dose treatment for scabies. Trends Parasitol. 35, 931–943 (2019).
Seiler, J. C. et al. Spinosad at 0.9% in the treatment of scabies: efficacy results from 2 multicenter, randomized, double-blind, vehicle-controlled studies. J. Am. Acad. Dermatol. 86, 97–103 (2022).
US National Library of Medicine. ClinicalTrials.gov https://www.clinicaltrials.gov/study/NCT05875441?cond=scabies&rank=8 (2023).
US National Library of Medicine. ClinicalTrials.gov https://www.clinicaltrials.gov/study/NCT03905265?cond=scabies&page=2&rank=12 (2023).
Weill, A. et al. Scabies-infested pregnant women: a critical therapeutic challenge. PLoS Negl. Trop. Dis. 15, e0008929 (2021).
Levy, M. et al. Ivermectin safety in infants and children under 15 kg treated for scabies: a multicentric observational study. Br. J. Dermatol. 182, 1003–1006 (2020).
Jittamala, P. et al. Correction: a systematic review and an individual patient data meta-analysis of ivermectin use in children weighing less than fifteen kilograms: is it time to reconsider the current contraindication? PLoS Negl. Trop. Dis. 17, e0011053 (2023).
Davis, J. S., McGloughlin, S., Tong, S. Y., Walton, S. F. & Currie, B. J. A novel clinical grading scale to guide the management of crusted scabies. PLoS Negl. Trop. Dis. 7, e2387 (2013).
Kim, H. S. et al. Scabies itch: an update on neuroimmune interactions and novel targets. J. Eur. Acad. Dermatol. Venereol. 35, 1765–1776 (2021).
Bowen, A. C. et al. Short-course oral co-trimoxazole versus intramuscular benzathine benzylpenicillin for impetigo in a highly endemic region: an open-label, randomised, controlled, non-inferiority trial. Lancet 384, 2132–2140 (2014).
Hotez, P. J., Fenwick, A., Ray, S. E., Hay, S. I. & Molyneux, D. H. “Rapid impact” 10 years after: the first “decade” (2006–2016) of integrated neglected tropical disease control. PLoS Negl. Trop. Dis. 12, e0006137 (2018).
Taplin, D. et al. Community control of scabies: a model based on use of permethrin cream. Lancet 337, 1016–1018 (1991).
Engelman, D. et al. A framework for scabies control. PLoS Negl. Trop. Dis. 15, e0009661 (2021).
Mounsey, K. E., Bernigaud, C., Chosidow, O. & McCarthy, J. S. Prospects for moxidectin as a new oral treatment for human scabies. PLoS Negl. Trop. Dis. 10, e0004389 (2016).
Elsner, E., Uhlmann, T., Krause, S. & Hartmann, R. Increase of scabies and therapy resistance among German military personnel : an 8-year follow-up study in the Department of Dermatology of the Armed Forces Hospital Berlin, Germany (2012-2019) [German]. Hautarzt 71, 447–454 (2020).
Hackenberg, B. et al. Scabies therapy in Germany : results of a nationwide survey with a special focus on the efficacy of first-line therapy with permethrin [German]. Hautarzt 71, 374–379 (2020).
Sunderkötter, C. et al. Increase of scabies in Germany and development of resistant mites? Evidence and consequences. J. Dtsch. Dermatol. Ges. 17, 15–23 (2019).
Mang, R., Kremer, A., Lehmann, P. & Assmann, T. Scabies-clinical resistance to permethrin therapy : case reports and a critical discussion of current treatment recommendations [German]. Hautarzt 72, 595–599 (2021).
Meyersburg, D., Kaiser, A. & Bauer, J. W. Loss of efficacy of topical 5% permethrin for treating scabies: an Austrian single-center study. J. Dermatol. Treat. 33, 774–777 (2022).
Balestri, R. et al. Scabies is becoming less sensitive to permethrin therapy. J. Eur. Acad. Dermatol. Venereol. 35, e889–e891 (2021).
Mazzatenta, C., Piccolo, V., Argenziano, G. & Bassi, A. Is scabies becoming less sensitive to permethrin therapy? J. Eur. Acad. Dermatol. Venereol. 35, e607–e609 (2021).
Coleman, H. & Sethi, G. C. Treatment failure in scabies: a single-centre 5-year retrospective review. Sex. Transm. Infect. https://doi.org/10.1136/sextrans-2023-055949 (2023).
Nemecek, R., Stockbauer, A., Lexa, M., Poeppl, W. & Mooseder, G. Application errors associated with topical treatment of scabies: an observational study. J. Dtsch Dermatol. Ges. 18, 554–559 (2020).
Riebenbauer, K. et al. Detection of a knockdown mutation in the voltage-sensitive sodium channel associated with permethrin tolerance in Sarcoptes scabiei var. hominis mites. J. Eur. Acad. Dermatol. Venereol. 37, 2355–2361 (2023).
Veraldi, S., Schianchi, R., Silvio, M. & Aromolo, I. F. Pseudoresistance to permethrin in scabies. J. Infect. Dev. Ctries 17, 713–715 (2023).
Pasay, C. et al. The effect of insecticide synergists on the response of scabies mites to pyrethroid acaricides. PLoS Negl. Trop. Dis. 3, e354 (2009).
Pasay, C., Walton, S., Fischer, K., Holt, D. & McCarthy, J. PCR-based assay to survey for knockdown resistance to pyrethroid acaricides in human scabies mites (Sarcoptes scabiei var hominis). Am. J. Trop. Med. Hyg. 74, 649–657 (2006).
Yürekli, A. Is there a really resistance to scabies treatment with permethrin? In vitro killing activity of permethrin on Sarcoptes scabiei from patients with resistant scabies. Dermatol. Ther. 35, e15260 (2022).
Fang, F. et al. Efficacy assessment of biocides or repellents for the control of Sarcoptes scabiei in the environment. Parasit. Vectors 8, 416 (2015).
Pallesen, K. et al. In vitro survival of scabies mites. Clin. Exp. Dermatol. 45, 712–715 (2020).
Fujimoto, K., Kawasaki, Y., Morimoto, K., Kikuchi, I. & Kawana, S. Treatment for crusted scabies: limitations and side effects of treatment with Ivermectin. J. Nippon. Med. Sch. 81, 157–163 (2014).
Currie, B. J., Harumal, P., McKinnon, M. & Walton, S. F. First documentation of in vivo and in vitro ivermectin resistance in Sarcoptes scabiei. Clin. Infect. Dis. 39, e8–e12 (2004).
Mounsey, K. E., Holt, D. C., McCarthy, J. S., Currie, B. J. & Walton, S. F. Longitudinal evidence of increasing in vitro tolerance of scabies mites to ivermectin in scabies-endemic communities. Arch. Dermatol. 145, 840–841 (2009).
Mounsey, K. E. et al. Increased transcription of Glutathione S-transferases in acaricide exposed scabies mites. Parasit. Vectors 3, 43 (2010).
Chang, A. Y. & Heukelbach, J. In: Scabies (eds Fischer, K. & Chosidow, O.) 471-482 (Springer, 2023).
Krotneva, S. P. et al. African program for onchocerciasis control 1995-2010: impact of annual ivermectin mass treatment on off-target infectious diseases. PLoS Negl. Trop. Dis. 9, e0004051 (2015).
Kearns, T. M. et al. Strongyloides seroprevalence before and after an ivermectin mass drug administration in a remote Australian Aboriginal community. PLoS Negl. Trop. Dis. 11, e0005607 (2017).
Mohammed, K. A., Deb, R. M., Stanton, M. C. & Molyneux, D. H. Soil transmitted helminths and scabies in Zanzibar, Tanzania following mass drug administration for lymphatic filariasis-a rapid assessment methodology to assess impact. Parasit. Vectors 5, 299 (2012).
Martin, D. et al. Impact of ivermectin mass drug administration for lymphatic filariasis on scabies in eight villages in Kongwa District, Tanzania. Am. J. Trop. Med. Hyg. 99, 937–939 (2018).
Heukelbach, J. et al. Selective mass treatment with ivermectin to control intestinal helminthiases and parasitic skin diseases in a severely affected population. Bull. World Health Organ. 82, 563–571 (2004).
Chosidow, O. & Fuller, L. C. Scratching the itch: is scabies a truly neglected disease. Lancet Infect. Dis. 17, 1220–1221 (2017).
Lake, S. J. et al. Health-related quality of life impact of scabies in the Solomon Islands. Trans. R. Soc. Trop. Med. Hyg. 116, 148–156 (2022).
Karadoğan, S. K. & Altay, B. U. Dermatology Quality of Life and Depression, Anxiety, and Stress-42 scale in scabies patients. Dermatol. pract. concept. 14, e2024112 (2024).
Paudel, S., Pudasaini, P., Adhikari, S., Pradhan, M. B. & Shekhar Babu, K. C. Quality of life in patients with scabies: a cross-sectional study using Dermatology Life Quality Index (DLQI) questionnaire. JEADV Clin. Pract. 2, 399–403 (2023).
Jin-gang, A. et al. Quality of life of patients with scabies. J. Eur. Acad. Dermatol. Venereol. 24, 1187–1191 (2010).
Nair, P. A., Vora, R. V., Jivani, N. B. & Gandhi, S. S. A study of clinical profile and quality of life in patients with scabies at a rural tertiary care centre. J. Clin. Diagn. Res. 10, WC01–WC05 (2016).
Worth, C. et al. Impaired quality of life in adults and children with scabies from an impoverished community in Brazil. Int. J. Dermatol. 51, 275–282 (2012).
Mohammed Ali, K. B., Othman, S. M. & Asaad, Y. A. Quality of life in patients with scabies in Erbil, Iraq. Zanco J. Med. Sci. 25, 638–648 (2021).
Koc Yildirim, S., Demirel Ogut, N., Erbagci, E. & Ogut, C. Scabies affects quality of life in correlation with depression and anxiety. Dermatol. Pract. Concept. 13, e2023144 (2023).
Menaldi, S. L. S. W., Surya, D., The, V. V. & Marissa, M. Impact of scabies on Indonesian public boarding school students’ quality of life: a mixed-method analysis. J. Gen. Proced. Dermatol. Venereol. Indones. https://doi.org/10.19100/jdvi.v5i2.264 (2021).
Duran, S. & YÜReklİ, A. Determination of drug compliance and quality of life in individuals diagnosed with scabies. Karya J. Health Sci. 4, 6–10 (2023).
Korycinska, J., Dzika, E. & Kloch, M. Epidemiology of scabies in relation to socio-economic and selected climatic factors in north-east Poland. Ann. Agric. Env. Med. 27, 374–378 (2020).
Chowdhry, S., Sheoran, P., D’souza, P., Yadav, M. K. & Rathore, S. Assessment of the quality of life in patients with scabies in an urban tertiary care centre in North India. Serb. J. Dermatol. Venerol. 12, 41–46 (2020).
Balcı, D. D., Sangün, Ö. & İnandı, T. Cross validation of the Turkish version of children’s Dermatology Life Quality Index. J. Turk. Acad. Dermatol. 1, 71402a (2007).
Temel, B. et al. Evaluation of dermatology life quality index, depression-anxiety-stress scores of patients with genital dermatoses. Indian J. Dermatol. 68, 399–404 (2023).
Makigami, K. et al. Risk factors for recurrence of scabies: a retrospective study of scabies patients in a long-term care hospital. J. Dermatol. 38, 874–879 (2011).
Hay, R. J. et al. Wastage of family income on skin disease in Mexico. BMJ 309, 848 (1994).
Campbell, M. et al. Health care cost of crusted scabies in Aboriginal communities in the Northern Territory, Australia. PLoS Negl. Trop. Dis. 16, e0010288 (2022).
Welch, E., Romani, L. & Whitfeld, M. J. Recent advances in understanding and treating scabies. Fac. Rev. 10, 28 (2021).
El-Moamly, A. A. Scabies as a part of the World Health Organization roadmap for neglected tropical diseases 2021-2030: what we know and what we need to do for global control. Trop. Med. Health 49, 64 (2021).
Cox, V., Fuller, L. C., Engelman, D., Steer, A. & Hay, R. J. Estimating the global burden of scabies: what else do we need? Br. J. Dermatol. 184, 237–242 (2021).
Romani, L. et al. Efficacy of mass drug administration with ivermectin for control of scabies and impetigo, with coadministration of azithromycin: a single-arm community intervention trial. Lancet Infect. Dis. 19, 510–518 (2019).
Romani, L. et al. Feasibility and safety of mass drug coadministration with azithromycin and ivermectin for the control of neglected tropical diseases: a single-arm intervention trial. Lancet Glob. Health 6, e1132–e1138 (2018).
Chosidow, O. & Hay, R. J. Control of scabies and secondary impetigo: optimising treatment effectiveness in endemic settings. Lancet Infect. Dis. 19, 454–456 (2019).
UKHSA guidance on the management of scabies cases and outbreaks in long-term care facilities and other closed settings. UKHSA https://www.gov.uk/government/publications/scabies-management-advice-for-health-professionals/ukhsa-guidance-on-the-management-of-scabies-cases-and-outbreaks-in-long-term-care-facilities-and-other-closed-settings (2023).
Marks, M. Why does treatment for scabies fail? J. Eur. Acad. Dermatol. Venereol. 38, 462–463 (2024).
Shelley, W. B., Shelley, E. D. & Burmeister, V. Staphylococcus aureus colonization of burrows in erythrodermic Norwegian scabies. A case study of iatrogenic contagion. J. Am. Acad. Dermatol. 19, 673–678 (1988).
Shelley, W. B. & Shelley, E. D. Scanning electron microscopy of the scabies burrow and its contents, with special reference to the Sarcoptes scabiei egg. J. Am. Acad. Dermatol. 9, 673–679 (1983).
Rapp, C. M., Morgan, M. S. & Arlian, L. G. Presence of host immunoglobulin in the gut of Sarcoptes scabiei (Acari: Sarcoptidae). J. Med. Entomol. 43, 539–542 (2006).
Willis, C., Fischer, K., Walton, S. F., Currie, B. J. & Kemp, D. J. Scabies mite inactivated serine protease paralogues are present both internally in the mite gut and externally in feces. Am. J. Trop. Med. Hyg. 75, 683–687 (2006).
Lambris, J. D., Ricklin, D. & Geisbrecht, B. V. Complement evasion by human pathogens. Nat. Rev. Microbiol. 6, 132–142 (2008).
Arlian, L. G., Vyszenski-Moher, D. L. & Pole, M. J. Survival of adults and development stages of Sarcoptes scabiei var. canis when off the host. Exp. Appl. Acarol. 6, 181–187 (1989).
Rodríguez-Cadenas, F., Carbajal-González, M. T., Fregeneda-Grandes, J. M., Aller-Gancedo, J. M. & Rojo-Vázquez, F. A. Clinical evaluation and antibody responses in sheep after primary and secondary experimental challenges with the mange mite Sarcoptes scabiei var. ovis. Vet. Immunol. Immunopathol. 133, 109–116 (2010).
Tarigan, S. & Huntley, J. F. Failure to protect goats following vaccination with soluble proteins of Sarcoptes scabiei: evidence for a role for IgE antibody in protection. Vet. Parasitol. 133, 101–109 (2005).
Zhang, R. et al. Characterization and evaluation of a Sarcoptes scabiei allergen as a candidate vaccine. Parasit. Vectors 5, 176 (2012).
Van Neste, D. J. & Staquet, M. J. Similar epidermal changes in hyperkeratotic scabies of humans and pigs. Am. J. Dermatopathol. 8, 267–273 (1986).
Bernigaud, C. et al. Preclinical study of single-dose moxidectin, a new oral treatment for scabies: efficacy, safety, and pharmacokinetics compared to two-dose ivermectin in a porcine model. PLoS Negl. Trop. Dis. 10, e0005030 (2016). This trial adapted an existing veterinary drug for use against human scabies.
Mounsey, K. E. et al. Quantitative PCR-based genome size estimation of the astigmatid mites Sarcoptes scabiei, Psoroptes ovis and Dermatophagoides pteronyssinus. Parasit Vectors 5, 3 (2012).
Mofiz, E. et al. Mitochondrial genome sequence of the scabies mite provides insight into the genetic diversity of individual scabies infections. PLoS Negl. Trop. Dis. 10, e0004384 (2016).
Fernando, D. D. et al. Gene silencing by RNA interference in Sarcoptes scabiei: a molecular tool to identify novel therapeutic targets. Parasit. Vectors 10, 289 (2017).
Fernando, D. D., Korhonen, P. K., Gasser, R. B. & Fischer, K. An RNA interference tool to silence genes in Sarcoptes scabiei eggs. Int. J. Mol. Sci. 23, 873 (2022).
Korhonen, P. K. et al. Evidence that transcriptional alterations in sarcoptes scabiei are under tight post-transcriptional (microRNA) control. Int. J. Mol. Sci. 23, 9719 (2022).
Mofiz, E. et al. Genomic resources and draft assemblies of the human and porcine varieties of scabies mites, Sarcoptes scabiei var. hominis and var. suis. GigaScience 5, 23 (2016).
Wang, T. et al. Proteomic analysis of Sarcoptes scabiei reveals that proteins differentially expressed between eggs and female adult stages are involved predominantly in genetic information processing, metabolism and/or host-parasite interactions. PLoS Negl. Trop. Dis. 16, e0010946 (2022).
Fischer, K. et al. Structural mechanisms of inactivation in scabies mite serine protease paralogues. J. Mol. Biol. 390, 635–645 (2009).
Taylor, S., Walther, D., Fernando, D. D., Swe-Kay, P. & Fischer, K. Investigating the antibacterial properties of prospective scabicides. Biomedicines 10, 3287 (2022).
Swe, P. M., Zakrzewski, M., Waddell, R., Sriprakash, K. S. & Fischer, K. High-throughput metagenome analysis of the Sarcoptes scabiei internal microbiota and in-situ identification of intestinal Streptomyces sp. Sci. Rep. 9, 11744 (2019).
Acknowledgements
The authors thank M. Flynn and M. Naghavi for help on the Figures. The authors also thank the anonymous patient for providing their experience.
Author information
Authors and Affiliations
Contributions
Introduction (K.F. and D.D.F.); Epidemiology (R.J.H. and B.J.C.); Mechanisms/pathophysiology (K.F., D.D.F. and K.E.M.); Diagnosis, screening and prevention (K.F. and C.B.); Management (K.E.M., C.B., G.E.E.C., B.J.C. and O.C.); Quality of life (K.F. and N.S.); Outlook (K.F. and O.C.); overview of the Primer (K.F.).
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Disease Primers thanks P. T. Campbell, R. R. Yotsu, A. Bowen and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fernando, D.D., Mounsey, K.E., Bernigaud, C. et al. Scabies. Nat Rev Dis Primers 10, 74 (2024). https://doi.org/10.1038/s41572-024-00552-8
Accepted:
Published:
DOI: https://doi.org/10.1038/s41572-024-00552-8
This article is cited by
-
Post-COVID-19 resurgence of scabies’ cases in the Lazio Region, Italy: a new emerging public health threat?
Infectious Diseases of Poverty (2025)