Abstract
Primary sclerosing cholangitis (PSC) is a chronic biliary inflammation associated with periductular fibrosis of the intrahepatic and extrahepatic bile ducts leading to strictures, bacterial cholangitis, decompensated liver disease and need for liver transplantation. This rare focal liver disease affects all races and ages, with a predominance of young males. There is an up to 88% association with inflammatory bowel disease. Although the aetiology is unknown and the pathophysiology is poorly understood, PSC is regarded as an autoimmune liver disease based on a strong immunogenetic background. Further, the associated risk for various malignancies, particularly cholangiocellular carcinoma, is also poorly understood. No medical therapy has been approved so far nor has been shown to improve transplant-free survival. However, ursodeoxycholic acid is widely used since it improves the biochemical parameters of cholestasis and is safe at low doses. MRI of the biliary tract is the primary imaging technology for diagnosis. Endoscopic interventions of the bile ducts should be limited to clinically relevant strictures for balloon dilatation, biopsy and brush cytology. End-stage liver disease with decompensation is an indication for liver transplantation with recurrent PSC in up to 38% of patients. Several novel therapeutic strategies are in various stages of development, including apical sodium-dependent bile acid transporter and ileal bile acid transporter inhibitors, integrin inhibitors, peroxisome proliferator-activated receptor agonists, CCL24 blockers, recombinant FGF19, CCR2/CCR5 inhibitors, farnesoid X receptor bile acid receptor agonists, and nor-ursodeoxycholic acid. Manipulation of the gut microbiome includes faecal microbiota transplantation. This article summarizes present knowledge and defines unmet medical needs to improve quality of life and survival.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 1 digital issues and online access to articles
$119.00 per year
only $119.00 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Chazouilleres, O. et al. EASL Clinical Practice Guidelines on sclerosing cholangitis. J. Hepatol. 77, 761–806 (2022). One of the latest clinical practice guidelines of the most important scientific societies in the field: EASL, AASLD and European Society of Gastrointestinal Endoscopy.
Bowlus, C. L. et al. AASLD practice guidance on primary sclerosing cholangitis and cholangiocarcinoma. Hepatology 77, 659–702 (2023). One of the latest clinical practice guidelines of the most important scientific societies in the field: EASL, AASLD and European Society of Gastrointestinal Endoscopy.
Mehta, T. I. et al. Global incidence, prevalence and features of primary sclerosing cholangitis: a systematic review and meta‐analysis. Liver Int. 41, 2418–2426 (2021).
Barberio, B. et al. Prevalence of primary sclerosing cholangitis in patients with inflammatory bowel disease: a systematic review and meta-analysis. Gastroenterology 161, 1865–1877 (2021). A global perspective on the association of PSC with IBD.
Karlsen, T. H. et al. Genome-wide association analysis in primary sclerosing cholangitis. Gastroenterology 138, 1102–1111 (2010).
Trivedi, P. J., Bowlus, C. L., Yimam, K. K., Razavi, H. & Estes, C. Epidemiology, natural history, and outcomes of primary sclerosing cholangitis: a systematic review of population-based studies. Clin. Gastroenterol. Hepatol. 20, 1687–1700.e4 (2022).
Tanaka, A. & Takikawa, H. Geoepidemiology of primary sclerosing cholangitis: a critical review. J. Autoimmun. 46, 35–40 (2013).
Zhang, Y. et al. Prevalence of inflammatory bowel disease in patients with primary sclerosing cholangitis: a systematic review and meta-analysis. Liver Int. 42, 1814–1822 (2022).
Lunder, A. K. et al. Prevalence of sclerosing cholangitis detected by magnetic resonance cholangiography in patients with long-term inflammatory bowel disease. Gastroenterology 151, 660–669.e4 (2016).
Deneau, M. R. et al. The natural history of primary sclerosing cholangitis in 781 children: a multicenter, international collaboration. Hepatology 66, 518–527 (2017).
Ng, S. C. et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet 390, 2769–2778 (2017).
Boonstra, K. et al. Population-based epidemiology, malignancy risk, and outcome of primary sclerosing cholangitis. Hepatology 58, 2045–2055 (2013).
Bakhshi, Z. et al. An update on primary sclerosing cholangitis epidemiology, outcomes and quantification of alkaline phosphatase variability in a population-based cohort. J. Gastroenterol. 55, 523–532 (2020).
Crothers, H. et al. Past, current, and future trends in the prevalence of primary sclerosing cholangitis and inflammatory bowel disease across England (2015-2027): a nationwide, population-based study. Lancet Reg. Health Eur. 44, 101002 (2024).
Goldberg, D. S. et al. Primary sclerosing cholangitis is not rare among blacks in a multicenter North American Consortium. Clin. Gastroenterol. Hepatol. 16, 591–593 (2018).
Bowlus, C. L., Li, C.-S., Karlsen, T. H., Lie, B. A. & Selmi, C. Primary sclerosing cholangitis in genetically diverse populations listed for liver transplantation: unique clinical and human leukocyte antigen associations. Liver Transpl. 16, 1324–1330 (2010).
Barner-Rasmussen, N., Pukkala, E., Jussila, A. & Färkkilä, M. Epidemiology, risk of malignancy and patient survival in primary sclerosing cholangitis: a population-based study in Finland. Scand. J. Gastroenterol. 55, 74–81 (2020).
Lindkvist, B., Benito De Valle, M., Gullberg, B. & Björnsson, E. Incidence and prevalence of primary sclerosing cholangitis in a defined adult population in sweden. Hepatology 52, 571–577 (2010).
Óli Guðnason, H. et al. Frumkomin trefjunargallgangabólga á Íslandi 1992-2012. Læknablaðið 2019, 371–376 (2019).
Takikawa, H., Takamori, Y., Tanaka, A., Kurihara, H. & Nakanuma, Y. Analysis of 388 cases of primary sclerosing cholangitis in Japan; presence of a subgroup without pancreatic involvement in older patients. Hepatol. Res. 29, 153–159 (2004).
Okolicsanyi, L. et al. Primary sclerosing cholangitis: clinical presentation, natural history and prognostic variables: an Italian multicentre study. The Italian PSC Study Group. Eur. J. Gastroenterol. Hepatol. 8, 685–691 (1996).
Jrgensen, K. K. et al. Inflammatory bowel disease in patients with primary sclerosing cholangitis: clinical characterization in liver transplanted and nontransplanted patients. Inflamm. Bowel Dis. 18, 536–545 (2012).
Bowlus, C. L., Lim, J. K. & Lindor, K. D. AGA clinical practice update on surveillance for hepatobiliary cancers in patients with primary sclerosing cholangitis: expert review. Clin. Gastroenterol. Hepatol. 17, 2416–2422 (2019).
Culver, E. L. et al. Prevalence and long-term outcome of sub-clinical primary sclerosing cholangitis in patients with ulcerative colitis. Liver Int. 40, 2744–2757 (2020).
Loftus, E. V. PSC-IBD: a unique form of inflammatory bowel disease associated with primary sclerosing cholangitis. Gut 54, 91–96 (2005).
Sørensen, J. Ø. et al. Inflammatory bowel disease with primary sclerosing cholangitis: a Danish population-based cohort study 1977-2011. Liver Int. 38, 532–541 (2018).
Trivedi, P. J. et al. Effects of primary sclerosing cholangitis on risks of cancer and death in people with inflammatory bowel disease, based on sex, race, and age. Gastroenterology 159, 915–928 (2020).
Wang, M.-H. et al. Unique phenotypic characteristics and clinical course in patients with ulcerative colitis and primary sclerosing cholangitis: a multicenter US experience. Inflamm. Bowel Dis. 26, 774–779 (2020).
Zheng, H.-H. & Jiang, X.-L. Increased risk of colorectal neoplasia in patients with primary sclerosing cholangitis and inflammatory bowel disease: a meta-analysis of 16 observational studies. Eur. J. Gastroenterol. Hepatol. 28, 383–390 (2016).
Lundberg Båve, A., von Seth, E., Ingre, M., Nordenvall, C. & Bergquist, A. Autoimmune diseases in primary sclerosing cholangitis and their first-degree relatives. Hepatology 80, 527–535 (2024).
Saarinen, S., Olerup, O. & Broome, U. Increased frequency of autoimmune diseases in patients with primary sclerosing cholangitis. Am. J. Gastroenterol. 95, 3195–3199 (2000).
Bergquist, A. et al. Hepatic and extrahepatic malignancies in primary sclerosing cholangitis. J. Hepatol. 36, 321–327 (2002).
Lundberg Båve, A. et al. Increased risk of cancer in patients with primary sclerosing cholangitis. Hepatol. Int. 15, 1174–1182 (2021).
Weismüller, T. J. et al. Patient age, sex, and inflammatory bowel disease phenotype associate with course of primary sclerosing cholangitis. Gastroenterology 152, 1975–1984.e8 (2017).
Fevery, J., Verslype, C., Lai, G., Aerts, R. & Van Steenbergen, W. Incidence, diagnosis, and therapy of cholangiocarcinoma in patients with primary sclerosing cholangitis. Dig. Dis. Sci. 52, 3123–3135 (2007).
Erichsen, R. et al. Hepatobiliary cancer risk in patients with inflammatory bowel disease: a Scandinavian population-based cohort study. Cancer Epidemiol. Biomarkers Prev. 30, 886–894 (2021).
Villard, C. et al. Prospective surveillance for cholangiocarcinoma in unselected individuals with primary sclerosing cholangitis. J. Hepatol. 78, 604–613 (2023).
Zenouzi, R. et al. Low risk of hepatocellular carcinoma in patients with primary sclerosing cholangitis with cirrhosis. Clin. Gastroenterol. Hepatol. 12, 1733–1738 (2014).
Ali, A. H. et al. Surveillance for hepatobiliary cancers in patients with primary sclerosing cholangitis. Hepatology 67, 2338–2351 (2018).
Bergquist, A. et al. Impact on follow‐up strategies in patients with primary sclerosing cholangitis. Liver Int. 43, 127–138 (2023).
Tan, N. et al. Surveillance MRI is associated with improved survival in patients with primary sclerosing cholangitis. Hepatol. Commun. 8, e0442 (2024).
Said, K., Glaumann, H. & Bergquist, A. Gallbladder disease in patients with primary sclerosing cholangitis. J. Hepatol. 48, 598–605 (2008).
Buckles, D. C., Lindor, K. D., Larusso, N. F., Petrovic, L. M. & Gores, G. J. In primary sclerosing cholangitis, gallbladder polyps are frequently malignant. Am. J. Gastroenterol. 97, 1138–1142 (2002).
Bergquist, A., Glaumann, H., Persson, B. & Broomé, U. Risk factors and clinical presentation of hepatobiliary carcinoma in patients with primary sclerosing cholangitis: a case-control study: risk factors and clinical presentation of hepatobiliary carcinoma in patients with primary sclerosing cholangitis: a case-control study. Hepatology 27, 311–316 (1998).
Boberg, K. M. et al. Cholangiocarcinoma in primary sclerosing cholangitis: risk factors and clinical presentation. Scand. J. Gastroenterol. 37, 1205–1211 (2002).
Burak, K. et al. Incidence and risk factors for cholangiocarcinoma in primary sclerosing cholangitis. Am. J. Gastroenterol. 99, 523–526 (2004).
Chalasani, N. et al. Cholangiocarcinoma in patients with primary sclerosing cholangitis: a multicenter case-control study. Hepatology 31, 7–11 (2000).
Gulamhusein, A. F. et al. Duration of inflammatory bowel disease is associated with increased risk of cholangiocarcinoma in patients with primary sclerosing cholangitis and IBD. Am. J. Gastroenterol. 111, 705–711 (2016).
Soetikno, R. M., Lin, O. S., Heidenreich, P. A., Young, H. S. & Blackstone, M. O. Increased risk of colorectal neoplasia in patients with primary sclerosing cholangitis and ulcerative colitis: a meta-analysis. Gastrointest. Endosc. 56, 48–54 (2002).
Halliday, J. S. et al. A unique clinical phenotype of primary sclerosing cholangitis associated with Crohn’s disease. J. Crohns Colitis 6, 174–181 (2012).
Björnsson, E. et al. The natural history of small-duct primary sclerosing cholangitis. Gastroenterology 134, 975–980 (2008).
Karlsen, T. H., Folseraas, T., Thorburn, D. & Vesterhus, M. Primary sclerosing cholangitis — a comprehensive review. J. Hepatol. 67, 1298–1323 (2017).
Weaver, G. H. Cirrhosis of liver of the guinea pig, produced by a bacterium and its products. Johns Hopkins Hosp. Rep. 9, 297 (1900).
Boden, R. W., Rankin, J. G., Goulston, S. J. M. & Morrow, W. The liver in ulcerative colitis the significance of raised serum-alkaline-phosphatase levels. Lancet 274, 245–248 (1959).
Thorpe, M. E., Scheuer, P. J. & Sherlock, S. Primary sclerosing cholangitis, the biliary tree, and ulcerative colitis. Gut 8, 435–448 (1967).
Lichtman, S. N., Keku, J., Clark, R. L., Schwab, J. H. & Sartor, R. B. Biliary tract disease in rats with experimental small bowel bacterial overgrowth. Hepatology 13, 766–772 (1991).
Lichtman, S. N., Keku, J., Schwab, J. H. & Sartor, R. B. Evidence for peptidoglycan absorption in rats with experimental small bowel bacterial overgrowth. Infect. Immun. 59, 555–562 (1991).
Fiorotto, R. et al. Loss of CFTR affects biliary epithelium innate immunity and causes TLR4–NF-κB-mediated inflammatory response in mice. Gastroenterology 141, 1498–1508.e5 (2011).
Nakamoto, N. et al. Gut pathobionts underlie intestinal barrier dysfunction and liver T helper 17 cell immune response in primary sclerosing cholangitis. Nat. Microbiol. 4, 492–503 (2019).
Björnsson, E. et al. Intestinal permeability and bacterial growth of the small bowel in patients with primary sclerosing cholangitis. Scand. J. Gastroenterol. 40, 1090–1094 (2005).
Dhillon, A. K. et al. Circulating markers of gut barrier function associated with disease severity in primary sclerosing cholangitis. Liver Int. 39, 371–381 (2019).
Beuers, U. Drug Insight: mechanisms and sites of action of ursodeoxycholic acid in cholestasis. Nat. Clin. Pract. Gastroenterol. Hepatol. 3, 318–328 (2006).
Vesterhus, M. & Karlsen, T. H. Emerging therapies in primary sclerosing cholangitis: pathophysiological basis and clinical opportunities. J. Gastroenterol. 55, 588–614 (2020).
Beuers, U., Trauner, M., Jansen, P. & Poupon, R. New paradigms in the treatment of hepatic cholestasis: from UDCA to FXR, PXR and beyond. J. Hepatol. 62, S25–S37 (2015).
Fuchs, C. D. & Trauner, M. Role of bile acids and their receptors in gastrointestinal and hepatic pathophysiology. Nat. Rev. Gastroenterol. Hepatol. 19, 432–450 (2022).
Fickert, P. et al. norUrsodeoxycholic acid improves cholestasis in primary sclerosing cholangitis. J. Hepatol. 67, 549–558 (2017).
Fickert, P. et al. 24-norUrsodeoxycholic acid is superior to ursodeoxycholic acid in the treatment of sclerosing cholangitis in Mdr2 (Abcb4) knockout mice. Gastroenterology 130, 465–481 (2006).
Van Niekerk, J., Kersten, R. & Beuers, U. Role of bile acids and the biliary HCO3− umbrella in the pathogenesis of primary biliary cholangitis. Clin. Liver Dis. 22, 457–479 (2018).
Hohenester, S. et al. A biliary HCO3− umbrella constitutes a protective mechanism against bile acid-induced injury in human cholangiocytes. Hepatology 55, 173–183 (2012).
Reich, M. et al. Downregulation of TGR5 (GPBAR1) in biliary epithelial cells contributes to the pathogenesis of sclerosing cholangitis. J. Hepatol. 75, 634–646 (2021).
Kowdley, K. V. et al. A randomized, placebo-controlled, phase II study of obeticholic acid for primary sclerosing cholangitis. J. Hepatol. 73, 94–101 (2020).
Hirschfield, G. M. et al. Effect of NGM282, an FGF19 analogue, in primary sclerosing cholangitis: a multicenter, randomized, double-blind, placebo-controlled phase II trial. J. Hepatol. 70, 483–493 (2019).
Königshofer, P. et al. Nuclear receptors in liver fibrosis. Biochim. Biophys. Acta Mol. Basis Dis. 1867, 166235 (2021).
Gadaleta, R. M. & Moschetta, A. Dark and bright side of targeting fibroblast growth factor receptor 4 in the liver. J. Hepatol. 75, 1440–1451 (2021).
Braadland, P. R. et al. Suppression of bile acid synthesis as a tipping point in the disease course of primary sclerosing cholangitis. JHEP Rep. 4, 100561 (2022).
Schneider, K. M. et al. Gut microbiota depletion exacerbates cholestatic liver injury via loss of FXR signalling. Nat. Metab. 3, 1228–1241 (2021).
Mousa, O. Y. et al. Bile acid profiles in primary sclerosing cholangitis and their ability to predict hepatic decompensation. Hepatology 74, 281–295 (2021).
Bowlus, C. L. et al. Safety, tolerability, and efficacy of maralixibat in adults with primary sclerosing cholangitis: open-label pilot study. Hepatol. Commun. 7, e0153 (2023).
Banales, J. M. et al. Cholangiocyte pathobiology. Nat. Rev. Gastroenterol. Hepatol. 16, 269–281 (2019).
O’Hara, S. P., Karlsen, T. H. & LaRusso, N. F. Cholangiocytes and the environment in primary sclerosing cholangitis: where is the link? Gut 66, 1873–1877 (2017).
Carpino, G. et al. Activation of biliary tree stem cells within peribiliary glands in primary sclerosing cholangitis. J. Hepatol. 63, 1220–1228 (2015).
Mederacke, I. et al. Fate tracing reveals hepatic stellate cells as dominant contributors to liver fibrosis independent of its aetiology. Nat. Commun. 4, 2823 (2013).
Lei, L. et al. Portal fibroblasts with mesenchymal stem cell features form a reservoir of proliferative myofibroblasts in liver fibrosis. Hepatology 76, 1360–1375 (2022).
Helgadottir, H. & Vesterhus, M. Noninvasive evaluation of fibrosis in adult biliary diseases. Curr. Opin. Gastroenterol. 39, 83–88 (2023).
Vesterhus, M. et al. Comprehensive assessment of ECM turnover using serum biomarkers establishes PBC as a high-turnover autoimmune liver disease. JHEP Rep. 3, 100178 (2021).
Vesterhus, M. et al. Enhanced liver fibrosis score predicts transplant‐free survival in primary sclerosing cholangitis. Hepatology 62, 188–197 (2015).
Heydtmann, M. et al. CXC chemokine ligand 16 promotes integrin-mediated adhesion of liver-infiltrating lymphocytes to cholangiocytes and hepatocytes within the inflamed human liver. J. Immunol. 174, 1055–1062 (2005).
Zimmer, C. L. et al. A biliary immune landscape map of primary sclerosing cholangitis reveals a dominant network of neutrophils and tissue-resident T cells. Sci. Transl. Med. 13, eabb3107 (2021).
Afford, S. C. The role of cholangiocytes in the development of chronic inflammatory liver disease. Front. Biosci. 7, e276–e285 (2002).
Locatelli, L. et al. Macrophage recruitment by fibrocystin‐defective biliary epithelial cells promotes portal fibrosis in congenital hepatic fibrosis. Hepatology 63, 965–982 (2016).
Vesterhus, M. et al. Novel serum and bile protein markers predict primary sclerosing cholangitis disease severity and prognosis. J. Hepatol. 66, 1214–1222 (2017).
Meng, F. et al. Ursodeoxycholate inhibits mast cell activation and reverses biliary injury and fibrosis in Mdr2−/− mice and human primary sclerosing cholangitis. Lab. Invest. 98, 1465–1477 (2018).
Zhou, T. et al. Mast cells selectively target large cholangiocytes during biliary injury via H2HR‐mediated cAMP/pERK1/2 signaling. Hepatol. Commun. 6, 2715–2731 (2022).
Meadows, V. et al. Mast cells regulate ductular reaction and intestinal inflammation in cholestasis through farnesoid X receptor signaling. Hepatology 74, 2684–2698 (2021).
Johnson, C. et al. Interleukin-6 and its receptor, key players in hepatobiliary inflammation and cancer. Transl. Gastrointest. Cancer 1, 58–70 (2012).
International Human Genome Sequencing Consortium. Finishing the euchromatic sequence of the human genome. Nature 431, 931–945 (2004).
Jiang, X. & Karlsen, T. H. Genetics of primary sclerosing cholangitis and pathophysiological implications. Nat. Rev. Gastroenterol. Hepatol. 14, 279–295 (2017).
Jiang, X. et al. A heterozygous germline CD100 mutation in a family with primary sclerosing cholangitis. Sci. Transl. Med. 13, eabb0036 (2021).
The UK-PSC Consortium et al. Genome-wide association study of primary sclerosing cholangitis identifies new risk loci and quantifies the genetic relationship with inflammatory bowel disease. Nat. Genet. 49, 269–273 (2017).
The UK-PSCSC Consortium et al. Dense genotyping of immune-related disease regions identifies nine new risk loci for primary sclerosing cholangitis. Nat. Genet. 45, 670–675 (2013).
Peng, X. et al. Immunosuppressive agents for the treatment of primary sclerosing cholangitis: a systematic review and meta-analysis. Dig. Dis. 35, 478–485 (2017).
Leung, K. K., Deeb, M., Fischer, S. E. & Gulamhusein, A. Recurrent primary sclerosing cholangitis: current understanding, management, and future directions. Semin. Liver Dis. 41, 409–420 (2021).
Ramachandran, P. et al. Resolving the fibrotic niche of human liver cirrhosis at single-cell level. Nature 575, 512–518 (2019).
Aizarani, N. et al. A human liver cell atlas reveals heterogeneity and epithelial progenitors. Nature 572, 199–204 (2019).
Hildebrandt, F. et al. Spatial transcriptomics to define transcriptional patterns of zonation and structural components in the mouse liver. Nat. Commun. 12, 7046 (2021).
Chung, B. K., Øgaard, J., Reims, H. M., Karlsen, T. H. & Melum, E. Spatial transcriptomics identifies enriched gene expression and cell types in human liver fibrosis. Hepatol. Commun. 6, 2538–2550 (2022).
Poch, T. et al. Single-cell atlas of hepatic T cells reveals expansion of liver-resident naive-like CD4+ cells in primary sclerosing cholangitis. J. Hepatol. 75, 414–423 (2021).
Shi, T. et al. Farnesoid X receptor antagonizes macrophage-dependent licensing of effector T lymphocytes and progression of sclerosing cholangitis. Sci. Transl. Med. 14, eabi4354 (2022).
Kunzmann, L. K. et al. Monocytes as potential mediators of pathogen‐induced T‐helper 17 differentiation in patients with primary sclerosing cholangitis (PSC). Hepatology 72, 1310–1326 (2020).
Katt, J. et al. Increased T helper type 17 response to pathogen stimulation in patients with primary sclerosing cholangitis: hepatology. Hepatology 58, 1084–1093 (2013).
Liaskou, E. et al. High‐throughput T‐cell receptor sequencing across chronic liver diseases reveals distinct disease‐associated repertoires. Hepatology 63, 1608–1619 (2016).
Henriksen, E. K. K. et al. Gut and liver T-cells of common clonal origin in primary sclerosing cholangitis-inflammatory bowel disease. J. Hepatol. 66, 116–122 (2017).
von Seth, E. et al. Primary sclerosing cholangitis leads to dysfunction and loss of MAIT cells. Eur. J. Immunol. 48, 1997–2004 (2018).
Valestrand, L. et al. Bile from patients with primary sclerosing cholangitis contains mucosal-associated invariant T-cell antigens. Am. J. Pathol. 192, 629–641 (2022).
Andrews, T. S. et al. Single-cell and spatial transcriptomics characterisation of the immunological landscape in the healthy and PSC human liver. J. Hepatol. 80, 730–743 (2024).
Hov, J. & Karlsen, T. The microbiome in primary sclerosing cholangitis: current evidence and potential concepts. Semin. Liver Dis. 37, 314–331 (2017).
Kummen, M. et al. The gut microbial profile in patients with primary sclerosing cholangitis is distinct from patients with ulcerative colitis without biliary disease and healthy controls. Gut 66, 611–619 (2017).
Hole, M. J. et al. A shared mucosal gut microbiota signature in primary sclerosing cholangitis before and after liver transplantation. Hepatology 77, 715–728 (2023).
Hov, J. R. & Karlsen, T. H. The microbiota and the gut–liver axis in primary sclerosing cholangitis. Nat. Rev. Gastroenterol. Hepatol. 20, 135–154 (2023).
Vieira-Silva, S. et al. Quantitative microbiome profiling disentangles inflammation- and bile duct obstruction-associated microbiota alterations across PSC/IBD diagnoses. Nat. Microbiol. 4, 1826–1831 (2019).
Liwinski, T. et al. Alterations of the bile microbiome in primary sclerosing cholangitis. Gut 69, 665–672 (2020).
Kummen, M. et al. Altered gut microbial metabolism of essential nutrients in primary sclerosing cholangitis. Gastroenterology 160, 1784–1798.e0 (2021).
Kummen, M. et al. Elevated trimethylamine‐ N‐oxide (TMAO) is associated with poor prognosis in primary sclerosing cholangitis patients with normal liver function. United European Gastroenterol. J. 5, 532–541 (2017).
Ponsioen, C. Y. et al. Defining primary sclerosing cholangitis: results from an international primary sclerosing cholangitis study group consensus process. Gastroenterology 161, 1764–1775.e5 (2021). An interdisciplinary group of experts defines PSC, which is helpful worldwide to increase awareness and facilitate early diagnosis.
Kozaka, K., Sheedy, S. P., Eaton, J. E., Venkatesh, S. K. & Heiken, J. P. Magnetic resonance imaging features of small-duct primary sclerosing cholangitis. Abdom. Radiol. 45, 2388–2399 (2020).
Ringe, K. I. et al. Clinical features and MRI progression of small duct primary sclerosing cholangitis (PSC). Eur. J. Radiol. 129, 109101 (2020).
Broome, U. et al. Natural history and prognostic factors in 305 Swedish patients with primary sclerosing cholangitis. Gut 38, 610–615 (1996).
Kuo, A. et al. Characteristics and outcomes reported by patients with primary sclerosing cholangitis through an online registry. Clin. Gastroenterol. Hepatol. 17, 1372–1378 (2019).
Valentino, P. L. et al. The natural history of primary sclerosing cholangitis in children: a large single-center longitudinal cohort study. J. Pediatr. Gastroenterol. Nutr. 63, 603–609 (2016).
Dave, M., Elmunzer, B. J., Dwamena, B. A. & Higgins, P. D. R. Primary sclerosing cholangitis: meta-analysis of diagnostic performance of MR cholangiopancreatography. Radiology 256, 387–396 (2010).
Alvarez, F. et al. International Autoimmune Hepatitis Group Report: review of criteria for diagnosis of autoimmune hepatitis. J. Hepatol. 31, 929–938 (1999).
van Buuren, H. R., van Hoogstraten, H. J. E., Terkivatan, T., Schalm, S. W. & Vleggaar, F. P. High prevalence of autoimmune hepatitis among patients with primary sclerosing cholangitis. J. Hepatol. 33, 543–548 (2000).
Ricciuto, A., Kamath, B. M., Hirschfield, G. M. & Trivedi, P. J. Primary sclerosing cholangitis and overlap features of autoimmune hepatitis: a coming of age or an age-ist problem? J. Hepatol. 79, 567–575 (2023).
Trivedi, P. J. & Hirschfield, G. M. Review article: overlap syndromes and autoimmune liver disease. Aliment. Pharmacol. Ther. 36, 517–533 (2012).
Wunsch, E. et al. Anti‐glycoprotein 2 (anti‐GP2) IgA and anti‐neutrophil cytoplasmic antibodies to serine proteinase 3 (PR3‐ANCA): antibodies to predict severe disease, poor survival and cholangiocarcinoma in primary sclerosing cholangitis. Aliment. Pharmacol. Ther. 53, 302–313 (2021).
Hunyady, P. et al. Secondary sclerosing cholangitis following coronavirus disease 2019 (COVID-19): a multicenter retrospective study. Clin. Infect. Dis. 76, e179–e187 (2023).
Pi, B. et al. Immune-related cholangitis induced by immune checkpoint inhibitors: a systematic review of clinical features and management. Eur. J. Gastroenterol. Hepatol. 33, e858–e867 (2021).
Peeraphatdit, T. B. et al. Hepatotoxicity from immune checkpoint inhibitors: a systematic review and management recommendation. Hepatology 72, 315–329 (2020).
Cazzagon, N. et al. The complementary value of magnetic resonance imaging and vibration-controlled transient elastography for risk stratification in primary sclerosing cholangitis. Am. J. Gastroenterol. 114, 1878–1885 (2019).
Muir, A. J. et al. Simtuzumab for primary sclerosing cholangitis: phase 2 study results with insights on the natural history of the disease. Hepatology 69, 684–698 (2019).
Eaton, J. E. et al. Changes in liver stiffness, measured by magnetic resonance elastography, associated with hepatic decompensation in patients with primary sclerosing cholangitis. Clin. Gastroenterol. Hepatol. 18, 1576–1583.e1 (2020).
Trivedi, P. J. et al. Inter- and intra-individual variation, and limited prognostic utility, of serum alkaline phosphatase in a trial of patients with primary sclerosing cholangitis. Clin. Gastroenterol. Hepatol. 19, 1248–1257 (2021).
De Vries, E. M. et al. A novel prognostic model for transplant-free survival in primary sclerosing cholangitis. Gut 67, 1864–1869 (2018).
Goode, E. C. et al. Factors associated with outcomes of patients with primary sclerosing cholangitis and development and validation of a risk scoring system. Hepatology 69, 2120–2135 (2019).
Eaton, J. E. et al. Primary sclerosing cholangitis risk estimate tool (PREsTo) predicts outcomes of the disease: a derivation and validation study using machine learning. Hepatology 71, 214–224 (2020).
Deneau, M. R. et al. The sclerosing cholangitis outcomes in pediatrics (SCOPE) index: a prognostic tool for children. Hepatology 73, 1074–1087 (2021).
Lundberg Båve, A. et al. Colectomy in patients with ulcerative colitis is not associated to future diagnosis of primary sclerosing cholangitis. United European Gastroenterol. J. 11, 471–481 (2023).
van Munster, K. N. et al. Disease burden in primary sclerosing cholangitis in the Netherlands: a long-term follow-up study. Liver Int. 43, 639–648 (2023).
Olsson, R. et al. High-dose ursodeoxycholic acid in primary sclerosing cholangitis: a 5-year multicenter, randomized, controlled study. Gastroenterology 129, 1464–1472 (2005).
Lindor, K. D. et al. High-dose ursodeoxycholic acid for the treatment of primary sclerosing cholangitis. Hepatology 50, 808–814 (2009).
Ponsioen, C. Y., Lindor, K. D., Mehta, R. & Dimick‐Santos, L. Design and endpoints for clinical trials in primary sclerosing cholangitis. Hepatology 68, 1174–1188 (2018).
Ponsioen, C. Y. et al. Surrogate endpoints for clinical trials in primary sclerosing cholangitis: review and results from an International PSC Study Group consensus process. Hepatology 63, 1357–1367 (2016).
Poupon, R. Liver alkaline phosphatase: a missing link between choleresis and biliary inflammation. Hepatology 61, 2080–2090 (2015).
Stanich, P. P. et al. Alkaline phosphatase normalization is associated with better prognosis in primary sclerosing cholangitis. Dig. Liver Dis. 43, 309–313 (2011).
Lindström, L., Hultcrantz, R., Boberg, K. M., Friis–Liby, I. & Bergquist, A. Association between reduced levels of alkaline phosphatase and survival times of patients with primary sclerosing cholangitis. Clin. Gastroenterol. Hepatol. 11, 841–846 (2013).
Rupp, C. et al. Reduction in alkaline phosphatase is associated with longer survival in primary sclerosing cholangitis, independent of dominant stenosis. Aliment. Pharmacol. Ther. 40, 1292–1301 (2014).
Al Mamari, S., Djordjevic, J., Halliday, J. S. & Chapman, R. W. Improvement of serum alkaline phosphatase to <1.5 upper limit of normal predicts better outcome and reduced risk of cholangiocarcinoma in primary sclerosing cholangitis. J. Hepatol. 58, 329–334 (2013).
De Vries, E. M. G. et al. Alkaline phosphatase at diagnosis of primary sclerosing cholangitis and 1 year later: evaluation of prognostic value. Liver Int. 36, 1867–1875 (2016).
Middelburg, T. et al. The association between cholestatic biochemical markers and clinical symptoms in patients with non-end-stage primary sclerosing cholangitis. J. Hepatol. 78, S408 (2023).
Corpechot, C. et al. Baseline values and changes in liver stiffness measured by transient elastography are associated with severity of fibrosis and outcomes of patients with primary sclerosing cholangitis. Gastroenterology 146, 970–979.e6 (2014).
Ehlken, H. et al. Validation of transient elastography and comparison with spleen length measurement for staging of fibrosis and clinical prognosis in primary sclerosing cholangitis. PLoS ONE 11, e0164224 (2016).
Chazouilleres, O. et al. Prospective validation of the prognostic value of liver stiffness (LS) assessed by Fibroscan in primary sclerosing cholangitis (PSC): interim analysis of the Ficus study. Hepatology 70, 33A–34A (2019).
Chazouillères, O. et al. GS-007 Prospective validation of the prognostic value of liver stiffness assessed by Fibroscan in primary sclerosing cholangitis: final results of the FICUS study. J. Hepatol. 80, S5 (2024).
Fossdal, G. et al. Fluctuating biomarkers in primary sclerosing cholangitis: a longitudinal comparison of alkaline phosphatase, liver stiffness, and ELF. JHEP Rep. Innov. Hepatol. 3, 100328 (2021).
de Vries, E. M. G. et al. Enhanced liver fibrosis test predicts transplant-free survival in primary sclerosing cholangitis, a multi-centre study. Liver Int. 37, 1554–1561 (2017).
Cadranel, J.-F., Rufat, P. & Degos, F. Practices of liver biopsy in France: results of a prospective nationwide survey. For the Group of Epidemiology of the French Association for the Study of the Liver (AFEF). Hepatology 32, 477–481 (2000).
Lindor, K. D. et al. The role of ultrasonography and automatic-needle biopsy in outpatient percutaneous liver biopsy. Hepatology 23, 1079–1083 (1996).
Olsson, R. et al. Sampling variability of percutaneous liver biopsy in primary sclerosing cholangitis. J. Clin. Pathol. 48, 933–935 (1995).
De Vries, E. M. G. et al. Applicability and prognostic value of histologic scoring systems in primary sclerosing cholangitis. J. Hepatol. 63, 1212–1219 (2015).
De Vries, E. M. G. et al. Validation of the prognostic value of histologic scoring systems in primary sclerosing cholangitis: an international cohort study. Hepatology 65, 907–919 (2017).
Kim, W. R. et al. A revised natural history model for primary sclerosing cholangitis. Mayo Clin. Proc. 75, 688–694 (2000).
Goet, J. C. et al. Validation, clinical utility and limitations of the Amsterdam-Oxford model for primary sclerosing cholangitis. J. Hepatol. 71, 992–999 (2019).
Ponsioen, C. Y., Lam, K., Van Milligen De Wit, A. W. M., Huibregtse, K. & Tytgat, G. N. J. Four years experience with short term stenting in primary sclerosing cholangitis. Am. J. Gastroenterol. 94, 2403–2407 (1999).
Ponsioen, C. Y. Endpoints in the design of clinical trials for primary sclerosing cholangitis. Biochim. Biophys. Acta Mol. Basis Dis. 1864, 1410–1414 (2018).
Younossi, Z. M. et al. Development and validation of a primary sclerosing cholangitis-specific patient‐reported outcomes instrument: the PSC PRO. Hepatology 68, 155–165 (2018).
Younossi, Z. M., Stepanova, M., Younossi, I. & Racila, A. Development and validation of a primary sclerosing cholangitis-specific health-related quality of life instrument: CLDQ-PSC. Hepatol. Commun. 7, e0049 (2023). PSC-specific HRQoL criteria to be used in future clinical trials for developing urgently needed novel therapies.
Munster, K. N., Dijkgraaf, M. G. W., Gennep, S., Beuers, U. & Ponsioen, C. Y. The simple cholestatic complaints score is a valid and quick patient‐reported outcome measure in primary sclerosing cholangitis. Liver Int. 40, 2758–2766 (2020).
Walmsley, R. S., Ayres, R. C., Pounder, R. E. & Allan, R. N. A simple clinical colitis activity index. Gut 43, 29–32 (1998).
Harvey, R. F. & Bradshaw, M. J. Measuring Crohn’s disease activity. Lancet 1, 1134–1135 (1980).
Stiehl, A., Rudolph, G., Klöters-Plachky, P., Sauer, P. & Walker, S. Development of dominant bile duct stenoses in patients with primary sclerosing cholangitis treated with ursodeoxycholic acid: outcome after endoscopic treatment. J. Hepatol. 36, 151–156 (2002).
Gotthardt, D. N., Rudolph, G., Klöters-Plachky, P., Kulaksiz, H. & Stiehl, A. Endoscopic dilation of dominant stenoses in primary sclerosing cholangitis: outcome after long-term treatment. Gastrointest. Endosc. 71, 527–534 (2010).
Aabakken, L. et al. Role of endoscopy in primary sclerosing cholangitis: European Society of Gastrointestinal Endoscopy (ESGE) and European Association for the Study of the Liver (EASL) Clinical Guideline. Endoscopy 49, 588–608 (2017). One of the latest clinical practice guidelines of the most important scientific societies in the field: EASL, AASLD and European Society of Gastrointestinal Endoscopy.
Venkatesh, S. K. et al. Reporting standards for primary sclerosing cholangitis using MRI and MR cholangiopancreatography: guidelines from MR Working Group of the International Primary Sclerosing Cholangitis Study Group. Eur. Radiol. 32, 923–937 (2022).
Ferreira, M. T. G. B. et al. Stent versus balloon dilation for the treatment of dominant strictures in primary sclerosing cholangitis: a systematic review and meta-analysis. Clin. Endosc. 54, 833–842 (2021).
Ponsioen, C. Y. et al. No superiority of stents vs balloon dilatation for dominant strictures in patients with primary sclerosing cholangitis. Gastroenterology 155, 752–759.e5 (2018).
Rupp, C. et al. Effect of scheduled endoscopic dilatation of dominant strictures on outcome in patients with primary sclerosing cholangitis. Gut 68, 2170–2178 (2019).
Jansen, P. L. M. et al. The ascending pathophysiology of cholestatic liver disease. Hepatology 65, 722–738 (2017).
Vartak, N. et al. On the mechanisms of biliary flux. Hepatology 74, 3497–3512 (2021).
Eaton, J. E. et al. Predictors of jaundice resolution and survival after endoscopic treatment of primary sclerosing cholangitis. Hepatol. Commun. 6, 809–820 (2022).
Peiseler, M. et al. Risk of endoscopic biliary interventions in primary sclerosing cholangitis is similar between patients with and without cirrhosis. PLoS ONE 13, e0202686 (2018).
Natt, N., Michael, F., Michael, H., Dubois, S. & Al Mazrou’i, A. ERCP-related adverse events in primary sclerosing cholangitis: a systematic review and meta-analysis. Can. J. Gastroenterol. Hepatol. 2022, 2372257 (2022).
El-Matary, W. et al. Colorectal dysplasia and cancer in pediatric-onset ulcerative colitis associated with primary sclerosing cholangitis. Clin. Gastroenterol. Hepatol. 19, 1067–1070.e2 (2021).
Navaneethan, U. et al. Random biopsies during surveillance colonoscopy increase dysplasia detection in patients with primary sclerosing cholangitis and ulcerative colitis. J. Crohns Colitis 7, 974–981 (2013).
Pouw, R. E. et al. Endoscopic tissue sampling – Part 2: lower gastrointestinal tract. European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy 53, 1261–1273 (2021).
Wanders, L. K. et al. Cancer risk after resection of polypoid dysplasia in patients with longstanding ulcerative colitis: a meta-analysis. Clin. Gastroenterol. Hep. 12, 756–764 (2014).
Laine, L. et al. SCENIC international consensus statement on surveillance and management of dysplasia in inflammatory bowel disease. Gastrointest. Endosc. 81, 489–501 (2015).
Eliasson, J. et al. Survey uncovering variations in the management of primary sclerosing cholangitis across Europe. JHEP Rep. Innov. Hepatol. 4, 100553 (2022).
Poropat, G., Giljaca, V., Stimac, D. & Gluud, C. Bile acids for primary sclerosing cholangitis. Cochrane Database Syst. Rev. 2011, CD003626 (2011).
Beuers, U. et al. Ursodeoxycholic acid for treatment of primary sclerosing cholangitis: a placebo-controlled trial. Hepatology 16, 707–714 (1992).
Lindor, K. D. Ursodiol for primary sclerosing cholangitis. N. Engl. J. Med. 336, 691–695 (1997).
Arizumi, T. et al. Ursodeoxycholic acid is associated with improved long-term outcome in patients with primary sclerosing cholangitis. J. Gastroenterol. 57, 902–912 (2022). Significant publication on the controversial use of UDCA in PSC.
Deneau, M. R. et al. Gamma glutamyltransferase reduction is associated with favorable outcomes in pediatric primary sclerosing cholangitis. Hepatol. Commun. 2, 1369–1378 (2018).
Wunsch, E. et al. Prospective evaluation of ursodeoxycholic acid withdrawal in patients with primary sclerosing cholangitis. Hepatology 60, 931–940 (2014).
Card, T. R., Solaymani-Dodaran, M. & West, J. Incidence and mortality of primary sclerosing cholangitis in the UK: a population-based cohort study. J. Hepatol. 48, 939–944 (2008).
Roberts, M. S., Angus, D. C., Bryce, C. L., Valenta, Z. & Weissfeld, L. Survival after liver transplantation in the United States: a disease-specific analysis of the UNOS database. Liver Transplant. 10, 886–897 (2004).
Organ Procurement and Transplantation Network Policies 188–189 (OPTN, 2024).
Ghali, P. et al. Liver transplantation for incidental cholangiocarcinoma: analysis of the Canadian experience. Liver Transpl. 11, 1412–1416 (2005).
Sierra, L. et al. Living-donor liver transplant and improved post-transplant survival in patients with primary sclerosing cholangitis. J. Clin. Med. 12, 2807 (2023).
Heinemann, M. et al. Long-term outcome after living donor liver transplantation compared to donation after brain death in autoimmune liver diseases: experience from the European Liver Transplant Registry. Am. J. Transplant. 22, 626–633 (2022).
Onofrio, F. et al. Living donor liver transplantation can address disparities in transplant access for patients with primary sclerosing cholangitis. Hepatol. Commun. 7, e0219 (2023).
Forman, L. M. et al. Reply: living donor liver transplantation for people with PSC. Hepatology 77, E97–E98 (2023).
Visseren, T. et al. Inflammatory conditions play a role in recurrence of PSC after liver transplantation: an international multicentre study. JHEP Rep. 4, 100599 (2022).
Pandanaboyana, S., Bell, R., Bartlett, A. J., McCall, J. & Hidalgo, E. Meta-analysis of duct-to-duct versus roux-en-Y biliary reconstruction following liver transplantation for primary sclerosing cholangitis. Transpl. Int. 28, 485–491 (2015).
Jonica, E. R., Han, S., Burton, J. R., Pomposelli, J. J. & Shah, R. J. Choledochoduodenostomy is associated with fewer post-transplant biliary complications compared to Roux-en-Y in primary sclerosing cholangitis patients. Clin. Transplant. 36, e14597 (2022).
Shamsaeefar, A. et al. Biliary reconstruction in liver transplant patients with primary sclerosing cholangitis, duct-to-duct or Roux-en-Y? Clin. Transplant. https://doi.org/10.1111/ctr.12964 (2017).
Fosby, B., Karlsen, T. H. & Melum, E. Recurrence and rejection in liver transplantation for primary sclerosing cholangitis. World J. Gastroenterol. 18, 1–15 (2012).
Narumi, S., Roberts, J. P., Emond, J. C., Lake, J. & Ascher, N. L. Liver transplantation for sclerosing cholangitis. Hepatology 22, 451–457 (1995).
Graziadei, I. W. et al. Recurrence of primary sclerosing cholangitis following liver transplantation. Hepatology 29, 1050–1056 (1999).
Hildebrand, T. et al. Biliary strictures and recurrence after liver transplantation for primary sclerosing cholangitis: a retrospective multicenter analysis. Liver Transpl. 22, 42–52 (2016).
Montano-Loza, A. J., Bhanji, R. A., Wasilenko, S. & Mason, A. L. Systematic review: recurrent autoimmune liver diseases after liver transplantation. Aliment. Pharmacol. Ther. 45, 485–500 (2017).
Alabraba, E. et al. A re-evaluation of the risk factors for the recurrence of primary sclerosing cholangitis in liver allografts: recurrence of primary sclerosing cholangitis. Liver Transpl. 15, 330–340 (2009).
Ravikumar, R. et al. Risk factors for recurrent primary sclerosing cholangitis after liver transplantation. J. Hepatol. 63, 1139–1146 (2015).
Joshi, D. et al. The impact of inflammatory bowel disease post-liver transplantation for primary sclerosing cholangitis. Liver Int. 33, 53–61 (2013).
Steenstraten, I. C. et al. Systematic review with meta-analysis: risk factors for recurrent primary sclerosing cholangitis after liver transplantation. Aliment. Pharmacol. Ther. 49, 636–643 (2019).
Trivedi, P. J. et al. The impact of ileal pouch-anal anastomosis on graft survival following liver transplantation for primary sclerosing cholangitis. Aliment. Pharmacol. Ther. 48, 322–332 (2018).
Rowe, I. A. et al. The impact of disease recurrence on graft survival following liver transplantation: a single centre experience. Transpl. Int. 21, 459–465 (2008).
Trauner, M., Fuchs, C. D., Halilbasic, E. & Paumgartner, G. New therapeutic concepts in bile acid transport and signaling for management of cholestasis. Hepatology 65, 1393–1404 (2017).
Nevens, F. et al. a placebo-controlled trial of obeticholic acid in primary biliary cholangitis. N. Engl. J. Med. 375, 631–643 (2016).
Murillo Perez, C. F. et al. Greater transplant-free survival in patients receiving obeticholic acid for primary biliary cholangitis in a clinical trial setting compared to real-world external controls. Gastroenterology 163, 1630–1642.e3 (2022).
Trauner, M. et al. The nonsteroidal farnesoid X receptor agonist cilofexor (GS-9674) improves markers of cholestasis and liver injury in patients with primary sclerosing cholangitis. Hepatology 70, 788–801 (2019).
Trauner, M. et al. Safety and sustained efficacy of the farnesoid X receptor (FXR) agonist cilofexor over a 96-week open-label extension in patients with PSC. Clin. Gastroenterol. Hepatol. 21, 1552–1560.e2 (2023).
Trauner, M. et al. PRIMIS: design of a pivotal, randomized, phase 3 study evaluating the safety and efficacy of the nonsteroidal farnesoid X receptor agonist cilofexor in noncirrhotic patients with primary sclerosing cholangitis. BMC Gastroenterol. 23, 75 (2023).
Trauner, M. et al. A phase 3, randomized, double-blind, placebo-controlled study evaluating the efficacy and safety of cilofexor in patients with non-cirrhotic primary sclerosing cholangitis (PRIMIS). J. Hepatol. 78, S12–S13 (2023).
Levy, C. et al. Safety and efficacy of the farnesoid X receptor (FXR) agonist cilofexor in a proof-of-concept study in patients with compensated cirrhosis due to primary sclerosing cholangitis (PSC). J. Hepatol. 77, S319–S320 (2022).
Karpen, S. J., Kelly, D., Mack, C. & Stein, P. Ileal bile acid transporter inhibition as an anticholestatic therapeutic target in biliary atresia and other cholestatic disorders. Hepatol. Int. 14, 677–689 (2020).
Kamath, B. M., Stein, P., Houwen, R. H. J. & Verkade, H. J. Potential of ileal bile acid transporter inhibition as a therapeutic target in Alagille syndrome and progressive familial intrahepatic cholestasis. Liver Int. 40, 1812–1822 (2020).
Hegade, V. S. et al. Effect of ileal bile acid transporter inhibitor GSK2330672 on pruritus in primary biliary cholangitis: a double-blind, randomised, placebo-controlled, crossover, phase 2a study. Lancet 389, 1114–1123 (2017).
Levy, C. et al. GLIMMER: a randomized phase 2b dose-ranging trial of linerixibat in primary biliary cholangitis patients with pruritus. Clin. Gastroenterol. Hepatol. 21, 1902–1912.e13 (2023).
Baghdasaryan, A. et al. Dual farnesoid X receptor/TGR5 agonist INT-767 reduces liver injury in the Mdr2−/− (Abcb4−/−) mouse cholangiopathy model by promoting biliary HCO−3 output. Hepatology 54, 1303–1312 (2011).
Miethke, A. G. et al. Pharmacological inhibition of apical sodium-dependent bile acid transporter changes bile composition and blocks progression of sclerosing cholangitis in multidrug resistance 2 knockout mice. Hepatology 63, 512–523 (2016).
Fuchs, C. D. et al. Colesevelam attenuates cholestatic liver and bile duct injury in Mdr2−/− mice by modulating composition, signalling and excretion of faecal bile acids. Gut 67, 1683–1691 (2018).
Yoon, Y. B. et al. Effect of side-chain shortening on the physiologic properties of bile acids: hepatic transport and effect on biliary secretion of 23-nor-ursodeoxycholate in rodents. Gastroenterology 90, 837–852 (1986).
Halilbasic, E. et al. Side chain structure determines unique physiologic and therapeutic properties of norursodeoxycholic acid in Mdr2−/− mice. Hepatology 49, 1972–1981 (2009).
Moustafa, T. et al. Alterations in lipid metabolism mediate inflammation, fibrosis, and proliferation in a mouse model of chronic cholestatic liver injury. Gastroenterology 142, 140–151.e12 (2012).
Zhu, C. et al. 24-Norursodeoxycholic acid reshapes immunometabolism in CD8+ T cells and alleviates hepatic inflammation. J. Hepatol. 75, 1164–1176 (2021).
Trauner, M. et al. Norucholic acid for the treatment of primary sclerosing cholangitis: baseline data from a phase III trial. J. Hepatol. 78, S403 (2023).
Kowdley, K. V. et al. A randomized, dose-finding, proof-of-concept study of berberine ursodeoxycholate in patients with primary sclerosing cholangitis. Am. J. Gastroenterol. 117, 1805–1815 (2022).
Mueller, M. et al. Ursodeoxycholic acid exerts farnesoid X receptor-antagonistic effects on bile acid and lipid metabolism in morbid obesity. J. Hepatol. 62, 1398–1404 (2015).
Nevens, F., Trauner, M. & Manns, M. P. Primary biliary cholangitis as a roadmap for the development of novel treatments for cholestatic liver diseases. J. Hepatol. 78, 430–441 (2023).
Corpechot, C. et al. A placebo-controlled trial of bezafibrate in primary biliary cholangitis. N. Engl. J. Med. 378, 2171–2181 (2018).
Tanaka, A. et al. Association of bezafibrate with transplant-free survival in patients with primary biliary cholangitis. J. Hepatol. 75, 565–571 (2021).
Mizuno, S. et al. Prospective study of bezafibrate for the treatment of primary sclerosing cholangitis. J. Hepatobiliary Pancreatic Sci. 22, 766–770 (2015).
Lemoinne, S. et al. Primary sclerosing cholangitis response to the combination of fibrates with ursodeoxycholic acid: French-Spanish experience. Clin. Res. Hepatol. Gastroenterol. 42, 521–528 (2018).
Ghonem, N. S. et al. Fenofibrate improves liver function and reduces the toxicity of the bile acid pool in patients with primary biliary cholangitis and primary sclerosing cholangitis who are partial responders to ursodiol. Clin. Pharmacol. Ther. 108, 1213–1223 (2020).
de Vries, E. et al. Fibrates for itch (FITCH) in fibrosing cholangiopathies: a double-blind, randomized, placebo-controlled trial. Gastroenterology 160, 734–743.e6 (2021).
Hatami, B. et al. Fenofibrate in primary sclerosing cholangitis; a randomized, double-blind, placebo-controlled trial. Pharmacol. Res. Perspect. 10, e00984 (2022).
Hirschfield, G. M. et al. A phase 3 trial of seladelpar in primary biliary cholangitis. N. Engl. J. Med. 390, 783–794 (2024).
Bowlus, C. L. et al. A phase II, randomized, open-label, 52-week study of seladelpar in patients with primary biliary cholangitis. J. Hepatol. 77, 353–364 (2022).
Kremer, A. E. et al. Seladelpar improved measures of pruritus, sleep, and fatigue and decreased serum bile acids in patients with primary biliary cholangitis. Liver Int. 42, 112–123 (2022).
Schattenberg, J. M. et al. A randomized placebo-controlled trial of elafibranor in patients with primary biliary cholangitis and incomplete response to UDCA. J. Hepatol. 74, 1344–1354 (2021).
Kowdley, K. V. et al. Efficacy and safety of elafibranor in primary biliary cholangitis. N. Engl. J. Med. 390, 795–805 (2024).
Trauner, M. & Fuchs, C. D. Novel therapeutic targets for cholestatic and fatty liver disease. Gut 71, 194–209 (2022).
Stokkeland, K., Höijer, J., Bottai, M., Söderberg-Löfdal, K. & Bergquist, A. Statin use is associated with improved outcomes of patients with primary sclerosing cholangitis. Clin. Gastroenterol. Hepatol. 17, 1860–1866.e1 (2019).
Dubrovsky, A. M. K. & Bowlus, C. L. Statins, fibrates, and other peroxisome proliferator-activated receptor agonists for the treatment of cholestatic liver diseases. Gastroenterol. Hepatol. 16, 31–38 (2020).
Eksteen, B. et al. Efficacy and safety of cenicriviroc in patients with primary sclerosing cholangitis: PERSEUS study. Hepatol. Commun. 5, 478–490 (2021).
Hirschfield, G. et al. INTEGRIS-PSC phase 2a study: evaluating the safety, tolerability, and pharmacokinetics of bexotegrast (PLN-74809) in participants with primary sclerosing cholangitis. J. Hepatol. 78, S356 (2023).
de Krijger, M., Wildenberg, M. E., de Jonge, W. J. & Ponsioen, C. Y. Return to sender: lymphocyte trafficking mechanisms as contributors to primary sclerosing cholangitis. J. Hepatol. 71, 603–615 (2019).
Graham, J. J. et al. Aberrant hepatic trafficking of gut-derived T cells is not specific to primary sclerosing cholangitis. Hepatology 75, 518–530 (2022).
Caron, B. et al. Vedolizumab therapy is ineffective for primary sclerosing cholangitis in patients with inflammatory bowel disease: a GETAID multicentre cohort study. J. Crohns Colitis 13, 1239–1247 (2019).
Lynch, K. D. et al. Effects of vedolizumab in patients with primary sclerosing cholangitis and inflammatory bowel diseases. Clin. Gastroenterol. Hepatol. 18, 179–187.e6 (2020).
Christensen, B. et al. Vedolizumab in patients with concurrent primary sclerosing cholangitis and inflammatory bowel disease does not improve liver biochemistry but is safe and effective for the bowel disease. Aliment. Pharmacol. Ther. 47, 753–762 (2018).
Tse, C. S., Loftus, E. V., Raffals, L. E., Gossard, A. A. & Lightner, A. L. Effects of vedolizumab, adalimumab and infliximab on biliary inflammation in individuals with primary sclerosing cholangitis and inflammatory bowel disease. Aliment. Pharmacol. Ther. 48, 190–195 (2018).
Hedin, C. R. H. et al. Effects of tumor necrosis factor antagonists in patients with primary sclerosing cholangitis. Clin. Gastroenterol. Hepatol. 18, 2295–2304.e2 (2020).
Tilg, H., Adolph, T. E. & Trauner, M. Gut-liver axis: pathophysiological concepts and clinical implications. Cell Metab. 34, 1700–1718 (2022).
Damman, J. L. et al. Review article: the evidence that vancomycin is a therapeutic option for primary sclerosing cholangitis. Aliment. Pharmacol. Ther. 47, 886–895 (2018).
Allegretti, J. R. et al. Fecal microbiota transplantation in patients with primary sclerosing cholangitis: a pilot clinical trial. Am. J. Gastroenterol. 114, 1071–1079 (2019).
Tabibian, J. H. et al. Absence of the intestinal microbiota exacerbates hepatobiliary disease in a murine model of primary sclerosing cholangitis. Hepatology 63, 185–196 (2016).
Ichikawa, M. et al. Bacteriophage therapy against pathological Klebsiella pneumoniae ameliorates the course of primary sclerosing cholangitis. Nat. Commun. 14, 3261 (2023).
van Munster, K. N., Dijkgraaf, M. G. W., Oude Elferink, R. P. J., Beuers, U. & Ponsioen, C. Y. Symptom patterns in the daily life of PSC patients. Liver Int. 42, 1562–1570 (2022).
Ponsioen, C. Diagnosis, prognosis, and management of primary sclerosing cholangitis. Gastroenterol. Hepatol. 9, 453–465 (2013).
Haapamäki, J., Tenca, A., Sintonen, H., Barner-Rasmussen, N. & Färkkilä, M. A. Health-related quality of life among patients with primary sclerosing cholangitis. Liver Int. 35, 2194–2201 (2015).
Cheung, A. C. et al. Factors that influence health-related quality of life in patients with primary sclerosing cholangitis. Dig. Dis. Sci. 61, 1692–1699 (2016).
Schmeltzer, P. A. & Russo, M. W. Systematic review of prognostic models compared to the Mayo Risk Score for primary sclerosing cholangitis. J. Clin. Med. 10, 4476 (2021).
Van Der Plas, S. M. et al. Generic and disease-specific health related quality of life of liver patients with various aetiologies: a survey. Qual. Life Res. 16, 375–388 (2007).
Hawthorne, G., Densley, K., Pallant, J. F., Mortimer, D. & Segal, L. Deriving utility scores from the SF-36 health instrument using Rasch analysis. Qual. Life Res. 17, 1183–1193 (2008).
Younossi, Z. M., Guyatt, G., Kiwi, M., Boparai, N. & King, D. Development of a disease specific questionnaire to measure health related quality of life in patients with chronic liver disease. Gut 45, 295–300 (1999).
Younossi, Z. M., Stepanova, M. & Henry, L. Performance and validation of chronic liver disease questionnaire-hepatitis C version (CLDQ-HCV) in clinical trials of patients with chronic hepatitis C. Value Health 19, 544–551 (2016).
Younossi, Z. M., Stepanova, M., Younossi, I. & Racila, A. Development and validation of a hepatitis B‐specific health‐related quality‐of‐life instrument: CLDQ‐HBV. J. Viral Hepat. 28, 484–492 (2021).
Younossi, Z. M. et al. A disease-specific quality of life instrument for non-alcoholic fatty liver disease and non-alcoholic steatohepatitis: CLDQ-NAFLD. Liver Int. 37, 1209–1218 (2017).
Hasan, I., Putra, R. P., Yunihastuti, E. & Kurniawan, J. The validity and reliability of the Indonesian version of the chronic liver disease questionnaire (CLDQ) in measuring quality of life in patients with liver cirrhosis. Acta Medica Indones. 54, 10–18 (2022).
Ai, X., Yang, X., Fu, H.-Y., Xu, J.-M. & Tang, Y.-M. Health-related quality of life questionnaires used in primary biliary cholangitis: a systematic review. Scand. J. Gastroenterol. 57, 333–339 (2022).
Younossi, Z. M. et al. Long-term patient-centered outcomes in cirrhotic patients with chronic hepatitis C after achieving sustained virologic response. Clin. Gastroenterol. Hepatol. 20, 438–446 (2022).
Plotogea, O.-M. et al. Assessment of sleep among patients with chronic liver disease: association with quality of life. J. Pers. Med. 11, 1387 (2021).
Khairullah, S. & Mahadeva, S. Translation, adaptation and validation of two versions of the Chronic Liver Disease Questionnaire in Malaysian patients for speakers of both English and Malay languages: a cross-sectional study. BMJ Open 7, e013873 (2017).
Elman, S., Hynan, L. S., Gabriel, V. & Mayo, M. J. The 5-D itch scale: a new measure of pruritus. Br. J. Dermatol. 162, 587–593 (2010).
Fisk, J. D. et al. Measuring the functional impact of fatigue: initial validation of the fatigue impact scale. Clin. Infect. Dis. 18, S79–S83 (1994).
Krupp, L. B., LaRocca, N. G., Muir-Nash, J. & Steinberg, A. D. The fatigue severity scale. Application to patients with multiple sclerosis and systemic lupus erythematosus. Arch. Neurol. 46, 1121–1123 (1989).
Isa, F. et al. Patient-reported outcome measures used in patients with primary sclerosing cholangitis: a systematic review. Health Qual. Life Outcomes 16, 133 (2018).
Dyson, J. K. et al. Fatigue in primary sclerosing cholangitis is associated with sympathetic over-activity and increased cardiac output. Liver Int. 35, 1633–1641 (2015).
Jopson, L., Dyson, J. K. & Jones, D. E. J. Understanding and treating fatigue in primary biliary cirrhosis and primary sclerosing cholangitis. Clin. Liver Dis. 20, 131–142 (2016).
Mol, B. et al. Health-related quality of life in patients with primary sclerosing cholangitis: a longitudinal population-based cohort study. Liver Int. 43, 1056–1067 (2023).
Reilly, M. C., Zbrozek, A. S. & Dukes, E. M. The validity and reproducibility of a work productivity and activity impairment instrument. PharmacoEconomics 4, 353–365 (1993).
Raszeja-Wyszomirska, J. et al. Prospective evaluation of PBC-specific health-related quality of life questionnaires in patients with primary sclerosing cholangitis. Liver Int. 35, 1764–1771 (2015).
Younossi, Z. M., Kiwi, M. L., Boparai, N., Price, L. L. & Guyatt, G. Cholestatic liver diseases and health-related quality of life. Am. J. Gastroenterol. 95, 497–502 (2000).
Marcus, E., Stone, P., Thorburn, D., Walmsley, M. & Vivat, B. Quality of life (QoL) for people with primary sclerosing cholangitis (PSC): a pragmatic strategy for identifying relevant QoL issues for rare disease. J. Patient Rep. Outcomes 6, 76 (2022).
Chapman, R. W. Primary sclerosing cholangitis — a long night’s journey into day. Clin. Liver Dis. 20, 21–32 (2022).
Bhat, P. & Aabakken, L. Role of endoscopy in primary sclerosing cholangitis. Clin. Endosc. 54, 193–201 (2021).
Fricker, Z. P. & Lichtenstein, D. R. Primary sclerosing cholangitis: a concise review of diagnosis and management. Dig. Dis. Sci. 64, 632–642 (2019).
Pria, H. D. et al. Practical guide for radiological diagnosis of primary and secondary sclerosing cholangitis. Semin. Ultrasound CT MR 43, 490–509 (2022).
Hartmann, P. & Schnabl, B. Can a mucosal microbiota signature predict disease severity, survival, and disease recurrence in PSC? Hepatology 77, 709–711 (2023). Understanding the role of the mucosal microbiota signature in PSC may lead to future novel therapies, including FMT.
Özdirik, B., Müller, T., Wree, A., Tacke, F. & Sigal, M. The role of microbiota in primary sclerosing cholangitis and related biliary malignancies. Int. J. Mol. Sci. 22, 6975 (2021).
Bambha, K. et al. Incidence, clinical spectrum, and outcomes of primary sclerosing cholangitis in a united states community. Gastroenterology 125, 1364–1369 (2003).
Byron, D. & Minuk, G. Y. Clinical hepatology: profile of an urban, hospital-based practice. Hepatology 24, 813–815 (1996).
Hurlburt, K. J. et al. Prevalence of autoimmune liver disease in Alaska natives. Am. J. Gastroenterol. 97, 2402–2407 (2002).
Toy, E., Balasubramanian, S., Selmi, C., Li, C.-S. & Bowlus, C. L. The prevalence, incidence and natural history of primary sclerosing cholangitis in an ethnically diverse population. BMC Gastroenterol. 11, 83 (2011).
Kaplan, G. G., Laupland, K. B., Butzner, D., Urbanski, S. J. & Lee, S. S. The burden of large and small duct primary sclerosing cholangitis in adults and children: a population-based analysis. Am. J. Gastroenterol. 102, 1042–1049 (2007).
Nardelli, M. J. et al. Clinical features and outcomes of primary sclerosing cholangitis in the highly admixed Brazilian population. Can. J. Gastroenterol. Hepatol. 2021, 7746401 (2021).
Xu, X. et al. Prevalence and clinical profiles of primary sclerosing cholangitis in China: data from electronic medical records and systematic literature retrieval. J. Autoimmun. 147, 103264 (2024).
Ang, T. L. et al. Clinical profile of primary sclerosing cholangitis in Singapore. J. Gastroenterol. Hepatol. 17, 908–913 (2002).
Tibdewal, P. et al. Clinical profile and outcome of primary sclerosing cholangitis: a single-centre experience from western India. Indian J. Gastroenterol. 38, 295–302 (2019).
Berdal, J. E., Ebbesen, J. & Rydning, A. Incidence and prevalence of autoimmune liver diseases [Norweigan]. Tidsskr Nor. Laegeforen. 118, 4517–4519 (1998).
Boberg, K. M. et al. Incidence and prevalence of primary biliary cirrhosis, primary sclerosing cholangitis, and autoimmune hepatitis in a Norwegian population. Scand. J. Gastroenterol. 33, 99–103 (1998).
Escorsell, A. et al. Epidemiology of primary sclerosing cholangitis in Spain. J. Hepatol. 21, 787–791 (1994).
Liang, H., Manne, S., Shick, J., Lissoos, T. & Dolin, P. Incidence, prevalence, and natural history of primary sclerosing cholangitis in the United Kingdom. Medicine 96, e7116 (2017).
Kingham, J. G. C., Kochar, N. & Gravenor, M. B. Incidence, clinical patterns, and outcomes of primary sclerosing cholangitis in South Wales, United Kingdom. Gastroenterology 126, 1929–1930 (2004).
Garioud, A. et al. Characteristics and clinical course of primary sclerosing cholangitis in France: a prospective cohort study. Eur. J. Gastroenterol. Hepatol. 22, 842–847 (2010).
Gudnason, H. O. et al. Primary sclerosing cholangitis in Iceland 1992-2012 [Icelandic]. Laeknabladid 105, 371–376 (2019).
Yanai, H. et al. Prognosis of primary sclerosing cholangitis in Israel is independent of coexisting inflammatory bowel disease. J. Crohns Colitis 9, 177–184 (2015).
Hadizadeh, M. et al. Prevalence of inflammatory bowel disease among patients with primary sclerosing cholangitis in Iran. Arab. J. Gastroenterol. 17, 17–19 (2016).
Ataseven, H. et al. Primary sclerosing cholangitis in Turkish patients: characteristic features and prognosis. Hepatobiliary Pancreat. Dis. Int. 8, 312–315 (2009).
Ngu, J. H., Gearry, R. B., Wright, A. J. & Stedman, C. A. M. Inflammatory bowel disease is associated with poor outcomes of patients with primary sclerosing cholangitis. Clin. Gastroenterol. Hepatol. 9, 1092–1097 (2011).
Liu, K. et al. Epidemiology and outcomes of primary sclerosing cholangitis with and without inflammatory bowel disease in an Australian cohort. Liver Int. 37, 442–448 (2017).
Lamba, M., Ngu, J. H. & Stedman, C. A. M. Trends in incidence of autoimmune liver diseases and increasing incidence of autoimmune hepatitis. Clin. Gastroenterol. Hepatol. 19, 573–579.e1 (2021).
Acknowledgements
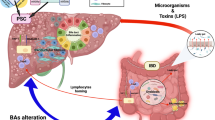
The authors thank J. Debarry and S. Riese for providing editorial assistance. They thank X. Jiang for help in developing Fig. 3.
Author information
Authors and Affiliations
Contributions
Introduction (M.P.M.); Epidemiology (A.B.); Mechanisms/pathophysiology (T.H.K.); Diagnosis, screening and prevention (A.J.M.); Management (C.L., C.P. and M.T.); Quality of life (Z.M.Y.); Outlook (G.W. and M.P.M.); overview of the Primer (M.P.M.). All authors have reviewed and provided input on all sections.
Corresponding author
Ethics declarations
Competing interests
M.P.M. received grants and or consulting fees from Falk Pharma GmbH (Freiburg, Germany), Gilead Sciences, Intercept and Novartis. A.B. received research grants from Gilead Sciences and is part of the Advisory Board Ipsen. T.H.K. received consulting fees from MSD, Albireo and Falk Pharma, and is Board Member and President Elect at Biomed Alliance. C.L. has received research grants and consulting fees from Gilead Science, Ipsen, High Tide, Mirum, Escient, Chemomab and Intercept. A.J.M. received research grants from Gilead Science and Pilant Therapeutics. C.P. received grants from Gilead Sciences, NGM and Perspectum, consultancy fees from Pliant and Chemomab, and speaker’s fees from Tillotts. M.T. has received research grants from Albireo, Alnylam, Cymabay, Falk, Genentech, Gilead, Intercept, MSD, Takeda and Ultragenyx, and travel grants from Abbvie, Falk, Gilead, Intercept and Jannsen. He has advised for Abbvie, Albireo, Agomab, BiomX, Boehringer Ingelheim, Chemomab, Falk Pharma GmbH, Genfit, Gilead, Hightide, Ipsen, Intercept, Janssen, MSD, Novartis, Phenex, Pliant, Regulus, Siemens and Shire, and has served as speaker for Albireo, BMS, Boehringer Ingelheim, Falk, Gilead, Intercept, Ipsen, Madrigal and MSD. He is a co-inventor (service inventions) of patents for the medical use of norUDCA (nor-ursodeoxycholic acid/norucholic acid)/NCA filed by the Medical Universities of Graz and Vienna. G.W. has served as an advisory committee member for AstraZeneca, Gilead Sciences, GlaxoSmithKline and Janssen, and as a speaker for Abbott, AbbVie, Ascletis, Bristol-Myers Squibb, Echosens, Gilead Sciences, Janssen and Roche. She has also received a research grant from Gilead Science. Z.M.Y. received research funding and/or serves as a consultant to Intercept, Cymabay, Boehringer Ingelheim, BMS, GSK, NovoNordisk, AstraZeneca, Ipsen, Siemens, Madrigal, Merck and Abbott.
Peer review
Peer review information
Nature Reviews Disease Primers thanks N. Cazzagon, A. M. Loza, A. Tanaka and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Manns, M.P., Bergquist, A., Karlsen, T.H. et al. Primary sclerosing cholangitis. Nat Rev Dis Primers 11, 17 (2025). https://doi.org/10.1038/s41572-025-00600-x
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41572-025-00600-x
This article is cited by
-
FDX1-mediated cuproptosis promotes cholestatic liver injury exacerbated by taurocholic acid-enhanced copper accumulation
Cell Death Discovery (2026)
-
IL-1 family of cytokines in gastrointestinal and liver disorders
Nature Reviews Gastroenterology & Hepatology (2026)
-
Harnessing organoid technology to model autoimmune pathways in rheumatological and inflammatory disorders
Naunyn-Schmiedeberg's Archives of Pharmacology (2025)
-
Shading light in the black box of hepatobiliary imaging
European Radiology (2025)