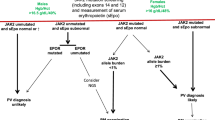
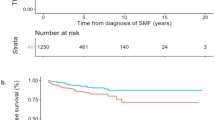
Abstract
Polycythaemia vera (PV) is a haematological malignancy in the myeloproliferative neoplasm family. PV is typically characterized by erythrocytosis and often leukocytosis and thrombocytosis1. Clinical features include reduced life expectancy due to hazards of thrombosis (often in atypical sites), haemorrhage and transformation to myelofibrosis and less frequently to a form of acute myeloid leukaemia called blast phase. Almost two decades ago, the JAK2V617F mutation in exon 14 of JAK2 was described, and is known to be present in more than 95% of patients with PV. Testing for the JAK2V617F mutation is used in the diagnosis of PV, and the quantity of the mutation (that is, the variant allele frequency) is linked to prognosis and the risk of complications. As such, reduction of JAK2V617F variant allele frequency is currently being evaluated as a treatment target. Recommendations for PV treatment include control of vascular risk factors, therapeutic phlebotomy and low-dose aspirin in all patients. Currently, patients at higher risk of thrombosis (aged over 60 years and/or with a history of thrombosis) are offered cytoreductive agents. Hydroxyurea or interferons remain the preferred first-line cytoreductive agents, with the JAK1 and JAK2 inhibitor, ruxolitinib, currently approved for the treatment of patients who are resistant to, or intolerant of, hydroxyurea. Future recommendations might be to treat the majority of patients with these agents as long-term benefits of treatment begin to emerge.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 1 digital issues and online access to articles
$119.00 per year
only $119.00 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Arber, D. A. et al. International Consensus Classification of Myeloid Neoplasms and Acute Leukemias: integrating morphologic, clinical, and genomic data. Blood 140, 1200–1228 (2022). The 2022 ICC diagnostic criteria for myeloid neoplasms and acute leukaemias.
James, C. et al. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature 434, 1144–1148 (2005). Credited as the first description of the JAK2V617F mutation.
Jaiswal, S. et al. Age-related clonal hematopoiesis associated with adverse outcomes. N. Engl. J. Med. 371, 2488–2498 (2014).
Jaiswal, S. et al. Clonal hematopoiesis and risk of atherosclerotic cardiovascular disease. N. Engl. J. Med. 377, 111–121 (2017).
Pearson, T. C. & Wetherley-Mein, G. Vascular occlusive episodes and venous haematocrit in primary proliferative polycythaemia. Lancet 2, 1219–1222 (1978).
Hultcrantz, M. et al. Risk for arterial and venous thrombosis in patients with myeloproliferative neoplasms: a population-based cohort study. Ann. Intern. Med. 168, 317–325 (2018).
Ferrari, A. et al. Clinical outcomes under hydroxyurea treatment in polycythemia vera: a systematic review and meta-analysis. Haematologica 104, 2391–2399 (2019).
Tefferi, A. & Barbui, T. Polycythemia vera: 2024 update on diagnosis, risk‐stratification, and management. Am. J. Hematol. 98, 1465–1487 (2023).
Barbui, T. et al. Philadelphia chromosome-negative classical myeloproliferative neoplasms: revised management recommendations from European LeukemiaNet. Leukemia 32, 1057–1069 (2018).
Hultcrantz, M. et al. Patterns of survival among patients with myeloproliferative neoplasms diagnosed in Sweden from 1973 to 2008: a population-based study. J. Clin. Oncol. 30, 2995–3001 (2012).
Hultcrantz, M. et al. Risk and cause of death in patients diagnosed with myeloproliferative neoplasms in Sweden between 1973 and 2005: a population-based study. J. Clin. Oncol. 33, 2288–2295 (2015).
Shallis, R. M. et al. Epidemiology of the classical myeloproliferative neoplasms: the four corners of an expansive and complex map. Blood Rev. 42, 100706 (2020).
Verstovsek, S. et al. Changes in the incidence and overall survival of patients with myeloproliferative neoplasms between 2002 and 2016 in the United States. Leuk. Lymphoma 63, 694–702 (2022).
James, C., Ugo, V., Casadevall, N., Constantinescu, S. N. & Vainchenker, W. A JAK2 mutation in myeloproliferative disorders: pathogenesis and therapeutic and scientific prospects. Trends Mol. Med. 11, 546–554 (2005).
Titmarsh, G. J. et al. How common are myeloproliferative neoplasms? A systematic review and meta-analysis. Am. J. Hematol. 89, 581–587 (2014).
Moulard, O. et al. Epidemiology of myelofibrosis, essential thrombocythemia, and polycythemia vera in the European Union. Eur. J. Haematol. 92, 289–297 (2014).
Hultcrantz, M. et al. Incidence of myeloproliferative neoplasms – trends by subgroup and age in a population-based study in Sweden. J. Intern. Med. 287, 448–454 (2020).
Mehta, J., Wang, H., Iqbal, S. U. & Mesa, R. Epidemiology of myeloproliferative neoplasms in the United States. Leuk. Lymphoma 55, 595–600 (2014).
Chievitz, E. & Thiede, T. Complications and causes of death in polycythaemia vera. Acta Med. Scand. 172, 513–523 (1962).
Marchioli, R. et al. Vascular and neoplastic risk in a large cohort of patients with polycythemia vera. J. Clin. Oncol. 23, 2224–2232 (2005).
Barbui, T. et al. In contemporary patients with polycythemia vera, rates of thrombosis and risk factors delineate a new clinical epidemiology. Blood 124, 3021–3023 (2014).
Grinfeld, J. et al. Classification and personalized prognosis in myeloproliferative neoplasms. N. Engl. J. Med. 379, 1416–1430 (2018).
Tefferi, A. et al. Mutation-enhanced international prognostic systems for essential thrombocythaemia and polycythaemia vera. Br. J. Haematol. 189, 291–302 (2020).
Luque Paz, D. et al. Leukemic evolution of polycythemia vera and essential thrombocythemia: genomic profiles predict time to transformation. Blood Adv. 4, 4887–4897 (2020).
Sobas, M. et al. Real-world study of children and young adults with myeloproliferative neoplasms: identifying risks and unmet needs. Blood Adv. 6, 5171–5183 (2022).
Goulart, H. et al. Myeloproliferative neoplasms in the adolescent and young adult population: a comprehensive review of the literature. Br. J. Haematol. 205, 48–60 (2024).
Heavner, K. et al. Working environment and myeloproliferative neoplasm: a population–based case-control study following a cluster investigation. Am. J. Ind. Med. 58, 595–604 (2015).
Anderson, L. A. et al. Environmental, lifestyle, and familial/ethnic factors associated with myeloproliferative neoplasms. Am. J. Hematol. 87, 175–182 (2012). A useful summary of the epidemiology of MPNs.
Merk, K. et al. The incidence of cancer among blood donors. Int. J. Epidemiol. 19, 505–509 (1990).
Najean, Y., Rain, J. D. & Billotey, C. Epidemiological data in polycythaemia vera: a study of 842 cases. Hematol. Cell Ther. 40, 159–165 (1998).
Edgren, G. et al. Blood donation and risk of polycythemia vera. Transfusion 56, 1622–1627 (2016).
Duncombe, A. S. et al. Modifiable lifestyle and medical risk factors associated with myeloproliferative neoplasms. Hemasphere 4, e327 (2020).
McMullin, M. F. & Anderson, L. A. Aetiology of myeloproliferative neoplasms. Cancers 12, 1810 (2020).
Pedersen, K. M. et al. Smoking is associated with increased risk of myeloproliferative neoplasms: a general population-based cohort study. Cancer Med. 7, 5796–5802 (2018).
Balandrán, J. C., Lasry, A. & Aifantis, I. The role of inflammation in the initiation and progression of myeloid neoplasms. Blood Cancer Discov. 4, 254–266 (2023). Important review highlighting the role of inflammation both extrinsic and intrinsic to the mutant clone.
Murphy, F. et al. Body size in relation to incidence of subtypes of haematological malignancy in the prospective Million Women Study. Br. J. Cancer 108, 2390–2398 (2013).
Podoltsev, N. A. et al. Diet and risk of myeloproliferative neoplasms in older individuals from the NIH-AARP cohort. Cancer Epidemiol. Biomark. Prev. 29, 2343–2350 (2020).
Baxter, E. J. et al. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet 365, 1054–1061 (2005).
Levine, R. L. et al. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell 7, 387–397 (2005).
Kralovics, R. et al. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N. Engl. J. Med. 352, 1779–1790 (2005).
Scott, L. M. et al. JAK2 exon 12 mutations in polycythemia vera and idiopathic erythrocytosis. N. Engl. J. Med. 356, 459–468 (2007). Credited as the first description of JAK2 exon 12 mutations.
Leroy, E. & Constantinescu, S. N. Rethinking JAK2 inhibition: towards novel strategies of more specific and versatile janus kinase inhibition. Leukemia 31, 1023–1038 (2017).
Constantinescu, S. N., Vainchenker, W., Levy, G. & Papadopoulos, N. Functional consequences of mutations in myeloproliferative neoplasms. HemaSphere 5, e578 (2021).
Wingelhofer, B. et al. Implications of STAT3 and STAT5 signaling on gene regulation and chromatin remodeling in hematopoietic cancer. Leukemia 32, 1713–1726 (2018).
Liu, F. et al. JAK2V617F-mediated phosphorylation of PRMT5 downregulates its methyltransferase activity and promotes myeloproliferation. Cancer Cell 19, 283–294 (2011).
Dawson, M. A. et al. JAK2 phosphorylates histone H3Y41 and excludes HP1α from chromatin. Nature 461, 819–822 (2009).
Bartalucci, N., Guglielmelli, P. & Vannucchi, A. M. Polycythemia vera: the current status of preclinical models and therapeutic targets. Expert. Opin. Ther. Targets 24, 615–628 (2020).
Lundberg, P. et al. Myeloproliferative neoplasms can be initiated from a single hematopoietic stem cell expressing JAK2-V617F. J. Exp. Med. 211, 2213–2230 (2014).
Grisouard, J. et al. JAK2 exon 12 mutant mice display isolated erythrocytosis and changes in iron metabolism favoring increased erythropoiesis. Blood 128, 839–851 (2016).
Passamonti, F. et al. Molecular and clinical features of the myeloproliferative neoplasm associated with JAK2 exon 12 mutations. Blood 117, 2813–2816 (2011).
Tefferi, A. et al. JAK2 exon 12 mutated polycythemia vera: Mayo-Careggi MPN Alliance study of 33 consecutive cases and comparison with JAK2V617F mutated disease. Am. J. Hematol. 93, E93–E96 (2018).
Spivak, J. L. Myeloproliferative neoplasms. N. Engl. J. Med. 376, 2168–2181 (2017).
Mullally, A. et al. Distinct roles for long-term hematopoietic stem cells and erythroid precursor cells in a murine model of Jak2V617F-mediated polycythemia vera. Blood 120, 166–172 (2012).
Spivak, J. L. et al. Thrombopoietin is required for full phenotype expression in a JAK2V617F transgenic mouse model of polycythemia vera. PLoS ONE 15, e0232801 (2020).
Moliterno, A. R., Hankins, W. D. & Spivak, J. L. Impaired expression of the thrombopoietin receptor by platelets from patients with polycythemia vera. N. Engl. J. Med. 338, 572–580 (1998).
Tapper, W. et al. Genetic variation at MECOM, TERT, JAK2 and HBS1L-MYB predisposes to myeloproliferative neoplasms. Nat. Commun. 6, 6691 (2015).
Lim, J., Ross, D. M., Brown, A. L., Scott, H. S. & Hahn, C. N. Germline genetic variants that predispose to myeloproliferative neoplasms and hereditary myeloproliferative phenotypes. Leuk. Res. 146, 107566 (2024).
Jones, A. V. et al. JAK2 haplotype is a major risk factor for the development of myeloproliferative neoplasms. Nat. Genet. 41, 446–449 (2009).
Olcaydu, D. et al. A common JAK2 haplotype confers susceptibility to myeloproliferative neoplasms. Nat. Genet. 41, 450–454 (2009).
Kilpivaara, O. et al. A germline JAK2 SNP is associated with predisposition to the development of JAK2V617F-positive myeloproliferative neoplasms. Nat. Genet. 41, 455–459 (2009).
Li, S. L., Zhang, P. J., Sun, G. X. & Lu, Z. J. The JAK2 46/1 haplotype (GGCC) in myeloproliferative neoplasms and splanchnic vein thrombosis: a pooled analysis of 26 observational studies. Ann. Hematol. 93, 1845–1852 (2014).
Tefferi, A. et al. Targeted deep sequencing in polycythemia vera and essential thrombocythemia. Blood Adv. 1, 21–30 (2016).
Ortmann, C. A. et al. Effect of mutation order on myeloproliferative neoplasms. N. Engl. J. Med. 372, 601–612 (2015).
Chen, E. et al. Distinct effects of concomitant Jak2V617F expression and Tet2 loss in mice combine to promote disease progression in myeloproliferative neoplasms. Blood 125, 327–335 (2015).
Knudsen, T. A. et al. Genomic profiling of a randomized trial of interferon-α vs hydroxyurea in MPN reveals mutation-specific responses. Blood Adv. 6, 2107–2119 (2022).
Abu-Zeinah, G., Erdos, K., Lee, N. Jr., Silver, R. T. & Scandura, J. M. DNMT3A mutations do not affect treatment response or outcomes for patients with polycythemia vera treated with interferon alpha [abstract]. Blood 144, 3190 (2024).
Shimizu, T. et al. Loss of Ezh2 synergizes with JAK2-V617F in initiating myeloproliferative neoplasms and promoting myelofibrosis. J. Exp. Med. 213, 1479–1496 (2016).
Usart, M. et al. Loss of Dnmt3a increases self-renewal and resistance to pegIFN-α in JAK2-V617F-positive myeloproliferative neoplasms. Blood 143, 2490–2503 (2024).
Theocharides, A. et al. Leukemic blasts in transformed JAK2-V617F-positive myeloproliferative disorders are frequently negative for the JAK2-V617F mutation. Blood 110, 375–379 (2007).
Calabresi, L. et al. Clonal dynamics and copy number variants by single‐cell analysis in leukemic evolution of myeloproliferative neoplasms. Am. J. Hematol. 98, 1520–1532 (2023).
Tefferi, A. et al. Survival and prognosis among 1545 patients with contemporary polycythemia vera: an international study. Leukemia 27, 1874–1881 (2013). Contemporary study of mortality of a cohort of patients with PV.
Tang, G. et al. Characteristics and clinical significance of cytogenetic abnormalities in polycythemia vera. Haematologica 102, 1511–1518 (2017).
Gangat, N. et al. Cytogenetic studies at diagnosis in polycythemia vera: clinical and JAK2V617F allele burden correlates. Eur. J. Haematol. 80, 197–200 (2008).
Fisher, D. A. C., Fowles, J. S., Zhou, A. & Oh, S. T. Inflammatory pathophysiology as a contributor to myeloproliferative neoplasms. Front. Immunol. 12, 683401 (2021).
Barbui, T. et al. Inflammation and thrombosis in essential thrombocythemia and polycythemia vera: different role of C-reactive protein and Pentraxin 3. Haematologica 96, 315–318 (2011).
Vaidya, R. et al. Plasma cytokines in polycythemia vera: phenotypic correlates, prognostic relevance, and comparison with myelofibrosis. Am. J. Hematol. 87, 1003–1005 (2012).
Tefferi, A. et al. Circulating interleukin (IL)-8, IL-2R, IL-12, and IL-15 levels are independently prognostic in primary myelofibrosis: a comprehensive cytokine profiling study. J. Clin. Oncol. 29, 1356–1363 (2011).
Laranjeira, A. B. A. et al. In vivo ablation of NFκB cascade effectors alleviates disease burden in myeloproliferative neoplasms. Blood J. 143, 2414–2424 (2024).
Rai, S. et al. IL-1β promotes MPN disease initiation by favoring early clonal expansion of JAK2-mutant hematopoietic stem cells. Blood Adv. 8, 1234–1249 (2024).
Rai, S. et al. Inhibition of interleukin-1β reduces myelofibrosis and osteosclerosis in mice with JAK2-V617F driven myeloproliferative neoplasm. Nat. Commun. 13, 5346 (2022).
Verstovsek, S. et al. Safety and efficacy of INCB018424, a JAK1 and JAK2 inhibitor, in myelofibrosis. N. Engl. J. Med. 363, 1117–1127 (2010). First description of the use of ruxolitinib in myelofibrosis.
Verstovsek, S. et al. A phase 2 study of ruxolitinib, an oral JAK1 and JAK2 inhibitor, in patients with advanced polycythemia vera who are refractory or intolerant to hydroxyurea. Cancer 120, 513–520 (2014). First study of ruxolitinib in PV.
Ramanathan, G. et al. Cigarette smoke stimulates clonal expansion of Jak2V617F and Tet2−/− cells. Front. Oncol. 13, 1210528 (2023).
Pratt, J. J. & Khan, K. S. Non-anaemic iron deficiency – a disease looking for recognition of diagnosis: a systematic review. Eur. J. Haematol. 96, 618–628 (2016).
Verstovsek, S. et al. Markers of iron deficiency in patients with polycythemia vera receiving ruxolitinib or best available therapy. Leuk. Res. 56, 52–59 (2017).
Song, J., Kim, S. J., Min, G., Lim, Y. & Prchal, J. T. Iron deficiency correction in myeloproliferative neoplasms reduces thrombosis risk via decreased P-selectin. Blood 144, 1758–1758 (2024).
Ganz, T. Anemia of inflammation. N. Engl. J. Med. 381, 1148–1157 (2019).
Kautz, L. et al. Identification of erythroferrone as an erythroid regulator of iron metabolism. Nat. Genet. 46, 678–684 (2014).
Handa, S., Ginzburg, Y., Hoffman, R. & Kremyanskaya, M. Hepcidin mimetics in polycythemia vera: resolving the irony of iron deficiency and erythrocytosis. Curr. Opin. Hematol. 30, 45–52 (2023).
Casu, C. et al. Minihepcidin peptides as disease modifiers in mice affected by β-thalassemia and polycythemia vera. Blood 128, 265–276 (2016).
Andrikovics, H. et al. HFE C282Y mutation as a genetic modifier influencing disease susceptibility for chronic myeloproliferative disease. Cancer Epidemiol. Biomark. Prev. 18, 929–934 (2009).
Stetka, J. et al. Iron is a modifier of the phenotypes of JAK2-mutant myeloproliferative neoplasms. Blood 141, 2127–2140 (2023).
van Genderen, P. J. & Michiels, J. J. Erythromelalgia: a pathognomonic microvascular thrombotic complication in essential thrombocythemia and polycythemia vera. Semin. Thromb. Hemost. 23, 357–363 (1997).
Kiladjian, J. J. & Cassinat, B. Myeloproliferative neoplasms and splanchnic vein thrombosis: contemporary diagnostic and therapeutic strategies. Am. J. Hematol. 98, 794–800 (2023).
Khoury, J. D. et al. The 5th edition of the World Health Organization classification of haematolymphoid tumours: myeloid and histiocytic/dendritic neoplasms. Leukemia 36, 1703–1719 (2022). The 2022 WHO criteria for diagnosis of haematolymphoid disorders.
Thiele, J. et al. The International Consensus Classification of Myeloid Neoplasms and Acute Leukemias: myeloproliferative neoplasms. Am. J. Hematol. 98, 166–179 (2023).
Maslah, N. et al. Masked polycythemia vera: analysis of a single center cohort of 2480 red cell masses. Haematologica 105, e95–e97 (2020).
Galtier, J. et al. Role of red cell mass evaluation in myeloproliferative neoplasms with splanchnic vein thrombosis and normal hemoglobin value: a study of the France Intergroupe des Syndromes myeloproliferatifs. Haematologica 109, 1989–1993 (2024).
Barbui, T. et al. Masked polycythemia Vera (mPV): results of an international study. Am. J. Hematol. 89, 52–54 (2014).
Barbui, T., Thiele, J., Vannucchi, A. M. & Tefferi, A. Rethinking the diagnostic criteria of polycythemia vera. Leukemia 28, 1191–1195 (2014).
Barbui, T. et al. Initial bone marrow reticulin fibrosis in polycythemia vera exerts an impact on clinical outcome. Blood 119, 2239–2241 (2012).
Wilkins, B. S. et al. Bone marrow pathology in essential thrombocythemia: interobserver reliability and utility for identifying disease subtypes. Blood 111, 60–70 (2008).
Madelung, A. B. et al. WHO-defined classification of myeloproliferative neoplasms: morphological reproducibility and clinical correlations – the Danish experience. Am. J. Hematol. 88, 1012–1016 (2013).
Ryou, H. et al. Continuous Indexing of Fibrosis (CIF): improving the assessment and classification of MPN patients. Leukemia 37, 348–358 (2023).
Scott, L. M. et al. The V617F JAK2 mutation is uncommon in cancers and in myeloid malignancies other than the classic myeloproliferative disorders. Blood 106, 2920–2921 (2005).
Ma, W. et al. Mutation profile of JAK2 transcripts in patients with chronic myeloproliferative neoplasias. J. Mol. Diagn. 11, 49–53 (2009).
McMullin, M. F. & Cario, H. LNK mutations and myeloproliferative disorders. Am. J. Hematol. 91, 248–251 (2016).
Milosevic Feenstra, J. D. et al. Whole-exome sequencing identifies novel MPL and JAK2 mutations in triple-negative myeloproliferative neoplasms. Blood 127, 325–332 (2016).
Williams, N. et al. Life histories of myeloproliferative neoplasms inferred from phylogenies. Nature 602, 162–168 (2022). Use of mathematical modelling to illustrate that a driver JAK2 mutation could arise several decades before the onset of disease.
How, J., Garcia, J. S. & Mullally, A. Biology and therapeutic targeting of molecular mechanisms in MPNs. Blood 141, 1922–1933 (2023).
Vannucchi, A. M. et al. Clinical profile of homozygous JAK2 617V>F mutation in patients with polycythemia vera or essential thrombocythemia. Blood 110, 840–846 (2007).
Passamonti, F. et al. A prospective study of 338 patients with polycythemia vera: the impact of JAK2 (V617F) allele burden and leukocytosis on fibrotic or leukemic disease transformation and vascular complications. Leukemia 24, 1574–1579 (2010).
Gisslinger, H. et al. Ropeginterferon alfa-2b versus standard therapy for polycythaemia vera (PROUD-PV and CONTINUATION-PV): a randomised, non-inferiority, phase 3 trial and its extension study. Lancet Haematol. 7, e196–e208 (2020). An important study (PROUD-PV) comparing ropeginterferon alfa-2b with hydroxycarbamide.
Kiladjian, J. J. et al. Pegylated interferon-alfa-2a induces complete hematological and molecular responses with low toxicity in polycythemia vera. Blood 112, 3065–3072 (2008).
Kiladjian, J. J. et al. Long-term efficacy and safety of ruxolitinib versus best available therapy in polycythaemia vera (RESPONSE): 5-year follow up of a phase 3 study. Lancet Haematol. 7, e226–e237 (2020). Long-term follow-up of the RESPONSE study in PV.
Harrison, C. N. et al. Ruxolitinib versus best available therapy for polycythemia vera intolerant or resistant to hydroxycarbamide in a randomized trial. J. Clin. Oncol. 41, 3534–3544 (2023). MAJIC-PV demonstrating the long-term benefit for ruxolitinib compared with best available therapy with improved event-free survival in patients treated with ruxolitinib, control of blood count and 50% reduction in JAK2V617F variant allele frequency.
Guglielmelli, P. et al. Clinical impact of mutated JAK2 allele burden reduction in polycythemia vera and essential thrombocythemia. Am. J. Hematol. 99, 1550–1559 (2024). Study demonstrating that reduction of JAK2V617F variant allele frequency correlates with lower risk of post-PV myelofibrosis.
Delhommeau, F. et al. Oncogenic mechanisms in myeloproliferative disorders. Cell Mol. Life Sci. 63, 2939–2953 (2006).
Pasquer, H. et al. Distinct clinico-molecular arterial and venous thrombosis scores for myeloproliferative neoplasms risk stratification. Leukemia 38, 326–339 (2024).
Santos, F. P. S. et al. Prognostic impact of RAS-pathway mutations in patients with myelofibrosis. Leukemia 34, 799–810 (2020).
O’Sullivan, J. M. et al. RAS-pathway mutations are common in patients with ruxolitinib refractory/intolerant myelofibrosis: molecular analysis of the PAC203 cohort. Leukemia 37, 2497–2501 (2023).
Quintás-Cardama, A. et al. Molecular analysis of patients with polycythemia vera or essential thrombocythemia receiving pegylated interferon alpha-2a. Blood 122, 893–901 (2013).
Bewersdorf, J. P. et al. Moving toward disease modification in polycythemia vera. Blood 142, 1859–1870 (2023).
McMullin, M. F. & Harrison, C. N. How I treat low-risk polycythemia vera patients who require cytoreduction. Blood https://doi.org/10.1182/blood.2023022418 (2024).
Gerds, A. T. et al. Myeloproliferative neoplasms, version 3.2022, NCCN clinical practice guidelines in oncology. J. Natl Compr. Cancer Netw. 20, 1033–1062 (2022).
Marchetti, M. et al. Appropriate management of polycythaemia vera with cytoreductive drug therapy: European LeukemiaNet 2021 recommendations. Lancet Haematol. 9, e301–e311 (2022).
Landolfi, R. et al. Leukocytosis as a major thrombotic risk factor in patients with polycythemia vera. Blood 109, 2446–2452 (2007).
Barbui, T. et al. White blood cell counts and thrombosis in polycythemia vera: a subanalysis of the CYTO-PV study. Blood 126, 560–561 (2015).
Ronner, L. et al. Persistent leukocytosis in polycythemia vera is associated with disease evolution but not thrombosis. Blood 135, 1696–1703 (2020).
Gerds, A. T. et al. Association between elevated white blood cell counts and thrombotic events in polycythemia vera: analysis from REVEAL. Blood 143, 1646–1655 (2024).
Carobbio, A. et al. Neutrophil-to-lymphocyte ratio is a novel predictor of venous thrombosis in polycythemia vera. Blood Cancer J. 12, 28 (2022).
Larsen, M. K. et al. Neutrophil-to-lymphocyte ratio and all-cause mortality with and without myeloproliferative neoplasms – a Danish longitudinal study. Blood Cancer J. 14, 28 (2024).
Moliterno, A. R., Kaizer, H. & Reeves, B. N. JAK2V617F allele burden in polycythemia vera: burden of proof. Blood 141, 1934–1942 (2023).
Vannucchi, A. M. et al. Prospective identification of high-risk polycythemia vera patients based on JAK2V617F allele burden. Leukemia 21, 1952–1959 (2007).
Guglielmelli, P. et al. JAK2V617F variant allele frequency >50% identifies patients with polycythemia vera at high risk for venous thrombosis. Blood Cancer J. 11, 199 (2021).
Marchioli, R. et al. Cardiovascular events and intensity of treatment in polycythemia vera. N. Engl. J. Med. 368, 22–33 (2013).
Landolfi, R. et al. Efficacy and safety of low-dose aspirin in polycythemia vera. N. Engl. J. Med. 350, 114–124 (2004).
Barbui, T. et al. A reappraisal of the benefit-risk profile of Hydroxyurea in polycythemia vera: a propensity-matched study. Am. J. Hematol. 92, 1131–1136 (2017).
Podoltsev, N. A. et al. The impact of phlebotomy and hydroxyurea on survival and risk of thrombosis among older patients with polycythemia vera. Blood Adv. 2, 2681–2690 (2018).
Barbui, T. et al. No correlation of intensity of phlebotomy regimen with risk of thrombosis in polycythemia vera: evidence from ECLAP and CYTO-PV clinical trials. Haematologica 102, e219–e221 (2017).
Alvarez-Larran, A. et al. Risk of thrombosis according to need of phlebotomies in patients with polycythemia vera treated with hydroxyurea. Haematologica 102, 103–109 (2017).
Finazzi, G. et al. Acute leukemia in polycythemia vera: an analysis of 1638 patients enrolled in a prospective observational study. Blood 105, 2664–2670 (2005).
Kiladjian, J.-J., Chevret, S., Dosquet, C., Chomienne, C. & Rain, J.-D. Treatment of polycythemia vera with hydroxyurea and pipobroman: final results of a randomized trial initiated in 1980. J. Clin. Oncol. 29, 3907–3913 (2011).
Landtblom, A. R. et al. Second malignancies in patients with myeloproliferative neoplasms: a population-based cohort study of 9379 patients. Leukemia 32, 2203–2210 (2018).
Barbui, T. et al. Second cancer in Philadelphia negative myeloproliferative neoplasms (MPN-K). A nested case-control study. Leukemia 33, 1996–2005 (2019).
Vachhani, P. et al. Interferons in the treatment of myeloproliferative neoplasms. Ther. Adv. Hematol. 15, 20406207241229588 (2024).
Gisslinger, H. et al. Event-free survival in patients with polycythemia vera treated with ropeginterferon alfa-2b versus best available treatment. Leukemia 37, 2129–2132 (2023). Demonstration that interferons lead to superior event-free survival in PV.
Barbui, T. et al. Ropeginterferon alfa-2b versus phlebotomy in low-risk patients with polycythaemia vera (Low-PV study): a multicentre, randomised phase 2 trial. Lancet Haematol. 8, e175–e184 (2021). The first study specifically evaluating interferon in low-risk patients with PV.
Barbui, T. et al. Ropeginterferon phase 2 randomized study in low-risk polycythemia vera: 5-year drug survival and efficacy outcomes. Ann. Hematol. 103, 437–442 (2024).
Mascarenhas, J. et al. A randomized phase 3 trial of interferon-α vs hydroxyurea in polycythemia vera and essential thrombocythemia. Blood 139, 2931–2941 (2022).
Abu-Zeinah, G. et al. Interferon-alpha for treating polycythemia vera yields improved myelofibrosis-free and overall survival. Leukemia 35, 2592–2601 (2021). Long-term study demonstrating benefits of interferon on myelofibrosis-free survival.
Kiladjian, J. J. et al. Clonal analysis of erythroid progenitors suggests that pegylated interferon α-2a treatment targets JAK2V617F clones without affecting TET2 mutant cells. Leukemia 24, 1519–1523 (2010).
Daltro De Oliveira, R. et al. Interferon-alpha (IFN) therapy discontinuation is feasible in myeloproliferative neoplasm (MPN) patients with complete hematological remission [abstract]. Blood 136, 35–36 (2020). Important demonstration that interferon can be discontinued in a proportion of patients with PV.
Masarova, L. et al. Pegylated interferon alfa-2a in patients with essential thrombocythaemia or polycythaemia vera: a post-hoc, median 83 month follow-up of an open-label, phase 2 trial. Lancet Haematol. 4, e165–e175 (2017).
Kiladjian, J. J. et al. Long-term outcomes of polycythemia vera patients treated with ropeginterferon alfa-2b. Leukemia 36, 1408–1411 (2022).
Barosi, G. et al. A unified definition of clinical resistance and intolerance to hydroxycarbamide in polycythaemia vera and primary myelofibrosis: results of a European LeukemiaNet (ELN) consensus process. Br. J. Haematol. 148, 961–963 (2010).
Alvarez-Larran, A. et al. Assessment and prognostic value of the European LeukemiaNet criteria for clinicohematologic response, resistance, and intolerance to hydroxyurea in polycythemia vera. Blood 119, 1363–1369 (2012).
Vannucchi, A. M. et al. Ruxolitinib versus standard therapy for the treatment of polycythemia vera. N. Engl. J. Med. 372, 426–435 (2015). Seminal study leading to the approval of ruxolitinib for the treatment of PV (the RESPONSE trial).
Passamonti, F. et al. Ruxolitinib for the treatment of inadequately controlled polycythaemia vera without splenomegaly (RESPONSE-2): a randomised, open-label, phase 3b study. Lancet Oncol. 18, 88–99 (2017). Second registration trial of ruxolitinib in the treatment of PV.
Masciulli, A., Ferrari, A., Carobbio, A., Ghirardi, A. & Barbui, T. Ruxolitinib for the prevention of thrombosis in polycythemia vera: a systematic review and meta-analysis. Blood Adv. 4, 380–386 (2020).
Vannucchi, A. M. et al. Ruxolitinib reduces JAK2 p.V617F allele burden in patients with polycythemia vera enrolled in the RESPONSE study. Ann. Hematol. 96, 1113–1120 (2017).
Koschmieder, S. et al. Firstline treatment with ruxolitinib versus best available therapy in patients with polycythemia vera: pre-specified interim analysis of the randomized phase 2b Ruxobeat clinical trial of the German Study Group for Myeloproliferative Neoplasms (GSG-MPN) [abstract]. Blood 142, 619 (2023).
Passamonti, F. et al. Ruxolitinib versus best available therapy in inadequately controlled polycythaemia vera without splenomegaly (RESPONSE-2): 5-year follow up of a randomised, phase 3b study. Lancet Haematol. 9, e480–e492 (2022).
Ginzburg, Y. Z. et al. Dysregulated iron metabolism in polycythemia vera: etiology and consequences. Leukemia 32, 2105–2116 (2018).
Casu, C., Nemeth, E. & Rivella, S. Hepcidin agonists as therapeutic tools. Blood 131, 1790–1794 (2018).
Kremyanskaya, M. et al. Rusfertide, a hepcidin mimetic, for control of erythrocytosis in polycythemia vera. N. Engl. J. Med. 390, 723–735 (2024). First description of the use of rusfertide, a hepcidin mimetic, in the management of PV.
Guerini, V. et al. The histone deacetylase inhibitor ITF2357 selectively targets cells bearing mutated JAK2V617F. Leukemia 22, 740–747 (2008).
Rambaldi, A. et al. A pilot study of the histone-deacetylase inhibitor givinostat in patients with JAK2V617F positive chronic myeloproliferative neoplasms. Br. J. Haematol. 150, 446–455 (2010).
Rambaldi, A. et al. Safety and efficacy of the maximum tolerated dose of givinostat in polycythemia vera: a two-part phase Ib/II study. Leukemia 34, 2234–2237 (2020).
Finazzi, G. et al. A phase II study of givinostat in combination with hydroxycarbamide in patients with polycythaemia vera unresponsive to hydroxycarbamide monotherapy. Br. J. Haematol. 161, 688–694 (2013).
Rambaldi, A. et al. Long-term safety and efficacy of givinostat in polycythemia vera: 4-year mean follow up of three phase 1/2 studies and a compassionate use program. Blood Cancer J. 11, 53 (2021).
Mesa, R. A. et al. The burden of fatigue and quality of life in myeloproliferative disorders (MPDs): an international Internet-based survey of 1179 MPD patients. Cancer 109, 68–76 (2007).
Mesa, R. A. et al. The Myelofibrosis Symptom Assessment Form (MFSAF): an evidence-based brief inventory to measure quality of life and symptomatic response to treatment in myelofibrosis. Leuk. Res. 33, 1199–1203 (2009).
Scherber, R. et al. The myeloproliferative neoplasm symptom assessment form (MPN-SAF): international prospective validation and reliability trial in 402 patients. Blood 118, 401–408 (2011). The development and validation of an international tool to measure patient symptoms.
Mesa, R. A. et al. NCCN guidelines insights: myeloproliferative neoplasms, version 2.2018. J. Natl Compr. Cancer Netw. 15, 1193–1207 (2017).
Mazza, G. L. et al. Symptom burden and quality of life in patients with high-risk essential thrombocythaemia and polycythaemia vera receiving hydroxyurea or pegylated interferon alfa-2a: a post-hoc analysis of the MPN-RC 111 and 112 trials. Lancet Haematol. 9, e38–e48 (2022).
Sørensen, A. L. et al. Combination therapy with ruxolitinib and pegylated interferon alfa-2a in newly diagnosed patients with polycythemia vera. Blood Adv. 8, 5416–5425 (2024).
Hansen, I. D. et al. Statins enhance the efficacy of pegylated interferon-alpha2 in patients with pH-negative chronic myeloproliferative neoplasms. results from a Danish single-institution cohort study [abstract]. Blood 144, 3184 (2024).
Dameshek, W. Some speculations on the myeloproliferative syndromes. Blood 6, 372–375 (1951). Seminal description by Dameshek of the family of disorders where he first used the term myeloproliferative.
Prchal, J. F. & Axelrad, A. A. Letter: bone-marrow responses in polycythemia vera. N. Engl. J. Med. 290, 1382 (1974).
Adamson, J. W., Fialkow, P. J., Murphy, S., Prchal, J. F. & Steinmann, L. Polycythemia vera: stem-cell and probable clonal origin of the disease. N. Engl. J. Med. 295, 913–916 (1976). Demonstration of clonal origin of PV using X-chromosome inactivation patterns.
Barbui, T. et al. Philadelphia-negative classical myeloproliferative neoplasms: critical concepts and management recommendations from European LeukemiaNet. J. Clin. Oncol. 29, 761–770 (2011).
Gu, W. et al. Prediction of thrombosis in polycythemia vera: development and validation of a multiple factor-based prognostic score system. Res. Pract. Thromb. Haemost. 7, 100132 (2023).
Yacoub, A. et al. Pegylated interferon alfa-2a for polycythemia vera or essential thrombocythemia resistant or intolerant to hydroxyurea. Blood 134, 1498–1509 (2019).
Samuelsson, J. et al. A phase II trial of pegylated interferon alpha-2b therapy for polycythemia vera and essential thrombocythemia: feasibility, clinical and biologic effects, and impact on quality of life. Cancer 106, 2397–2405 (2006).
Knudsen, T. A. Three-year analysis of the DALIAH trial – a randomized controlled phase III clinical trial comparing recombinant interferon alpha-2 vs. hydroxyurea in patients with myeloproliferative neoplasms [abstract S1609]. Hemasphere 3, 741–742 (2019).
Gisslinger, H. et al. Ropeginterferon alfa-2b, a novel IFNα-2b, induces high response rates with low toxicity in patients with polycythemia vera. Blood 126, 1762–1769 (2015).
Barbui, T. et al. Ropeginterferon versus standard therapy for low-risk patients with polycythemia vera. NEJM Evid. 2, EVIDoa2200335 (2023).
Robinson, S., Ragheb, M. & Harrison, C. How I treat myeloproliferative neoplasms in pregnancy. Blood 143, 777–785 (2024).
Author information
Authors and Affiliations
Contributions
Introduction (T.B.); Epidemiology (M.F.M.); Mechanisms/pathophysiology (A.M.V.); Diagnosis, screening and prevention (J.-J.K.); Management (P.B. and J.M.); Quality of life (R.M.); Outlook (C.N.H.); overview of the Primer (C.N.H. and A.M.V.).
Corresponding author
Ethics declarations
Competing interests
C.N.H. discloses research support from Celgene (BMS), Constellation, GSK and Novartis; and honoraria/consulting fees from Abbvie, AOP, BMS, CTI, IMAGO, Incyte, Novartis, Galacteo, Geron, GSK, Janssen, Keros, MSD, SOBI and Morphosys. T.B. discloses research support ftom GSK and AOP; and honoraria/consulting fees from AOP, Italfarmaco, Ionis and Novartis. P.B. discloses research support from Incyte, BMS, CTI, Morphosys, Sumitomo, Karyopharm, Kartos, Telios, Ionis, Disc, Ajax, Geron, Janssen, Blueprint and Cogent; and honoraria/consulting fees from Incyte, BMS, CTI, GSK, Abbvie, Morphosys, Sumitomo, Karyopharm, Ionis, Disc, Geron, Keros, Pharma Essentia, Jubilant, Morphic, Novartis, Blueprint, Ono, Raythera and Cogent. J.-J.K. discloses honoraria/consulting fees from Novartis, GSK, Abbvie, BMS, Incyte, AOP Health and PharmaEssentia. J.M. discloses research funding from Incyte, Novartis, BMS, CTI/SOBI, Abbvie, Geron, Kartos, Karyopharm AJAX, Italfarmaco Spa, Disc and PharmaEssentia; and consulting fees from Incyte, Novartis, BMS, Geron, Karyopharm, Kartos, GSK, PharmaEssentia, Italfarmaco Spa, Abbvie, Roche, Merck, Pfizer, Galecto, MorphoSys, Disc, Keros and Sumitomo. M.F.M. discloses research support from BMS and AOP; and honorarium/consulting fees from Novartis, GSK, Incyte, BMS and AOP. R.M. discloses research support from Abbvie, Blueprint, BMS, CTI, Genentech, Incyte, Morphosys and Sierra; and honoraria/consulting fees from Abbvie, Blueprint, BMS, CTI, Genentech, Geron, GSK, Incyte, Novartis, Sierra, Sierra Oncology and Telios. A.M.V. discloses honoraria/consulting fees from Incyte, Novartis, AOP, Italfarmaco, BMS, GSK, Abbvie, Blueprint and Ionis Disc.
Peer review
Peer review information
Nature Reviews Disease Primers thanks H. Hasselbalch; N. Komatsu, who co-reviewed with Y. Edahiro; H. L. Pahl; and J. Prchal, who co-reviewed with J. Song, for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Harrison, C.N., Barbui, T., Bose, P. et al. Polycythaemia vera. Nat Rev Dis Primers 11, 26 (2025). https://doi.org/10.1038/s41572-025-00608-3
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41572-025-00608-3
This article is cited by
-
Emerging Significance and Implications of a Durable Complete Molecular Remission in the Treatment of Polycythemia Vera
Current Hematologic Malignancy Reports (2025)