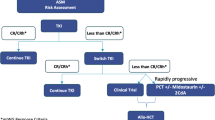
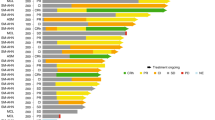
Abstract
Mastocytosis is a spectrum of clonal myeloid disorders defined by abnormal growth and accumulation of mast cells in various organ systems. The disease is divided into cutaneous mastocytosis, systemic mastocytosis (SM) and mast cell sarcoma. SM is further categorized into several non-advanced and advanced forms. The prognosis of cutaneous mastocytosis and non-advanced SM is mostly favourable, whereas prognosis and survival in advanced SM and mast cell sarcoma are poor. During the past 15 years, major advances have been made in the diagnosis, prognosis and management of patients with mast cell neoplasms. Management of mastocytosis consists of symptomatic therapy, including anti-mast cell mediator drugs, and cytoreductive agents for patients with advanced disease and selected individuals with non-advanced disease, as well as recognition and prevention of comorbidities such as osteoporosis and anaphylaxis. The preclinical and clinical development of KIT-D816V-targeting drugs, such as midostaurin or avapritinib, mark a milestone in improving management, the quality of life and survival in patients with SM. These agents induce major responses or even remission in people with advanced SM and lead to rapid improvement of mediator-related symptoms and quality of life in symptomatic patients.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 1 digital issues and online access to articles
$119.00 per year
only $119.00 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Theoharides, T. C., Valent, P. & Akin, C. Mast cells, mastocytosis, and related disorders. N. Engl. J. Med. 373, 163–172 (2015).
Ustun, C. et al. Advanced systemic mastocytosis: from molecular and genetic progress to clinical practice. Haematologica 101, 1133–1143 (2016).
Valent, P., Akin, C. & Metcalfe, D. D. Mastocytosis: 2016 updated WHO classification and novel emerging treatment concepts. Blood 129, 1420–1427 (2017).
Valent, P. et al. New insights into the pathogenesis of mastocytosis: emerging concepts in diagnosis and therapy. Annu. Rev. Pathol. 18, 361–386 (2023).
Valent, P. et al. Diagnostic criteria and classification of mastocytosis: a consensus proposal. Leuk. Res. 25, 603–625 (2001). Basic diagnostic consensus criteria and classification of mastocytosis: these criteria were reviewed, discussed and approved in the Year 2000 Working Conference on Mastocytosis.
Metcalfe, D. D. Mast cells and mastocytosis. Blood 112, 946–956 (2008).
Hartmann, K. et al. Cutaneous manifestations in patients with mastocytosis: consensus report of the European Competence Network on Mastocytosis; the American Academy of Allergy, Asthma & Immunology; and the European Academy of Allergology and Clinical Immunology. J. Allergy Clin. Immunol. 137, 35–45 (2016).
Valent, P. et al. Advances in the classification and treatment of mastocytosis: current status and outlook toward the future. Cancer Res. 77, 1261–1270 (2017).
Radia, D. H. & Moonim, M. T. Update on diagnostic approaches and therapeutic strategies in systemic mastocytosis. Best Pract. Res. Clin. Haematol. 35, 101380 (2022).
Lim, K. H. et al. Systemic mastocytosis in 342 consecutive adults: survival studies and prognostic factors. Blood 113, 5727–5736 (2009).
Pardanani, A. et al. Prognostically relevant breakdown of 123 patients with systemic mastocytosis associated with other myeloid malignancies. Blood 114, 3769–3772 (2009).
Sperr, W. R. et al. International Prognostic Scoring System for Mastocytosis (IPSM): a retrospective cohort study. Lancet Haematol. 6, e638–e649 (2019). Comprehensive international prognostic scoring system for SM, including non-advanced and advanced forms of the disease.
Reiter, A., George, T. I. & Gotlib, J. New developments in diagnosis, prognostication, and treatment of advanced systemic mastocytosis. Blood 135, 1365–1376 (2020).
Valent, P., Akin, C., Sperr, W. R., Horny, H. P. & Metcalfe, D. D. Smouldering mastocytosis: a novel subtype of systemic mastocytosis with slow progression. Int. Arch. Allergy Immunol. 127, 137–139 (2002).
Tefferi, A., Shah, S., Reichard, K. K., Hanson, C. A. & Pardanani, A. Smoldering mastocytosis: survival comparisons with indolent and aggressive mastocytosis. Am. J. Hematol. 94, E1–E2 (2019).
Zanotti, R. et al. Refined diagnostic criteria for bone marrow mastocytosis: a proposal of the European competence network on mastocytosis. Leukemia 36, 516–524 (2022). This article provides revised definitions and refined diagnostic consensus criteria for bone marrow mastocytosis: based on this study, bone marrow mastocytosis becomes an independent separate variant of SM in the updated WHO classification 2022.
Valent, P. et al. Updated diagnostic criteria and classification of mast cell disorders: a consensus proposal. Hemasphere 5, e646 (2021). Updated diagnostic consensus criteria and classification of mastocytosis: this paper provided the basis for the revised WHO classification of mastocytosis 2022.
Akin, C. et al. A novel form of mastocytosis associated with a transmembrane c-kit mutation and response to imatinib. Blood 103, 3222–3225 (2004). This article is the first description of WDSM and reports that imatinib can induce major clinical responses in patients with KIT-D816V-negative mastocytosis: in this particular responder, neoplastic mast cells displayed the KIT germline mutation Phe522Cys.
Alvarez-Twose, I. et al. Clinical, immunophenotypic, and molecular characteristics of well-differentiated systemic mastocytosis. J. Allergy Clin. Immunol. 137, 168–178.e161 (2016).
Khoury, J. D. et al. The 5th edition of the World Health Organization classification of haematolymphoid tumours: myeloid and histiocytic/dendritic neoplasms. Leukemia 36, 1703–1719 (2022). Revised WHO classification of myeloid neoplasms including mastocytosis 2022.
Loghavi, S. et al. Fifth Edition of the World Health Classification of tumors of the hematopoietic and lymphoid tissue: myeloid neoplasms. Mod. Pathol. 37, 100397 (2024). Revised WHO classification of myeloid neoplasms (WHO blue book) including mastocytosis.
Arber, D. A. et al. International Consensus Classification of Myeloid Neoplasms and Acute Leukemias: integrating morphologic, clinical, and genomic data. Blood 140, 1200–1228 (2022).
Wang, S. A. et al. The international consensus classification of eosinophilic disorders and systemic mastocytosis. Am. J. Hematol. 98, 1286–1306 (2023). An international consensus classification provides an alternative classification of mastocytosis with slightly modified diagnostic criteria deviating marginally from the WHO classification.
Nagata, H. et al. Identification of a point mutation in the catalytic domain of the protooncogene c-kit in peripheral blood mononuclear cells of patients who have mastocytosis with an associated hematologic disorder. Proc. Natl Acad. Sci. USA 92, 10560–10564 (1995).
Longley, B. J. et al. Somatic c-KIT activating mutation in urticaria pigmentosa and aggressive mastocytosis: establishment of clonality in a human mast cell neoplasm. Nat. Genet. 12, 312–314 (1996).
Fritsche-Polanz, R. et al. Mutation analysis of C-KIT in patients with myelodysplastic syndromes without mastocytosis and cases of systemic mastocytosis. Br. J. Haematol. 113, 357–364 (2001).
Arock, M. et al. KIT mutation analysis in mast cell neoplasms: recommendations of the European Competence Network on Mastocytosis. Leukemia 29, 1223–1232 (2015). Basic consensus recommendations for the use and application of molecular markers and assays, including KIT mutational studies, in patients with mastocytosis.
Muñoz-González, J. I. et al. Frequency and prognostic impact of KIT and other genetic variants in indolent systemic mastocytosis. Blood 134, 456–468 (2019).
Hoermann, G. et al. Standards of genetic testing in the diagnosis and prognostication of systemic mastocytosis in 2022: recommendations of the EU-US cooperative group. J. Allergy Clin. Immunol. Pract. 10, 1953–1963 (2022).
Bergstrom, A., Hagglund, H., Berglund, A., Nilsson, G. & Lambe, M. Epidemiology of mastocytosis: a population-based study (Sweden). Acta Oncol. 63, 44–50 (2024).
Schuler, C. F. T. et al. Prevalence of mastocytosis and Hymenoptera venom allergy in the United States. J. Allergy Clin. Immunol. 148, 1316–1323 (2021).
Zanotti, R. et al. A multidisciplinary diagnostic approach reveals a higher prevalence of indolent systemic mastocytosis: 15-years’ experience of the GISM network. Cancers 13, 6380 (2021).
Jorgensen, M. P. et al. Prevalence and incidence of mastocytosis in adults: a Danish nationwide register study. Eur. J. Epidemiol. 40, 43–53 (2025).
Kaakati, R. N., Khokhar, D. & Akin, C. Demographics, types of patient-reported allergic diseases, and anaphylaxis in mastocytosis: a single-center US experience. J. Allergy Clinic. Immunologist. Pract. 13, 398–406 (2025).
Aldama, L. N. D. et al. Prevalence and impact of the KIT M541L variant in patients with mastocytosis. Oncotarget 15, 521–531 (2024).
Jennings, S. V. et al. Mast cell diseases in practice and research: issues and perspectives raised by patients and their recommendations to the scientific community and beyond. J. Allergy Clin. Immunol. Pract. 10, 2039–2051 (2022).
Trizuljak, J. et al. Clinical features and survival of patients with indolent systemic mastocytosis defined by the updated WHO classification. Allergy 75, 1927–1938 (2020).
Kluin-Nelemans, H. C. et al. Cytogenetic and molecular aberrations and worse outcome for male patients in systemic mastocytosis. Theranostics 11, 292–303 (2021).
Galatà, G. et al. Genome-wide association study identifies novel susceptibility loci for KIT D816V positive mastocytosis. Am. J. Hum. Genet. 108, 284–294 (2021).
Popadic, S. et al. Mastocytosis in children: a single-center long-term follow-up study. Int. J. Dermatol. 62, 616–620 (2023).
Castells, M. & Austen, K. F. Mastocytosis: mediator-related signs and symptoms. Int. Arch. Allergy Immunol. 127, 147–152 (2002).
Valent, P. et al. Global classification of mast cell activation disorders: an ICD-10-CM-adjusted proposal of the ECNM-AIM consortium. J. Allergy Clin. Immunol. Pract. 10, 1941–1950 (2022).
Broesby-Olsen, S. et al. Risk of solid cancer, cardiovascular disease, anaphylaxis, osteoporosis and fractures in patients with systemic mastocytosis: a nationwide population-based study. Am. J. Hematol. 91, 1069–1075 (2016).
Kaszuba, A. et al. Mastocytosis and skin cancer: the current state of knowledge. Int. J. Mol. Sci. 24, 9840 (2023).
Meni, C. et al. Paediatric mastocytosis: a systematic review of 1747 cases. Br. J. Dermatol. 172, 642–651 (2015).
Czarny, J., Renke, J., Żawrocki, A., Nowicki, R. J. & Lange, M. Natural evolution in pediatric cutaneous mastocytosis: 10-year follow-up. Int. J. Dermatol. 60, 1253–1257 (2021).
Auquit-Auckbur, I. et al. Malignant transformation of mastocytoma developed on skin mastocytosis into cutaneous mast cell sarcoma. Am. J. Surg. Pathol. 36, 779–782 (2012).
Chantorn, R. & Shwayder, T. Death from mast cell leukemia: a young patient with longstanding cutaneous mastocytosis evolving into fatal mast cell leukemia. Pediatr. Dermatol. 29, 605–609 (2012).
Bautista-Quach, M. A. et al. Mast cell sarcoma in an infant: a case report and review of the literature. J. Pediatr. Hematol. Oncol. 35, 315–320 (2013).
Wimazal, F., Geissler, P., Shnawa, P., Sperr, W. R. & Valent, P. Severe life-threatening or disabling anaphylaxis in patients with systemic mastocytosis: a single-center experience. Int. Arch. Allergy Immunol. 157, 399–405 (2012).
Niedoszytko, M. et al. Prevalence of hypersensitivity reactions in various forms of mastocytosis: a pilot study of 2485 adult patients with mastocytosis collected in the ECNM registry. Allergy 79, 2470–2481 (2024).
Alvarez-Twose, I. et al. Clinical, biological, and molecular characteristics of clonal mast cell disorders presenting with systemic mast cell activation symptoms. J. Allergy Clin. Immunol. 125, 1269–1278.e1262 (2010).
Bonadonna, P. et al. Clonal mast cell disorders in patients with systemic reactions to Hymenoptera stings and increased serum tryptase levels. J. Allergy Clin. Immunol. 123, 680–686 (2009).
Niedoszytko, M., Bonadonna, P., Oude Elberink, J. N. & Golden, D. B. Epidemiology, diagnosis, and treatment of Hymenoptera venom allergy in mastocytosis patients. Immunol. Allergy Clin. North Am. 34, 365–381 (2014).
Castells, M. C., Hornick, J. L. & Akin, C. Anaphylaxis after hymenoptera sting: is it venom allergy, a clonal disorder, or both? J. Allergy Clin. Immunol. Pract. 3, 350–355 (2015).
Rossini, M. et al. Bone involvement and osteoporosis in mastocytosis. Immunol. Allergy Clin. North Am. 34, 383–396 (2014).
Degboe, Y. et al. Prevalence and risk factors for fragility fracture in systemic mastocytosis. Bone 105, 219–225 (2017).
Greene, L. W., Asadipooya, K., Corradi, P. F. & Akin, C. Endocrine manifestations of systemic mastocytosis in bone. Rev. Endocr. Metab. Disord. 17, 419–431 (2016).
Fritsche-Polanz, R. et al. High frequency of concomitant mastocytosis in patients with acute myeloid leukemia exhibiting the transforming KIT mutation D816V. Mol. Oncol. 4, 335–346 (2010).
Ustun, C. et al. Core-binding factor acute myeloid leukemia with t(8;21): risk factors and a novel scoring system (I-CBFit). Cancer Med. 7, 4447–4455 (2018).
Craig, J. W. et al. Detection of the KIT(D816V) mutation in myelodysplastic and/or myeloproliferative neoplasms and acute myeloid leukemia with myelodysplasia-related changes predicts concurrent systemic mastocytosis. Mod. Pathol. 33, 1135–1145 (2020).
Jahn, N. et al. Genomic heterogeneity in core-binding factor acute myeloid leukemia and its clinical implication. Blood Adv. 4, 6342–6352 (2020).
Jawhar, M. et al. Additional mutations in SRSF2, ASXL1 and/or RUNX1 identify a high-risk group of patients with KIT D816V+ advanced systemic mastocytosis. Leukemia 30, 136–143 (2016). This article describes the prognostic impact of multiple somatic mutations in critical target genes in patients with SM.
Pardanani, A. D. et al. ASXL1 and CBL mutations are independently predictive of inferior survival in advanced systemic mastocytosis. Br. J. Haematol. 175, 534–536 (2016).
Jawhar, M. et al. MARS: Mutation-Adjusted Risk Score for advanced systemic mastocytosis. J. Clin. Oncol. 37, 2846–2856 (2019).
Pardanani, A. et al. Mayo Alliance Prognostic System for mastocytosis: clinical and hybrid clinical-molecular models. Blood Adv. 2, 2964–2972 (2018).
Muñoz-González, J. I. et al. Proposed Global Prognostic Score for Systemic Mastocytosis: a retrospective prognostic modelling study. Lancet Haematol. 8, e194–e204 (2021).
Pardanani, A. Systemic mastocytosis in adults: 2023 update on diagnosis, risk stratification and management. Am. J. Hematol. 98, 1097–1116 (2023).
Gulen, T., Ljung, C., Nilsson, G. & Akin, C. Risk factor analysis of anaphylactic reactions in patients with systemic mastocytosis. J. Allergy Clin. Immunol. Pract. 5, 1248–1255 (2017).
Brockow, K., Jofer, C., Behrendt, H. & Ring, J. Anaphylaxis in patients with mastocytosis: a study on history, clinical features and risk factors in 120 patients. Allergy 63, 226–232 (2008).
Gonzalez de Olano, D. et al. Prevalence of allergy and anaphylactic symptoms in 210 adult and pediatric patients with mastocytosis in Spain: a study of the Spanish Network on Mastocytosis (REMA). Clin. Exp. Allergy 37, 1547–1555 (2007).
Vos, B., van Anrooij, B., van Doormaal, J. J., Dubois, A. E. J. & Oude Elberink, J. N. G. Fatal anaphylaxis to yellow jacket stings in mastocytosis: options for identification and treatment of at-risk patients. J. Allergy Clin. Immunol. Pract. 5, 1264–1271 (2017).
Bonadonna, P. et al. Anaphylactic reactions after discontinuation of hymenoptera venom immunotherapy: a clonal mast cell disorder should be suspected. J. Allergy Clin. Immunol. Pract. 6, 1368–1372 (2018).
Fodinger, M. et al. Origin of human mast cells: development from transplanted hematopoietic stem cells after allogeneic bone marrow transplantation. Blood 84, 2954–2959 (1994). Report demonstrating that allogeneic stem cell transplantation in a patient with advanced SM is a potentially curative treatment approach and that neoplastic mast cells are replaced by normal donor-derived mast cells several months after transplantation.
Valent, P. Cytokines involved in growth and differentiation of human basophils and mast cells. Exp. Dermatol. 4, 255–259 (1995).
Valent, P. et al. Mast cells as a unique hematopoietic lineage and cell system: from Paul Ehrlich’s visions to precision medicine concepts. Theranostics 10, 10743–10768 (2020).
Boyce, J. A. Advances in mast cell biology. J. Allergy Clin. Immunol. 149, 1919–1925 (2022).
Dahlin, J. S. et al. The ingenious mast cell: contemporary insights into mast cell behavior and function. Allergy 77, 83–99 (2022).
Valent, P. The riddle of the mast cell: kit(CD117)-ligand as the missing link? Immunol. Today 15, 111–114 (1994).
Galli, S. J., Tsai, M. & Wershil, B. K. The c-kit receptor, stem cell factor, and mast cells. What each is teaching us about the others. Am. J. Pathol. 142, 965–974 (1993).
Costa, J. J. et al. Recombinant human stem cell factor (kit ligand) promotes human mast cell and melanocyte hyperplasia and functional activation in vivo. J. Exp. Med. 183, 2681–2686 (1996).
Chatterjea, D. et al. Adoptive transfer of mast cells does not enhance the impaired survival of Kit(W)/Kit(W-v) mice in a model of low dose intraperitoneal infection with bioluminescent Salmonella typhimurium. Immunol. Lett. 99, 122–129 (2005).
Maurer, M. et al. The c-kit ligand, stem cell factor, can enhance innate immunity through effects on mast cells. J. Exp. Med. 188, 2343–2348 (1998).
Akahoshi, M. et al. Mast cell chymase reduces the toxicity of Gila monster venom, scorpion venom, and vasoactive intestinal polypeptide in mice. J. Clin. Invest. 121, 4180–4191 (2011).
Starkl, P. et al. IgE antibodies increase honeybee venom responsiveness and detoxification efficiency of mast cells. Allergy 77, 499–512 (2022).
Abraham, S. N. & Malaviya, R. Mast cells in infection and immunity. Infect. Immun. 65, 3501–3508 (1997).
Malaviya, R., Ikeda, T., Ross, E. & Abraham, S. N. Mast cell modulation of neutrophil influx and bacterial clearance at sites of infection through TNF-α. Nature 381, 77–80 (1996).
Cerny-Reiterer, S. et al. Long-term treatment with imatinib results in profound mast cell deficiency in Ph+ chronic myeloid leukemia. Oncotarget 6, 3071–3084 (2015).
Lev, S., Yarden, Y. & Givol, D. Dimerization and activation of the kit receptor by monovalent and bivalent binding of the stem cell factor. J. Biol. Chem. 267, 15970–15977 (1992).
Ashman, L. K. The biology of stem cell factor and its receptor c-kit. Int. J. Biochem. Cell Biol. 31, 1037–1051 (1999).
Qiu, F. H. et al. Primary structure of c-kit: relationship with the CSF-1/PDGF receptor kinase family-oncogenic activation of v-kit involves deletion of extracellular domain and C terminus. EMBO J. 7, 1003–1011 (1988).
Nocka, K., Buck, J., Levi, E. & Besmer, P. Candidate ligand for the c-kit transmembrane kinase receptor: KL, a fibroblast derived growth factor stimulates mast cells and erythroid progenitors. EMBO J. 9, 3287–3294 (1990).
Roskoski, R. Jr. Structure and regulation of Kit protein-tyrosine kinase-the stem cell factor receptor. Biochem. Biophys. Res. Commun. 338, 1307–1315 (2005).
Kuriu, A. et al. Proliferation of human myeloid leukemia cell line associated with the tyrosine-phosphorylation and activation of the proto-oncogene c-kit product. Blood 78, 2834–2840 (1991).
Blechman, J. M., Lev, S., Givol, D. & Yarden, Y. Structure-function analyses of the kit receptor for the steel factor. Stem Cell 11, 12–21 (1993).
Blechman, J. M. & Yarden, Y. Structural aspects of receptor dimerization. c-kit as an example. Ann. N. Y. Acad. Sci. 766, 344–362 (1995).
Lev, S., Yarden, Y. & Givol, D. A recombinant ectodomain of the receptor for the stem cell factor (SCF) retains ligand-induced receptor dimerization and antagonizes SCF-stimulated cellular responses. J. Biol. Chem. 267, 10866–10873 (1992).
Lemmon, M. A., Pinchasi, D., Zhou, M., Lax, I. & Schlessinger, J. Kit receptor dimerization is driven by bivalent binding of stem cell factor. J. Biol. Chem. 272, 6311–6317 (1997).
Roskoski, R. Jr. Signaling by Kit protein-tyrosine kinase—the stem cell factor receptor. Biochem. Biophys. Res. Commun. 337, 1–13 (2005).
Harir, N. et al. Oncogenic Kit controls neoplastic mast cell growth through a Stat5/PI3-kinase signaling cascade. Blood 112, 2463–2473 (2008).
Tsai, M., Valent, P. & Galli, S. J. KIT as a master regulator of the mast cell lineage. J. Allergy Clin. Immunol. 149, 1845–1854 (2022).
Baumgartner, C. et al. Expression of activated STAT5 in neoplastic mast cells in systemic mastocytosis: subcellular distribution and role of the transforming oncoprotein KIT D816V. Am. J. Pathol. 175, 2416–2429 (2009).
Bibi, S. et al. Co-operating STAT5 and AKT signaling pathways in chronic myeloid leukemia and mastocytosis: possible new targets of therapy. Haematologica 99, 417–429 (2014).
Agarwal, S., Kazi, J. U., Mohlin, S., Pahlman, S. & Ronnstrand, L. The activation loop tyrosine 823 is essential for the transforming capacity of the c-Kit oncogenic mutant D816V. Oncogene 34, 4581–4590 (2015).
Park, H., Kim, D., Koh, Y. & Yoon, S. S. The upregulation of Pim kinases is essential in coordinating the survival, proliferation, and migration of KIT D816V-mutated neoplastic mast cells. Leuk. Res. 83, 106166 (2019).
Eisenwort, G. et al. Identification of a leukemia-initiating stem cell in human mast cell leukemia. Leukemia 33, 2673–2684 (2019). This study describes that MCL and other forms of advanced SM originate from a small pool of CD34+/CD38−/CD117+ disease-initiating (leukaemia-initiating) neoplastic stem cells.
Akin, C. & Metcalfe, D. D. The biology of Kit in disease and the application of pharmacogenetics. J. Allergy Clin. Immunol. 114, 13–19 (2004).
Arock, M. et al. Clinical impact and proposed application of molecular markers, genetic variants, and cytogenetic analysis in mast cell neoplasms: status 2022. J. Allergy Clin. Immunol. 149, 1855–1865 (2022).
Chantran, Y., Valent, P. & Arock, M. KIT mutations and other genetic defects in mastocytosis: implications for disease pathology and targeted therapies. Immunol. Allergy Clin. North Am. 43, 651–664 (2023).
Bodemer, C. et al. Pediatric mastocytosis is a clonal disease associated with D816V and other activating c-KIT mutations. J. Invest. Dermatol. 130, 804–815 (2010). This article describes the broad spectrum of diverse KIT mutations that can be detected in patients with childhood-onset mastocytosis.
Meni, C. et al. Paediatric mastocytosis: long-term follow-up of 53 patients with whole sequencing of KIT. A prospective study. Br. J. Dermatol. 179, 925–932 (2018).
Bibi, S. et al. Molecular defects in mastocytosis: KIT and beyond KIT. Immunol. Allergy Clin. North Am. 34, 239–262 (2014).
Kitayama, H. et al. Constitutively activating mutations of c-kit receptor tyrosine kinase confer factor-independent growth and tumorigenicity of factor-dependent hematopoietic cell lines. Blood 85, 790–798 (1995).
Kitamura, Y., Tsujimura, T., Jippo, T., Kasugai, T. & Kanakura, Y. Regulation of development, survival and neoplastic growth of mast cells through the c-kit receptor. Int. Arch. Allergy Immunol. 107, 54–56 (1995).
Mayerhofer, M. et al. Unique effects of KIT D816V in BaF3 cells: induction of cluster formation, histamine synthesis, and early mast cell differentiation antigens. J. Immunol. 180, 5466–5476 (2008). This study shows that KIT-D816V alone is a strong differentiation-inducing oncoprotein (mast cell differentiation), but only a relatively weak oncogene regarding its capacity to trigger proliferation.
Rajan, V. et al. KIT D816V is dimerization-independent and activates downstream pathways frequently perturbed in mastocytosis. Br. J. Haematol. 202, 960–970 (2023).
Hartmann, K. et al. Expression of Bcl-2 and Bcl-xL in cutaneous and bone marrow lesions of mastocytosis. Am. J. Pathol. 163, 819–826 (2003).
Aichberger, K. J. et al. Identification of proapoptotic Bim as a tumor suppressor in neoplastic mast cells: role of KIT D816V and effects of various targeted drugs. Blood 114, 5342–5351 (2009).
Aichberger, K. J. et al. Identification of MCL1 as a novel target in neoplastic mast cells in systemic mastocytosis: inhibition of mast cell survival by MCL1 antisense oligonucleotides and synergism with PKC412. Blood 109, 3031–3041 (2007).
Longley, B. J., Reguera, M. J. & Ma, Y. Classes of c-KIT activating mutations: proposed mechanisms of action and implications for disease classification and therapy. Leuk. Res. 25, 571–576 (2001).
Conde-Fernandes, I. et al. Systemic mastocytosis with KIT V560G mutation presenting as recurrent episodes of vascular collapse: response to disodium cromoglycate and disease outcome. Allergy Asthma Clin. Immunol. 13, 21 (2017).
Joris, M. et al. Mast cell leukaemia: c-KIT mutations are not always positive. Case Rep. Hematol. 2012, 517546 (2012).
Kennedy, V. E. et al. Mast cell leukemia: clinical and molecular features and survival outcomes of patients in the ECNM registry. Blood Adv. 7, 1713–1724 (2023).
Matsumoto, N. P. et al. Mast cell sarcoma: clinicopathologic and molecular analysis of 10 new cases and review of literature. Mod. Pathol. 35, 865–874 (2022).
Ryan, R. J. et al. Mast cell sarcoma: a rare and potentially under-recognized diagnostic entity with specific therapeutic implications. Mod. Pathol. 26, 533–543 (2013).
Lyons, J. J. et al. Elevated basal serum tryptase identifies a multisystem disorder associated with increased TPSAB1 copy number. Nat. Genet. 48, 1564–1569 (2016).
Lyons, J. J., Greiner, G., Hoermann, G. & Metcalfe, D. D. Incorporating tryptase genotyping into the workup and diagnosis of mast cell diseases and reactions. J. Allergy Clin. Immunol. Pract. 10, 1964–1973 (2022).
Khoury, P. & Lyons, J. J. Mast cell activation in the context of elevated basal serum tryptase: genetics and presentations. Curr. Allergy Asthma Rep. 19, 55 (2019).
Greiner, G. et al. Hereditary α tryptasemia is a valid genetic biomarker for severe mediator-related symptoms in mastocytosis. Blood 137, 238–247 (2021). This article describes a relationship between hereditary α-tryptasaemia and severe mediator-related symptoms in patients with mastocytosis.
Chollet, M. B. & Akin, C. Hereditary alpha tryptasemia is not associated with specific clinical phenotypes. J. Allergy Clin. Immunol. 149, 728–735.e722 (2022).
Sordi, B. et al. Disease correlates and clinical relevance of hereditary α-tryptasemia in patients with systemic mastocytosis. J. Allergy Clin. Immunol. 151, 485–493.e411 (2023).
Polivka, L. et al. Pathophysiologic implications of elevated prevalence of hereditary alpha-tryptasemia in all mastocytosis subtypes. J. Allergy Clin. Immunol. 153, 349–353.e344 (2024).
Gonzalez-de-Olano, D. et al. Clinical impact of the TPSAB1 genotype in mast cell diseases: a REMA study in a cohort of 959 individuals. Allergy 79, 711–723 (2024).
Serafin, W. E. & Austen, K. F. Mediators of immediate hypersensitivity reactions. N. Engl. J. Med. 317, 30–34 (1987).
Schwartz, L. B. Mediators of human mast cells and human mast cell subsets. Ann. Allergy 58, 226–235 (1987).
Gordon, J. R. & Galli, S. J. Mast cells as a source of both preformed and immunologically inducible TNF-α/cachectin. Nature 346, 274–276 (1990).
Boyce, J. A. Mast cells and eicosanoid mediators: a system of reciprocal paracrine and autocrine regulation. Immunol. Rev. 217, 168–185 (2007).
Kalesnikoff, J. & Galli, S. J. New developments in mast cell biology. Nat. Immunol. 9, 1215–1223 (2008).
Elieh Ali Komi, D., Wohrl, S. & Bielory, L. Mast cell biology at molecular level: a comprehensive review. Clin. Rev. Allergy Immunol. 58, 342–365 (2020).
Marcella, S. et al. Vascular endothelial growth factors and angiopoietins as new players in mastocytosis. Clin. Exp. Med. 21, 415–427 (2021).
Caughey, G. H. Update on mast cell proteases as drug targets. Immunol. Allergy Clin. North Am. 43, 777–787 (2023).
Greiner, G. et al. Tumor necrosis factor alpha promotes clonal dominance of KIT D816V+ cells in mastocytosis: role of survivin and impact on prognosis. Blood 143, 1006–1017 (2024).
Chia, S. L., Kapoor, S., Carvalho, C., Bajenoff, M. & Gentek, R. Mast cell ontogeny: from fetal development to life-long health and disease. Immunol. Rev. 315, 31–53 (2023).
Tomita, Y., Maeda, K. & Tagami, H. Mechanisms for hyperpigmentation in postinflammatory pigmentation, urticaria pigmentosa and sunburn. Dermatologica 179, 49–53 (1989).
Longley, B. J. Jr. et al. Altered metabolism of mast-cell growth factor (c-kit ligand) in cutaneous mastocytosis. N. Engl. J. Med. 328, 1302–1307 (1993).
Brockow, K. et al. Levels of mast-cell growth factors in plasma and in suction skin blister fluid in adults with mastocytosis: correlation with dermal mast-cell numbers and mast-cell tryptase. J. Allergy Clin. Immunol. 109, 82–88 (2002).
Aberer, E. et al. Clinical impact of skin lesions in mastocytosis: a multicenter study of the european competence network on mastocytosis. J. Invest. Dermatol. 141, 1719–1727 (2021).
Escribano, L. et al. Indolent systemic mastocytosis without skin involvement vs. isolated bone marrow mastocytosis. Haematologica 96, e26 (2011).
Akin, C., Scott, L. M. & Metcalfe, D. D. Slowly progressive systemic mastocytosis with high mast-cell burden and no evidence of a non-mast-cell hematologic disorder: an example of a smoldering case? Leuk. Res. 25, 635–638 (2001).
Akin, C. et al. Analysis of the surface expression of c-kit and occurrence of the c-kit Asp816Val activating mutation in T cells, B cells, and myelomonocytic cells in patients with mastocytosis. Exp. Hematol. 28, 140–147 (2000). Demonstration of multilineage involvement with KITD816V mutation in SM proving the haematopietic stem cell nature of the disease.
Grootens, J. et al. Single-cell analysis reveals the KIT D816V mutation in haematopoietic stem and progenitor cells in systemic mastocytosis. EBioMedicine 43, 150–158 (2019).
Jara-Acevedo, M. et al. Detection of the KIT D816V mutation in peripheral blood of systemic mastocytosis: diagnostic implications. Mod. Pathol. 28, 1138–1149 (2015).
Yavuz, A. S., Lipsky, P. E., Yavuz, S., Metcalfe, D. D. & Akin, C. Evidence for the involvement of a hematopoietic progenitor cell in systemic mastocytosis from single-cell analysis of mutations in the c-kit gene. Blood 100, 661–665 (2002).
Hoermann, G. et al. The KIT D816V allele burden predicts survival in patients with mastocytosis and correlates with the WHO type of the disease. Allergy 69, 810–813 (2014).
Teodosio, C. et al. An immature immunophenotype of bone marrow mast cells predicts for multilineage D816V KIT mutation in systemic mastocytosis. Leukemia 26, 951–958 (2012).
Schwaab, J. et al. Comprehensive mutational profiling in advanced systemic mastocytosis. Blood 122, 2460–2466 (2013).
Damaj, G. et al. ASXL1 but not TET2 mutations adversely impact overall survival of patients suffering systemic mastocytosis with associated clonal hematologic non-mast-cell diseases. PLoS ONE 9, e85362 (2014).
Shah, S. et al. Cytogenetic abnormalities in systemic mastocytosis: WHO subcategory-specific incidence and prognostic impact among 348 informative cases. Am. J. Hematol. 93, 1461–1466 (2018).
Naumann, N. et al. Incidence and prognostic impact of cytogenetic aberrations in patients with systemic mastocytosis. Genes Chromosomes Cancer 57, 252–259 (2018).
Soucie, E. et al. In aggressive forms of mastocytosis, TET2 loss cooperates with c-KITD816V to transform mast cells. Blood 120, 4846–4849 (2012).
Ellis, J. M. Urticaria pigmentosa; a report of a case with autopsy. Arch. Pathol. 48, 426–435 (1949).
Wolff, K., Komar, M. & Petzelbauer, P. Clinical and histopathological aspects of cutaneous mastocytosis. Leuk. Res. 25, 519–528 (2001).
Lennert, K. & Parwaresch, M. R. Mast cells and mast cell neoplasia: a review. Histopathology 3, 349–365 (1979).
Akin, C. How to evaluate the patient with a suspected mast cell disorder and how/when to manage symptoms. Hematol. Am. Soc. Hematol. Educ. Program. 2022, 55–63 (2022).
Carter, M. C. et al. A distinct biomolecular profile identifies monoclonal mast cell disorders in patients with idiopathic anaphylaxis. J. Allergy Clin. Immunol. 141, 180–188.e183 (2018).
Fuchs, D. et al. Scoring the risk of having systemic mastocytosis in adult patients with mastocytosis in the skin. J. Allergy Clin. Immunol. Pract. 9, 1705–1712.e1704 (2021).
Gulen, T., Hagglund, H., Sander, B., Dahlen, B. & Nilsson, G. The presence of mast cell clonality in patients with unexplained anaphylaxis. Clin. Exp. Allergy 44, 1179–1187 (2014).
Chovanec, J. et al. Genetically defined individual reference ranges for tryptase limit unnecessary procedures and unmask myeloid neoplasms. Blood Adv. 7, 1796–1810 (2023).
Rossini, M. et al. Prevalence, pathogenesis, and treatment options for mastocytosis-related osteoporosis. Osteoporos. Int. 27, 2411–2421 (2016).
Makovoz, A. et al. Assessment of osteoporosis and fracture risk in mastocytosis within a North American Cohort. J. Allergy Clinic. Immunologist. Pract. 9, 4459–4467 (2021).
Tanasi, I. et al. Underlying systemic mastocytosis in patients with unexplained osteoporosis: score proposal. Bone 186, 117141 (2024).
Valent, P. et al. Standards and standardization in mastocytosis: consensus statements on diagnostics, treatment recommendations and response criteria. Eur. J. Clin. Invest. 37, 435–453 (2007).
Valent, P., Sotlar, K., Horny, H. P., Arock, M. & Akin, C. World Health Organization classification and diagnosis of mastocytosis: update 2023 and future perspectives. Immunol. Allergy Clin. North Am. 43, 627–649 (2023).
Valent, P. et al. Refined diagnostic criteria and classification of mast cell leukemia (MCL) and myelomastocytic leukemia (MML): a consensus proposal. Ann. Oncol. 25, 1691–1700 (2014). This article describes updated consensus criteria and a revised consensus classification of MCL.
Sperr, W. R. et al. Morphologic properties of neoplastic mast cells: delineation of stages of maturation and implication for cytological grading of mastocytosis. Leuk. Res. 25, 529–536 (2001). This article provides the basic morphological criteria to identify and delineate various types of abnormal (neoplastic) mast cells in bone marrow smears in patients with mastocytosis.
Escribano, L. et al. Indolent systemic mast cell disease in adults: immunophenotypic characterization of bone marrow mast cells and its diagnostic implications. Blood 91, 2731–2736 (1998).
Valent, P. et al. Variable expression of activation-linked surface antigens on human mast cells in health and disease. Immunol. Rev. 179, 74–81 (2001).
Escribano, L. et al. Immunophenotypic analysis of mast cells in mastocytosis: when and how to do it. Proposals of the Spanish Network on Mastocytosis (REMA). Cytom. B Clin. Cytom. 58, 1–8 (2004).
Krauth, M. T. et al. Effects of the CD33-targeted drug gemtuzumab ozogamicin (Mylotarg) on growth and mediator secretion in human mast cells and blood basophils. Exp. Hematol. 35, 108–116 (2007).
Mueller, N. et al. CD44 is a RAS/STAT5-regulated invasion receptor that triggers disease expansion in advanced mastocytosis. Blood 132, 1936–1950 (2018).
Morgado, J. M. et al. CD30 expression by bone marrow mast cells from different diagnostic variants of systemic mastocytosis. Histopathology 63, 780–787 (2013).
Huang, L. et al. Well-differentiated systemic mastocytosis showed excellent clinical response to imatinib in the absence of known molecular genetic abnormalities: a case report. Medicine 95, e4934 (2016).
Blatt, K. et al. Identification of the Ki-1 antigen (CD30) as a novel therapeutic target in systemic mastocytosis. Blood 126, 2832–2841 (2015).
Greiner, G. et al. Digital PCR: a sensitive and precise method for KIT D816V quantification in mastocytosis. Clin. Chem. 64, 547–555 (2018).
Kristensen, T., Vestergaard, H. & Moller, M. B. Improved detection of the KIT D816V mutation in patients with systemic mastocytosis using a quantitative and highly sensitive real-time qPCR assay. J. Mol. Diagn. 13, 180–188 (2011).
Akin, C. Molecular diagnosis of mast cell disorders: a paper from the 2005 William Beaumont Hospital Symposium on Molecular Pathology. J. Mol. Diagn. 8, 412–419 (2006).
Zhang, L. Y. et al. A novel K509I mutation of KIT identified in familial mastocytosis-in vitro and in vivo responsiveness to imatinib therapy. Leuk. Res. 30, 373–378 (2006).
de Melo Campos, P. et al. Familial systemic mastocytosis with germline KIT K509I mutation is sensitive to treatment with imatinib, dasatinib and PKC412. Leuk. Res. 38, 1245–1251 (2014).
Chan, E. C. et al. Mastocytosis associated with a rare germline KIT K509I mutation displays a well-differentiated mast cell phenotype. J. Allergy Clin. Immunol. 134, 178–187 (2014).
Schwartz, L. B., Metcalfe, D. D., Miller, J. S., Earl, H. & Sullivan, T. Tryptase levels as an indicator of mast-cell activation in systemic anaphylaxis and mastocytosis. N. Engl. J. Med. 316, 1622–1626 (1987). This study shows that the basal serum tryptase level is a solid biomarker for the total body burden of normal and neoplastic mast cells and that the event-induced increase in tryptase over the individual´s baseline is indicative of anaphylaxis caused by systemic mast cell activation.
Schwartz, L. B. & Irani, A. M. Serum tryptase and the laboratory diagnosis of systemic mastocytosis. Hematol. Oncol. Clin. North Am. 14, 641–657 (2000).
Sperr, W. R. et al. Serum tryptase levels in patients with mastocytosis: correlation with mast cell burden and implication for defining the category of disease. Int. Arch. Allergy Immunol. 128, 136–141 (2002).
Akin, C., Valent, P. & Metcalfe, D. D. Mast cell activation syndrome: proposed diagnostic criteria. J. Allergy Clin. Immunol. 126, 1099–1104.e1094 (2010).
Valent, P. et al. Definitions, criteria and global classification of mast cell disorders with special reference to mast cell activation syndromes: a consensus proposal. Int. Arch. Allergy Immunol. 157, 215–225 (2012).
Valent, P. et al. Proposed diagnostic algorithm for patients with suspected mast cell activation syndrome. J. Allergy Clin. Immunol. Pract. 7, 1125–1133.e1121 (2019).
Lyons, J. J. Inherited and acquired determinants of serum tryptase levels in humans. Ann. Allergy Asthma Immunol. 127, 420–426 (2021).
Lyons, J. J. et al. Heritable risk for severe anaphylaxis associated with increased α-tryptase-encoding germline copy number at TPSAB1. J. Allergy Clin. Immunol. 147, 622–632 (2021).
Valent, P. et al. The normal range of baseline tryptase should be 1 to 15 ng/mL and covers healthy individuals with HαT. J. Allergy Clin. Immunol. Pract. 11, 3010–3020 (2023).
Hagen, W. et al. A case of bone marrow mastocytosis associated with multiple myeloma. Ann. Hematol. 76, 167–174 (1998).
Sotlar, K. et al. “Occult” mastocytosis with activating c-kit point mutation evolving into systemic mastocytosis associated with plasma cell myeloma and secondary amyloidosis. J. Clin. Pathol. 59, 875–878 (2006).
Prabahran, A. A. & Juneja, S. K. Systemic mastocytosis with concurrent multiple myeloma. Blood 131, 1494 (2018).
Gadde, R. & Oduro, K. A. Jr. Systemic mastocytosis with an associated plasma cell myeloma. Int. J. Lab. Hematol. 45, 409–410 (2023).
Valent, P. et al. Chronic mast cell leukemia (MCL) with KIT S476I: a rare entity defined by leukemic expansion of mature mast cells and absence of organ damage. Ann. Hematol. 94, 223–231 (2015).
Jain, P. et al. Mast cell leukemia (MCL): clinico-pathologic and molecular features and survival outcome. Leuk. Res. 59, 105–109 (2017).
Galura, G. M., Cherukuri, S. V., Hakim, N., Gaur, S. & Orazi, A. Acute aleukemic mast cell leukemia: report of a case and review of the literature. Leuk. Res. Rep. 14, 100230 (2020).
Zanelli, M. et al. Mast cell leukemia: an update with a practical review. Cancers 15, 1664–1680 (2023).
Akin, C. Mast cell activation syndromes. J. Allergy Clin. Immunol. 140, 349–355 (2017).
Wiechers, T. et al. Large maculopapular cutaneous lesions are associated with favorable outcome in childhood-onset mastocytosis. J. Allergy Clin. Immunol. 136, 1581–1590.e1583 (2015). This article describes the prognostic impact of two types of skin lesion in childhood patients with MPCM: in patients with small-sized symmetrical lesions the disease usually persists into adulthood, whereas large polymorphic skin lesions are mostly seen in children as these lesions disappear during or shortly after puberty.
Galen, B. T. & Rose, M. G. Darier’s sign in mastocytosis. Blood 123, 1127 (2014).
Carter, M. C. & Metcalfe, D. D. Paediatric mastocytosis. Arch. Dis. Child. 86, 315–319 (2002).
Carter, M. C. et al. Assessment of clinical findings, tryptase levels, and bone marrow histopathology in the management of pediatric mastocytosis. J. Allergy Clin. Immunol. 136, 1673–1679.e1673 (2015).
Alvarez-Twose, I. et al. Increased serum baseline tryptase levels and extensive skin involvement are predictors for the severity of mast cell activation episodes in children with mastocytosis. Allergy 67, 813–821 (2012).
Carter, M. C. et al. Detection of KIT D816V in peripheral blood of children with manifestations of cutaneous mastocytosis suggests systemic disease. Br. J. Haematol. 183, 775–782 (2018).
Voelker, D., Bednarski, J. J., Nieman, E., Carter, M. C. & Polk, B. Hematopoietic KIT D816Y mutation presenting as in utero aggressive systemic mastocytosis with response to midostaurin. J. Allergy Clin. Immunol. Pract. 11, 1323–1325.e1321 (2023).
Carter, M. C., Metcalfe, D. D., Clark, A. S., Wayne, A. S. & Maric, I. Abnormal bone marrow histopathology in paediatric mastocytosis. Br. J. Haematol. 168, 865–873 (2015).
Ke, H., Kazi, J. U., Zhao, H. & Sun, J. Germline mutations of KIT in gastrointestinal stromal tumor (GIST) and mastocytosis. Cell Biosci. 6, 55 (2016).
Tanasi, I. et al. Familial occurrence of systemic and cutaneous mastocytosis in an adult multicentre series. Br. J. Haematol. 193, 845–848 (2021).
Wasag, B. et al. Novel, activating KIT-N822I mutation in familial cutaneous mastocytosis. Exp. Hematol. 39, 859–865.e852 (2011).
Wohrl, S. et al. A c-kit mutation in exon 18 in familial mastocytosis. J. Invest. Dermatol. 133, 839–841 (2013).
Hartmann, K. et al. Novel germline mutation of KIT associated with familial gastrointestinal stromal tumors and mastocytosis. Gastroenterology 129, 1042–1046 (2005).
Brockow, K. et al. Mediator-related symptoms and anaphylaxis in children with mastocytosis. Int. J. Mol. Sci. 22, 2684 (2021).
Valent, P. et al. Personalized management strategies in mast cell disorders: ECNM-AIM user’s guide for daily clinical practice. J. Allergy Clin. Immunol. Pract. 10, 1999–2012.e1996 (2022).
Shibata, Y., Hirota, S., Saito, I. & Asahina, A. Diffuse cutaneous mastocytosis: identification of KIT mutation and long-term follow-up with serum tryptase level. J. Dermatol. 48, 672–675 (2021).
Carter, M. C. et al. Adverse reactions to drugs and biologics in patients with clonal mast cell disorders: a work group report of the mast cells disorder committee, american academy of allergy, asthma & immunology. J. Allergy Clin. Immunol. 143, 880–893 (2019).
Akin, C. et al. Demonstration of an aberrant mast-cell population with clonal markers in a subset of patients with “idiopathic” anaphylaxis. Blood 110, 2331–2333 (2007). Report of demonstration of mastocytosis as the underlying pathology in patients presenting with ‘idiopathic’ anaphylaxis.
Castells, M. & Butterfield, J. Mast cell activation syndrome and mastocytosis: initial treatment options and long-term management. J. Allergy Clin. Immunol. Pract. 7, 1097–1106 (2019).
Gulen, T. & Akin, C. Pharmacotherapy of mast cell disorders. Curr. Opin. Allergy Clin. Immunol. 17, 295–303 (2017).
Tolar, J., Tope, W. D. & Neglia, J. P. Leukotriene-receptor inhibition for the treatment of systemic mastocytosis. N. Engl. J. Med. 350, 735–736 (2004).
Butterfield, J. H. Survey of aspirin administration in systemic mastocytosis. Prostaglandins Other Lipid Mediat. 88, 122–124 (2009).
Horan, R. F., Sheffer, A. L. & Austen, K. F. Cromolyn sodium in the management of systemic mastocytosis. J. Allergy Clin. Immunol. 85, 852–855 (1990).
Dykewicz, M. S., Wong, S. S., Patterson, R. & Harris, K. E. Evaluation of ketotifen in corticosteroid-dependent idiopathic anaphylaxis. Ann. Allergy 65, 406–410 (1990).
Akin, C. Omalizumab for mast cell disorders. J. Allergy Clin. Immunol. 155, 81–83 (2025).
Jendoubi, F. et al. Omalizumab in the treatment of adult patients with mastocytosis: a systematic review. Clin. Exp. Allergy 50, 654–661 (2020).
Joshi, S. R., Anstey, K. M. & Khan, D. A. Chronic spontaneous urticaria: an update on the evaluation and management. Immunol. Allergy Clin. North Am. 44, 503–515 (2024).
Brazzelli, V. et al. Narrow-band UVB phototherapy and psoralen-ultraviolet A photochemotherapy in the treatment of cutaneous mastocytosis: a study in 20 patients. Photodermatol. Photoimmunol. Photomed. 32, 238–246 (2016).
Czarnetzki, B. M., Rosenbach, T., Kolde, G. & Frosch, P. J. Phototherapy of urticaria pigmentosa: clinical response and changes of cutaneous reactivity, histamine and chemotactic leukotrienes. Arch. Dermatol. Res. 277, 105–113 (1985).
Czarnetzki, B. M. & Behrendt, H. Urticaria pigmentosa: clinical picture and response to oral disodium cromoglycate. Br. J. Dermatol. 105, 563–567 (1981).
Czarnetzki, B. M. A double-blind cross-over study of the effect of ketotifen in urticaria pigmentosa. Dermatologica 166, 44–47 (1983).
Kettelhut, B. V., Berkebile, C., Bradley, D. & Metcalfe, D. D. A double-blind, placebo-controlled, crossover trial of ketotifen versus hydroxyzine in the treatment of pediatric mastocytosis. J. Allergy Clin. Immunol. 83, 866–870 (1989).
Lemal, R. et al. Omalizumab therapy for mast cell-mediator symptoms in patients with ISM, CM, MMAS, and MCAS. J. Allergy Clin. Immunol. Pract. 7, 2387–2395.e2383 (2019).
Broesby-Olsen, S. et al. Omalizumab prevents anaphylaxis and improves symptoms in systemic mastocytosis: efficacy and safety observations. Allergy 73, 230–238 (2018).
Distler, M. et al. Efficacy of omalizumab in mastocytosis: allusive indication obtained from a prospective, double-blind, multicenter study (XOLMA Study). Dermatology 236, 529–539 (2020).
McComish, J. S. et al. Randomized controlled trial of omalizumab in treatment-resistant systemic and cutaneous mastocytosis (ROAM). J. Allergy Clin. Immunol. Pract. 11, 2248–2250.e2243 (2023).
Akin, C., Arock, M. & Valent, P. Tyrosine kinase inhibitors for the treatment of indolent systemic mastocytosis: are we there yet. J. Allergy Clin. Immunol. 149, 1912–1918 (2022).
Alvarez-Twose, I. et al. Complete response after imatinib mesylate therapy in a patient with well-differentiated systemic mastocytosis. J. Clin. Oncol. 30, e126–e129 (2012).
Gotlib, J. et al. Efficacy and safety of avapritinib in advanced systemic mastocytosis: interim analysis of the phase 2 PATHFINDER trial. Nat. Med. 27, 2192–2199 (2021).
DeAngelo, D. J. et al. Safety and efficacy of avapritinib in advanced systemic mastocytosis: the phase 1 EXPLORER trial. Nat. Med. 27, 2183–2191 (2021). This study describes that avapritinib is a disease-modifying drug that can induce major clinical responses and haematological remission in a subset of patients with SM.
Reiter, A. et al. Efficacy and safety of avapritinib in previously treated patients with advanced systemic mastocytosis. Blood Adv. 6, 5750–5762 (2022).
Pardanani, A., Reichard, K. & Tefferi, A. Advanced systemic mastocytosis-Revised classification, new drugs and how we treat. Br. J. Haematol. 204, 402–414 (2024).
Costa, A. et al. Systemic mastocytosis: 2023 update on diagnosis and management in adults. Expert Opin. Emerg. Drugs 28, 153–165 (2023).
Ma, Y. et al. The c-KIT mutation causing human mastocytosis is resistant to STI571 and other KIT kinase inhibitors; kinases with enzymatic site mutations show different inhibitor sensitivity profiles than wild-type kinases and those with regulatory-type mutations. Blood 99, 1741–1744 (2002).
Akin, C. et al. Effects of tyrosine kinase inhibitor STI571 on human mast cells bearing wild-type or mutated c-kit. Exp. Hematol. 31, 686–692 (2003).
Gotlib, J. et al. Avapritinib versus placebo in indolent systemic mastocytosis. NEJM Evid. 2, EVIDoa2200339 (2023). This clinical trial shows that avapritinib is an effective drug that can decrease the symptom burden and increase the QoL in patients with indolent SM.
Gotlib, J. et al. Efficacy and safety of midostaurin in advanced systemic mastocytosis. N. Engl. J. Med. 374, 2530–2541 (2016). This study documents the clinical efficacy and disease-modifying effect of midostaurin in patients with advanced SM.
Akin, C. Tyrosine kinase inhibitors in non-advanced systemic mastocytosis. Immunol. Allergy Clin. North Am. 43, 743–750 (2023).
Growney, J. D. et al. Activation mutations of human c-KIT resistant to imatinib mesylate are sensitive to the tyrosine kinase inhibitor PKC412. Blood 106, 721–724 (2005).
Degenfeld-Schonburg, L. et al. Antineoplastic efficacy profiles of avapritinib and nintedanib in KIT D816V+ systemic mastocytosis: a preclinical study. Am. J. Cancer Res. 13, 355–378 (2023).
Frost, M. J., Ferrao, P. T., Hughes, T. P. & Ashman, L. K. Juxtamembrane mutant V560GKit is more sensitive to Imatinib (STI571) compared with wild-type c-kit whereas the kinase domain mutant D816VKit is resistant. Mol. Cancer Ther. 1, 1115–1124 (2002).
Peter, B. et al. Target interaction profiling of midostaurin and its metabolites in neoplastic mast cells predicts distinct effects on activation and growth. Leukemia 30, 464–472 (2016).
Krauth, M. T. et al. Midostaurin (PKC412) inhibits immunoglobulin E-dependent activation and mediator release in human blood basophils and mast cells. Clin. Exp. Allergy 39, 1711–1720 (2009).
Valent, P. et al. Midostaurin: a magic bullet that blocks mast cell expansion and activation. Ann. Oncol. 28, 2367–2376 (2017).
Ebeling, P. R., Daly, R. M., Kerr, D. A. & Kimlin, M. G. An evidence-informed strategy to prevent osteoporosis in Australia. Med. J. Aust. 198, 90–91 (2013).
Kluin-Nelemans, H. C. et al. Response to interferon alfa-2b in a patient with systemic mastocytosis. N. Engl. J. Med. 326, 619–623 (1992).
Tefferi, A., Li, C. Y., Butterfield, J. H. & Hoagland, H. C. Treatment of systemic mast-cell disease with cladribine. N. Engl. J. Med. 344, 307–309 (2001).
Casassus, P. et al. Treatment of adult systemic mastocytosis with interferon-alpha: results of a multicentre phase II trial on 20 patients. Br. J. Haematol. 119, 1090–1097 (2002).
Tefferi, A. et al. Cladribine therapy for advanced and indolent systemic mastocytosis: Mayo Clinic experience in 42 consecutive cases. Br. J. Haematol. 196, 975–983 (2022).
Valent, P., Sperr, W. R. & Akin, C. How I treat patients with advanced systemic mastocytosis. Blood 116, 5812–5817 (2010).
Kluin-Nelemans, H. C. et al. Cladribine therapy for systemic mastocytosis. Blood 102, 4270–4276 (2003).
Gotlib, J. et al. Activity of the tyrosine kinase inhibitor PKC412 in a patient with mast cell leukemia with the D816V KIT mutation. Blood 106, 2865–2870 (2005).
Valent, P. et al. Aggressive systemic mastocytosis and related mast cell disorders: current treatment options and proposed response criteria. Leuk. Res. 27, 635–641 (2003).
Gotlib, J. et al. International working group-myeloproliferative neoplasms research and treatment (IWG-MRT) & European Competence Network on Mastocytosis (ECNM) consensus response criteria in advanced systemic mastocytosis. Blood 121, 2393–2401 (2013).
Castells, M. & Akin, C. Finding the right KIT inhibitor for advanced systemic mastocytosis. Nat. Med. 27, 2081–2082 (2021).
Gotlib, J., Reiter, A. & DeAngelo, D. J. Avapritinib for advanced systemic mastocytosis. Blood 140, 1667–1673 (2022).
Tashi, T. & Deininger, M. W. Management of advanced systemic mastocytosis and associated myeloid neoplasms. Immunol. Allergy Clin. North Am. 43, 723–741 (2023).
Ustun, C. et al. Hematopoietic stem-cell transplantation for advanced systemic mastocytosis. J. Clin. Oncol. 32, 3264–3274 (2014). This multicentre study provides definitive evidence that allogeneic HSCT is a potentially curative treatment approach in patients with advanced mastocytosis.
Ustun, C. et al. Consensus opinion on allogeneic hematopoietic cell transplantation in advanced systemic mastocytosis. Biol. Blood Marrow Transpl. 22, 1348–1356 (2016).
McLornan, D. P. et al. Allogeneic haematopoietic cell transplantation for advanced systemic mastocytosis: best practice recommendations on behalf of the EBMT Practice Harmonisation and Guidelines Committee. Leukemia 38, 699–711 (2024).
Lubke, J. et al. Allogeneic hematopoietic cell transplantation in advanced systemic mastocytosis: a retrospective analysis of the DRST and GREM registries. Leukemia 38, 810–821 (2024).
Jennings, S. et al. The mastocytosis society survey on mast cell disorders: patient experiences and perceptions. J. Allergy Clin. Immunol. Pract. 2, 70–76 (2014).
Russell, N. et al. The mastocytosis society survey on mast cell disorders: part 2-patient clinical experiences and beyond. J. Allergy Clin. Immunol. Pract. 7, 1157–1165.e1156 (2019).
Pulfer, S. et al. Health-related quality of life and influencing factors in adults with nonadvanced mastocytosis-a cross-sectional study and qualitative approach. J. Allergy Clin. Immunol. Pract. 9, 3166–3175.e3162 (2021).
Pyatilova, P. & Siebenhaar, F. Measuring symptom severity and quality of life in mastocytosis. Immunol. Allergy Clin. North Am. 43, 751–762 (2023).
Vermeiren, M. R., Kranenburg, L. W., van Daele, P. L. A., Gerth van Wijk, R. & Hermans, M. A. W. Psychological functioning and quality of life in patients with mastocytosis: a cross-sectional study. Ann. Allergy Asthma Immunol. 124, 373–378.e372 (2020).
Sagues-Sese, E., Garcia-Casares, N. & Alvarez-Twose, I. Cognitive, neuropsychiatric and neurological alterations in mastocytosis: a systematic review. Clin. Transl. Allergy 13, e12319 (2023).
Siebenhaar, F. et al. Development and validation of the mastocytosis quality of life questionnaire: MC-QoL. Allergy 71, 869–877 (2016).
Taylor, F. et al. Psychometric evaluation of the advanced systemic mastocytosis symptom assessment form (AdvSM-SAF). Leuk. Res. 108, 106606 (2021).
Hartmann, K. et al. Midostaurin improves quality of life and mediator-related symptoms in advanced systemic mastocytosis. J. Allergy Clin. Immunol. 146, 356–366.e354 (2020).
Gonzalez de Olano, D., Cain, W. V., Bernstein, J. A. & Akin, C. Disease spectrum of anaphylaxis disorders. J. Allergy Clin. Immunol. Pract. 11, 1989–1996 (2023).
Akin, C., Siebenhaar, F., Wechsler, J. B., Youngblood, B. A. & Maurer, M. Detecting changes in mast cell numbers versus activation in human disease: a roadblock for current biomarkers? J. Allergy Clin. Immunol. Pract. 12, 1727–1737 (2024).
Acknowledgements
P.V. was supported in part by the Austrian Science Fund (FWF) project PAT6394523.
Author information
Authors and Affiliations
Contributions
Introduction (C.A. and P.V.); Epidemiology (C.A. and M.C.C.); Mechanisms/pathophysiology (M.A., C.A. and P.V.); Diagnosis, screening and prevention (M.C.C., T.I.G., C.A. and P.V.); Management (M.C.C., C.A. and P.V.); Quality of life (C.A. and P.V.); Outlook (C.A. and P.V.); overview of the Primer (C.A. and P.V.). All authors have read and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
P.V. declares consultancy (honoraria) for Novartis, Blueprint, Cogent, Incyte, AOP Orphan, Stemline and Daiichi Sankyo. M.A. receives research grants from Blueprint; honoraria from AB Science, Blueprint and Novartis. T.I.G. declares consultancy for Incyte, Blueprint, Celgene/BMS and Cogent Biosciences. C.A. declares consultancy (honoraria) for Blueprint, Novartis, Cogent and Telios; research grant from Blueprint, Cogent and Telios; Investigator in clinical trial for Blueprint, Cogent and Telios. M.C.C. declares no competing interests.
Peer review
Peer review information
Nature Reviews Disease Primers thanks M. Bonifacio, S. Broesby-Olsen, D. Radia, P. Van Daele and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Informed consent
The authors affirm that human research participants provided informed consent for publication of the images in Fig. 4.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Akin, C., Arock, M., Carter, M.C. et al. Mastocytosis. Nat Rev Dis Primers 11, 30 (2025). https://doi.org/10.1038/s41572-025-00611-8
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41572-025-00611-8
This article is cited by
-
Novel KIT mutation, D816_N819delinsll, in a patient with systemic mastocytosis: a case report
Virchows Archiv (2025)
-
Bone marrow mastocytosis associated with primary cutaneous follicle center lymphoma: an unusual case report
Annals of Hematology (2025)