Abstract
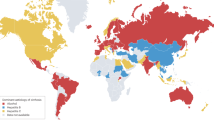
Heavy alcohol consumption is a major cause of morbidity and mortality. Globally, alcohol per-capita consumption rose from 5.5 litres in 2005 to 6.4 litres in 2016 and is projected to increase further to 7.6 litres in 2030. In 2019, an estimated 25% of global cirrhosis deaths were associated with alcohol. The global estimated age-standardized death rate (ASDR) of alcohol-associated cirrhosis was 4.5 per 100,000 population, with the highest and lowest ASDR in Africa and the Western Pacific, respectively. The annual incidence of hepatocellular carcinoma (HCC) among patients with alcohol-associated cirrhosis ranged from 0.9% to 5.6%. Alcohol was associated with approximately one-fifth of global HCC-related deaths in 2019. Between 2012 and 2017, the global estimated ASDR for alcohol-associated cirrhosis declined, but the ASDR for alcohol-associated liver cancer increased. Measures are required to curb heavy alcohol consumption to reduce the burden of alcohol-associated cirrhosis and HCC. Degree of alcohol intake, sex, older age, obesity, type 2 diabetes mellitus, gut microbial dysbiosis and genetic variants are key factors in the development of alcohol-associated cirrhosis and HCC. In this Review, we discuss the global epidemiology, projections and risk factors for alcohol-associated cirrhosis and HCC.
Key points
-
Global alcohol consumption per capita rose from 5.5 litres in 2005 to 6.4 litres in 2016 and is projected to increase further to 7.6 litres in 2030.
-
Currently, Europe has the highest levels of alcohol consumption; however, it is projected to be surpassed by countries/regions in the Western Pacific region by 2030.
-
Alcohol was estimated to be associated with one-quarter of global cirrhosis deaths and one-fifth of liver cancer deaths in 2019.
-
Alcohol was the second-fastest-growing cause of liver-cancer deaths from 2010 to 2019.
-
Patients with alcohol-associated hepatocellular carcinoma (HCC) tend to present with advanced tumours, which relates at least in part to late diagnosis and limited access to HCC screening in comparison to other aetiologies of liver disease.
-
The risk factors for the development of cirrhosis and HCC include the amount of alcohol consumed, age, obesity, diabetes, smoking and PNPLA3 variants.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Rehm, J., Samokhvalov, A. V. & Shield, K. D. Global burden of alcoholic liver diseases. J. Hepatol. 59, 160–168 (2013).
Seitz, H. K. et al. Alcoholic liver disease. Nat. Rev. Dis. Prim. 4, 16 (2018).
World Health Organization. Global Status Report On Alcohol And Health 2018. WHO https://www.who.int/publications/i/item/9789241565639 (2018). Comprehensive work from the World Health Organization that provides global and country/region-level data on alcohol consumption and burden.
Manthey, J. et al. Global alcohol exposure between 1990 and 2017 and forecasts until 2030: a modelling study. Lancet 393, 2493–2502 (2019). An important study that forecasts alcohol exposure in 2030.
GBD 2016 Alcohol Collaborators. Alcohol use and burden for 195 countries and territories, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 392, 1015–1035 (2018).
Crabb, D. W., Im, G. Y., Szabo, G., Mellinger, J. L. & Lucey, M. R. Diagnosis and treatment of alcohol-associated liver diseases: 2019 practice guidance from the American Association for the Study of Liver Diseases. Hepatology 71, 306–333 (2020).
Mathurin, P. & Bataller, R. Trends in the management and burden of alcoholic liver disease. J. Hepatol. 62, S38–S46 (2015).
European Association for the Study of the Liver. EASL Clinical Practice Guidelines: management of alcohol-related liver disease. J. Hepatol. 69, 154–181 (2018).
Rehm, J. et al. Alcohol as a risk factor for liver cirrhosis: a systematic review and meta-analysis. Drug Alcohol Rev. 29, 437–445 (2010).
Mathurin, P. et al. Fibrosis progression occurs in a subgroup of heavy drinkers with typical histological features. Aliment. Pharmacol. Ther. 25, 1047–1054 (2007).
Singal, A. K. & Mathurin, P. Diagnosis and treatment of alcohol-associated liver disease: a review. JAMA 326, 165–176 (2021).
Collaborators, G. D. A. I. Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet 396, 1204–1222 (2020). An important source of data providing peer-reviewed estimates for the burden of liver disease associated with alcohol.
Sung, H. et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71, 209–249 (2021).
Huang, D. Q. et al. Changing global epidemiology of liver cancer from 2010 to 2019: NASH is the fastest growing cause of liver cancer. Cell Metab. 34, 969–977.e962 (2022). This study utilized data from the Global Burden of Disease Study and determined that alcohol was the second fastest growing cause of liver cancer deaths.
Paik, J. M., Golabi, P., Younossi, Y., Mishra, A. & Younossi, Z. M. Changes in the global burden of chronic liver diseases from 2012 to 2017: the growing impact of NAFLD. Hepatology 72, 1605–1616 (2020).
WHO. Global Information System on Alcohol and Health (GISAH). Global Health Observatory https://www.who.int/data/gho/data/themes/global-information-system-on-alcohol-and-health (2016).
Blas, E. and Sivasankara Kurup, A. (eds) Equity, social determinants and public health programmes. Social Determinants of Health (WHO) https://www.who.int/publications/i/item/9789241563970 (2015).
WHO. European action plan to reduce the harmful use of alcohol 2012–2020. WHO https://www.euro.who.int/__data/assets/pdf_file/0008/178163/E96726.pdf (2012).
Schmidt, L. A. & Room, R. Alcohol and inequity in the process of development: contributions from ethnographic research. Int. J. Alcohol Drug Res. 1, 41–55 (2013).
Wang, H., Ma, L., Yin, Q., Zhang, X. & Zhang, C. Prevalence of alcoholic liver disease and its association with socioeconomic status in north-eastern China. Alcohol. Clin. Exp. Res. 38, 1035–1041 (2014).
Charatcharoenwitthaya, P., Liangpunsakul, S. & Piratvisuth, T. Alcohol-associated liver disease: East versus West. Clin. Liver Dis. 16, 231–235 (2020).
Rehm, J. et al. The relationship between different dimensions of alcohol use and the burden of disease-an update. Addiction 112, 968–1001 (2017).
US Department of Health and Human Services and US Department of Agriculture. 2015–2020 Dietary Guidelines for Americans. health.gov https://health.gov/our-work/nutrition-physical-activity/dietary-guidelines/previous-dietary-guidelines/2015 (2015).
Ritchie, H. Alcohol Consumption (Our World in Data, 2019).
Bellentani, S. et al. Drinking habits as cofactors of risk for alcohol induced liver damage. The Dionysos Study Group. Gut 41, 845–850 (1997).
The global, regional, and national burden of cirrhosis by cause in 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol. 5, 245-266, (2020).
Tapper, E. B. & Parikh, N. D. Mortality due to cirrhosis and liver cancer in the United States, 1999-2016: observational study. Br. Med. J. 362, k2817 (2018).
Julien, J., Ayer, T., Bethea, E. D., Tapper, E. B. & Chhatwal, J. Projected prevalence and mortality associated with alcohol-related liver disease in the USA, 2019-40: a modelling study. Lancet Public Health 5, e316–e323 (2020).
Toyoda, H., Huang, D. Q., Le, M. H. & Nguyen, M. H. Liver care and surveillance: the global impact of the COVID-19 pandemic. Hepatol. Commun. 4, 1751–1757 (2020).
Tan, E. X.-X. et al. Impact of COVID-19 on liver transplantation in Hong Kong and Singapore: a modelling study. Lancet Regional Health West. Pacif. https://doi.org/10.1016/j.lanwpc.2021.100262 (2021).
Boettler, T. et al. Impact of COVID-19 on the care of patients with liver disease: EASL-ESCMID position paper after 6 months of the pandemic. JHEP Rep. 2, 100169 (2020).
Li, J. et al. Epidemiology of COVID-19: a systematic review and meta-analysis of clinical characteristics, risk factors, and outcomes. J. Med. Virol. 93, 1449–1458 (2021).
Pollard, M. S., Tucker, J. S. & Green, H. D. Jr Changes in adult alcohol use and consequences during the COVID-19 pandemic in the US. JAMA Netw. Open 3, e2022942 (2020).
The Lancet Gastroenterology, H. Drinking alone: COVID-19, lockdown, and alcohol-related harm. Lancet Gastroenterol. Hepatol. 5, 625 (2020).
Bittermann, T., Mahmud, N. & Abt, P. Trends in liver transplantation for acute alcohol-associated hepatitis during the COVID-19 pandemic in the US. JAMA Netw. Open 4, e2118713–e2118713 (2021).
Marjot, T. et al. Outcomes following SARS-CoV-2 infection in patients with chronic liver disease: an international registry study. J. Hepatol. 74, 567–577 (2021).
Julien, J. et al. Effect of increased alcohol consumption during COVID-19 pandemic on alcohol-associated liver disease: a modeling study. Hepatology 75, 1480–1490 (2022).
Akinyemiju, T. et al. The burden of primary liver cancer and underlying etiologies from 1990 to 2015 at the global, regional, and national level: results from the Global Burden of Disease Study 2015. JAMA Oncol. 3, 1683–1691 (2017).
Altekruse, S. F., Devesa, S. S., Dickie, L. A., McGlynn, K. A. & Kleiner, D. E. Histological classification of liver and intrahepatic bile duct cancers in SEER registries. J. Regist. Manag. 38, 201–205 (2011).
Percy, C., Ries, L. G. & Van Holten, V. D. The accuracy of liver cancer as the underlying cause of death on death certificates. Public Health Rep. 105, 361–367 (1990).
Polednak, A. P. Using cancer registries to assess the accuracy of primary liver or intrahepatic bile duct cancer as the underlying cause of death, 1999–2010. J. Regist. Manag. 40, 168–175 (2013).
Hagström, H. et al. Risk of cancer in biopsy-proven alcohol-related liver disease: a population-based cohort study of 3,410 persons. Clin. Gastroenterol. Hepatol. https://doi.org/10.1016/j.cgh.2021.01.005 (2021).
N’Kontchou, G. et al. Risk factors for hepatocellular carcinoma in patients with alcoholic or viral C cirrhosis. Clin. Gastroenterol. Hepatol. 4, 1062–1068 (2006).
Huang, D. Q. et al. Hepatocellular carcinoma incidence in alcohol-associated cirrhosis: systematic review and meta-analysis. Clin. Gastroenterol. Hepatol. https://doi.org/10.1016/j.cgh.2022.06.032 (2022).
Jepsen, P., Ott, P., Andersen, P. K., Sørensen, H. T. & Vilstrup, H. Risk for hepatocellular carcinoma in patients with alcoholic cirrhosis: a Danish nationwide cohort study. Ann. Intern. Med. 156, 841–847 (2012).
Ioannou, G. N., Green, P., Kerr, K. F. & Berry, K. Models estimating risk of hepatocellular carcinoma in patients with alcohol or NAFLD-related cirrhosis for risk stratification. J. Hepatol. 71, 523–533 (2019).
Ganne-Carrié, N. et al. Estimate of hepatocellular carcinoma incidence in patients with alcoholic cirrhosis. J. Hepatol. 69, 1274–1283 (2018).
Lin, C. W. et al. Heavy alcohol consumption increases the incidence of hepatocellular carcinoma in hepatitis B virus-related cirrhosis. J. Hepatol. 58, 730–735 (2013).
Aguilera, V. et al. Cirrhosis of mixed etiology (hepatitis C virus and alcohol): posttransplantation outcome — comparison with hepatitis C virus-related cirrhosis and alcoholic-related cirrhosis. Liver Transpl. 15, 79–87 (2009).
Asrani, S. K., Larson, J. J., Yawn, B., Therneau, T. M. & Kim, W. R. Underestimation of liver-related mortality in the United States. Gastroenterology 145, 375–382 (2013).
Bucci, L. et al. Comparison between alcohol- and hepatitis C virus-related hepatocellular carcinoma: clinical presentation, treatment and outcome. Aliment. Pharmacol. Ther. 43, 385–399 (2016).
Schutte, K. et al. Delayed diagnosis of HCC with chronic alcoholic liver disease. Liver Cancer 1, 257–266 (2012).
Costentin, C. E. et al. Hepatocellular carcinoma is diagnosed at a later stage in alcoholic patients: results of a prospective, nationwide study. Cancer 124, 1964–1972 (2018).
Costentin, C. E. et al. Geographical disparities of outcomes of hepatocellular carcinoma in France: the heavier burden of alcohol compared to hepatitis C. Dig. Dis. Sci. 65, 301–311 (2020).
Eskesen, A. N., Bjøro, K., Aandahl, E. M., Line, P. D. & Melum, E. Low use of surveillance and early diagnosis of hepatocellular carcinoma in Norway — a population-based cohort study. Cancer Epidemiol. 38, 741–747 (2014).
Singal, A. G. et al. Failure rates in the hepatocellular carcinoma surveillance process. Cancer Prev. Res. 5, 1124–1130 (2012).
Goutté, N. et al. Geographical variations in incidence, management and survival of hepatocellular carcinoma in a Western country. J. Hepatol. 66, 537–544 (2017).
Younossi, Z. et al. Nonalcoholic steatohepatitis is the fastest growing cause of hepatocellular carcinoma in liver transplant candidates. Clin. Gastroenterol. Hepatol. 17, 748–755.e743 (2019).
Mathurin, P. & Lucey, M. R. Liver transplantation in patients with alcohol-related liver disease: current status and future directions. Lancet Gastroenterol. Hepatol. 5, 507–514 (2020).
Sangro, B., Sarobe, P., Hervás-Stubbs, S. & Melero, I. Advances in immunotherapy for hepatocellular carcinoma. Nat. Rev. Gastroenterol. Hepatol. 18, 525–543 (2021).
Finn, R. S. et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N. Engl. J. Med. 382, 1894–1905 (2020).
Pfister, D. et al. NASH limits anti-tumour surveillance in immunotherapy-treated HCC. Nature 592, 450–456 (2021).
Scheiner, B. et al. Prognosis of patients with hepatocellular carcinoma treated with immunotherapy — development and validation of the CRAFITY score. J. Hepatol. https://doi.org/10.1016/j.jhep.2021.09.035 (2021).
O’Shea, R. S., Dasarathy, S. & McCullough, A. J., Practice Guideline Committee of the American Association for the Study of Liver Diseases & Practice Parameters Committee of the American College of Gastroenterology. Alcoholic liver disease. Hepatology 51, 307–328 (2010).
Christoffersen, P. & Nielsen, K. Histological changes in human liver biopsies from chronic alcoholics. Acta Pathol. Microbiol. Scand. A 80, 557–565 (1972).
Ganne-Carrié, N. & Nahon, P. Hepatocellular carcinoma in the setting of alcohol-related liver disease. J. Hepatol. 70, 284–293 (2019).
Mancebo, A. et al. Annual incidence of hepatocellular carcinoma among patients with alcoholic cirrhosis and identification of risk groups. Clin. Gastroenterol. Hepatol. 11, 95–101 (2013).
Janele, D. et al. Effects of testosterone, 17β-estradiol, and downstream estrogens on cytokine secretion from human leukocytes in the presence and absence of cortisol. Ann. N. Y. Acad. Sci. 1069, 168–182 (2006).
Yin, M. et al. Estrogen is involved in early alcohol-induced liver injury in a rat enteral feeding model. Hepatology 31, 117–123 (2000).
Baraona, E. et al. Gender differences in pharmacokinetics of alcohol. Alcohol. Clin. Exp. Res. 25, 502–507 (2001).
Ikejima, K. et al. Estrogen increases sensitivity of hepatic Kupffer cells to endotoxin. Am. J. Physiol. 274, G669–G676 (1998).
Becker, U. et al. Prediction of risk of liver disease by alcohol intake, sex, and age: a prospective population study. Hepatology 23, 1025–1029 (1996).
Simpson, R. F. et al. Alcohol drinking patterns and liver cirrhosis risk: analysis of the prospective UK Million Women Study. Lancet Public Health 4, e41–e48 (2019).
Askgaard, G., Grønbæk, M., Kjær, M. S., Tjønneland, A. & Tolstrup, J. S. Alcohol drinking pattern and risk of alcoholic liver cirrhosis: a prospective cohort study. J. Hepatol. 62, 1061–1067 (2015).
Tan, D. J. H. et al. Global burden of liver cancer in males and females: changing etiological basis and the growing contribution of NASH. Hepatology https://doi.org/10.1002/hep.32758 (2022).
Hart, C. L., Morrison, D. S., Batty, G. D., Mitchell, R. J. & Davey Smith, G. Effect of body mass index and alcohol consumption on liver disease: analysis of data from two prospective cohort studies. BMJ 340, c1240 (2010).
Loomba, R., Bettencourt, R. & Barrett-Connor, E. Synergistic association between alcohol intake and body mass index with serum alanine and aspartate aminotransferase levels in older adults: the Rancho Bernardo Study. Aliment. Pharmacol. Ther. 30, 1137–1149 (2009).
Ruhl, C. E. & Everhart, J. E. Joint effects of body weight and alcohol on elevated serum alanine aminotransferase in the United States population. Clin. Gastroenterol. Hepatol. 3, 1260–1268 (2005).
Raynard, B. et al. Risk factors of fibrosis in alcohol-induced liver disease. Hepatology 35, 635–638 (2002).
Loomba, R. et al. Synergism between obesity and alcohol in increasing the risk of hepatocellular carcinoma: a prospective cohort study. Am. J. Epidemiol. 177, 333–342 (2013).
Loomba, R. et al. Obesity and alcohol synergize to increase the risk of incident hepatocellular carcinoma in men. Clin. Gastroenterol. Hepatol. 8, 891–898 (2010).
Hassan, M. M. et al. Obesity early in adulthood increases risk but does not affect outcomes of hepatocellular carcinoma. Gastroenterology 149, 119–129 (2015).
Nair, S., Mason, A., Eason, J., Loss, G. & Perrillo, R. P. Is obesity an independent risk factor for hepatocellular carcinoma in cirrhosis? Hepatology 36, 150–155 (2002).
Elkrief, L. et al. Diabetes mellitus in patients with cirrhosis: clinical implications and management. Liver Int. 36, 936–948 (2016).
Fehrenbach, H., Weiskirchen, R., Kasper, M. & Gressner, A. M. Up-regulated expression of the receptor for advanced glycation end products in cultured rat hepatic stellate cells during transdifferentiation to myofibroblasts. Hepatology 34, 943–952 (2001).
Roerecke, M. et al. Alcohol consumption and risk of liver cirrhosis: a systematic review and meta-analysis. Am. J. Gastroenterol. 114, 1574–1586 (2019).
Gentry, R. T. Effect of food on the pharmacokinetics of alcohol absorption. Alcohol. Clin. Exp. Res. 24, 403–404 (2000).
Munaka, M. et al. Genetic polymorphisms of tobacco- and alcohol-related metabolizing enzymes and the risk of hepatocellular carcinoma. J. Cancer Res. Clin. Oncol. 129, 355–360 (2003).
Pimpin, L. et al. Burden of liver disease in Europe: epidemiology and analysis of risk factors to identify prevention policies. J. Hepatol. 69, 718–735 (2018).
Sheron, N. Alcohol and liver disease in Europe — simple measures have the potential to prevent tens of thousands of premature deaths. J. Hepatol. 64, 957–967 (2016).
Anderson, P. et al. Improving the delivery of brief interventions for heavy drinking in primary health care: outcome results of the optimizing delivery of health care intervention (ODHIN) five-country cluster randomized factorial trial. Addiction 111, 1935–1945 (2016).
Manns, M. P., Burra, P., Sargent, J., Horton, R. & Karlsen, T. H. The Lancet-EASL commission on liver diseases in Europe: overcoming unmet needs, stigma, and inequities. Lancet 392, 621–622 (2018).
El-Zayadi, A. R., Selim, O., Hamdy, H., El-Tawil, A. & Moustafa, H. Heavy cigarette smoking induces hypoxic polycythemia (erythrocytosis) and hyperuricemia in chronic hepatitis C patients with reversal of clinical symptoms and laboratory parameters with therapeutic phlebotomy. Am. J. Gastroenterol. 97, 1264–1265 (2002).
Wang, L. Y. et al. 4-Aminobiphenyl DNA damage in liver tissue of hepatocellular carcinoma patients and controls. Am. J. Epidemiol. 147, 315–323 (1998).
Dam, M. K., Flensborg-Madsen, T., Eliasen, M., Becker, U. & Tolstrup, J. S. Smoking and risk of liver cirrhosis: a population-based cohort study. Scand. J. Gastroenterol. 48, 585–591 (2013).
Klatsky, A. L. & Armstrong, M. A. Alcohol, smoking, coffee, and cirrhosis. Am. J. Epidemiol. 136, 1248–1257 (1992).
Petrick, J. L. et al. Tobacco, alcohol use and risk of hepatocellular carcinoma and intrahepatic cholangiocarcinoma: the Liver Cancer Pooling Project. Br. J. Cancer 118, 1005–1012 (2018).
Abdel-Rahman, O. et al. Cigarette smoking as a risk factor for the development of and mortality from hepatocellular carcinoma: an updated systematic review of 81 epidemiological studies. J. Evidence-Based Med. 10, 245–254 (2017).
Kuper, H. et al. Tobacco smoking, alcohol consumption and their interaction in the causation of hepatocellular carcinoma. Int. J. Cancer 85, 498–502 (2000).
Jee, S. H., Ohrr, H., Sull, J. W. & Samet, J. M. Cigarette smoking, alcohol drinking, hepatitis B, and risk for hepatocellular carcinoma in Korea. J. Natl Cancer Inst. 96, 1851–1856 (2004).
Buch, S. et al. A genome-wide association study confirms PNPLA3 and identifies TM6SF2 and MBOAT7 as risk loci for alcohol-related cirrhosis. Nat. Genet. 47, 1443–1448 (2015).
Salameh, H. et al. PNPLA3 gene polymorphism is associated with predisposition to and severity of alcoholic liver disease. Am. J. Gastroenterol. 110, 846–856 (2015).
Romeo, S. et al. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat. Genet. 40, 1461–1465 (2008).
Schwantes-An, T. H. et al. Genome-wide association study and meta-analysis on alcohol-related liver cirrhosis identifies novel genetic risk factors. Hepatology https://doi.org/10.1002/hep.31535 (2020).
Trépo, E. et al. Common genetic variation in alcohol-related hepatocellular carcinoma: a case-control genome-wide association study. Lancet Oncol. https://doi.org/10.1016/s1470-2045(21)00603-3 (2021).
Schulze, K. et al. Exome sequencing of hepatocellular carcinomas identifies new mutational signatures and potential therapeutic targets. Nat. Genet. 47, 505–511 (2015).
Nahon, P. & Nault, J. C. Constitutional and functional genetics of human alcohol-related hepatocellular carcinoma. Liver Int. 37, 1591–1601 (2017).
Bajaj, J. S. Alcohol, liver disease and the gut microbiota. Nat. Rev. Gastroenterol. Hepatol. 16, 235–246 (2019).
Dubinkina, V. B. et al. Links of gut microbiota composition with alcohol dependence syndrome and alcoholic liver disease. Microbiome 5, 141 (2017).
Leclercq, S. et al. Intestinal permeability, gut-bacterial dysbiosis, and behavioral markers of alcohol-dependence severity. Proc. Natl Acad. Sci. USA 111, E4485–E4493 (2014).
Llopis, M. et al. Intestinal microbiota contributes to individual susceptibility to alcoholic liver disease. Gut 65, 830–839 (2016).
Hartmann, P. et al. Modulation of the intestinal bile acid/farnesoid X receptor/fibroblast growth factor 15 axis improves alcoholic liver disease in mice. Hepatology 67, 2150–2166 (2018).
Saunders, J. B., Aasland, O. G., Babor, T. F., de la Fuente, J. R. & Grant, M. Development of the alcohol use disorders identification test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption-II. Addiction 88, 791–804 (1993).
Bajaj, J. S. et al. Continued alcohol misuse in human cirrhosis is associated with an impaired gut-liver axis. Alcohol. Clin. Exp. Res. 41, 1857–1865 (2017).
Sarin, S. K., Pande, A. & Schnabl, B. Microbiome as a therapeutic target in alcohol-related liver disease. J. Hepatol. 70, 260–272 (2019).
Ren, Z. et al. Gut microbiome analysis as a tool towards targeted non-invasive biomarkers for early hepatocellular carcinoma. Gut 68, 1014–1023 (2019).
Dapito, D. H. et al. Promotion of hepatocellular carcinoma by the intestinal microbiota and TLR4. Cancer Cell 21, 504–516 (2012).
Schwabe, R. F. & Greten, T. F. Gut microbiome in HCC — mechanisms, diagnosis and therapy. J. Hepatol. 72, 230–238 (2020).
GHDx GBD Results Tool. Global Burden of Disease Study https://ghdx.healthdata.org/gbd-results-tool (2019).
Sharma, S. A. et al. Toronto HCC risk index: a validated scoring system to predict 10-year risk of HCC in patients with cirrhosis. J. Hepatol. https://doi.org/10.1016/j.jhep.2017.07.033 (2017).
Toshikuni, N. et al. Comparison of outcomes between patients with alcoholic cirrhosis and those with hepatitis C virus-related cirrhosis. J. Gastroenterol. Hepatol. 24, 1276–1283 (2009).
Kodama, K., Tokushige, K., Hashimoto, E., Taniai, M. & Shiratori, K. Hepatic and extrahepatic malignancies in cirrhosis caused by nonalcoholic steatohepatitis and alcoholic liver disease. Alcohol. Clin. Exp. Res. 37 (suppl. 1), E247–E252 (2013).
The SURF Report 2. WHO https://apps.who.int/iris/bitstream/handle/10665/43190/9241593024_eng.pdf (2005).
Lieber, C. S., Rubin, E. & DeCarli, L. M. Hepatic microsomal ethanol oxidizing system (MEOS): differentiation from alcohol dehydrogenase and NADPH oxidase. Biochem. Biophys. Res. Commun. 40, 858–865 (1970).
Seitz, H. K. & Stickel, F. Molecular mechanisms of alcohol-mediated carcinogenesis. Nat. Rev. Cancer 7, 599–612 (2007).
Linhart, K., Bartsch, H. & Seitz, H. K. The role of reactive oxygen species (ROS) and cytochrome P-450 2E1 in the generation of carcinogenic etheno-DNA adducts. Redox Biol. 3, 56–62 (2014).
Chiba, T., Marusawa, H. & Ushijima, T. Inflammation-associated cancer development in digestive organs: mechanisms and roles for genetic and epigenetic modulation. Gastroenterology 143, 550–563 (2012).
Callinan, S. & Livingston, M. Increases in alcohol consumption in middle-income countries will lead to increased harms. Lancet 393, 2471–2472 (2019).
COVID-19 and increased alcohol consumption: NANOS poll summary report. Canadian Centre on Substance Use and Addiction (CCSA) https://www.ccsa.ca/covid-19-and-increased-alcohol-consumption-nanos-poll-summary-report (2020).
Vanderbruggen, N. et al. Self-reported alcohol, tobacco, and cannabis use during COVID-19 lockdown measures: results from a web-based survey. Eur. Addict. Res. 26, 309–315 (2020).
Sidor, A. & Rzymski, P. Dietary choices and habits during COVID-19 lockdown: experience from Poland. Nutrients https://doi.org/10.3390/nu12061657 (2020).
Kim, J. U. et al. Effect of COVID-19 lockdown on alcohol consumption in patients with pre-existing alcohol use disorder. Lancet Gastroenterol. Hepatol. 5, 886–887 (2020).
Mahmud, N., Hubbard, R. A., Kaplan, D. E. & Serper, M. Declining cirrhosis hospitalizations in the wake of the COVID-19 pandemic: a national cohort study. Gastroenterology 159, 1134–1136.e1133 (2020).
White, A. M., Castle, I.-J. P., Powell, P. A., Hingson, R. W. & Koob, G. F. Alcohol-related deaths during the COVID-19 pandemic. JAMA https://doi.org/10.1001/jama.2022.4308 (2022).
Marjot, T. et al. COVID-19 and liver disease: mechanistic and clinical perspectives. Nat. Rev. Gastroenterol. Hepatol. 18, 348–364 (2021).
Acknowledgements
R.L. receives funding support from the NIAAA (U01AA029019), the NIEHS (5P42ES010337), the NCATS (5UL1TR001442), the NIDDK (U01DK130190, U01DK061734, R01DK106419, P30DK120515, R01DK121378 and R01DK124318), the NHLBI (P01HL147835) and the DOD PRCRP (W81XWH-18-2-0026). D.Q.H. receives funding support from Singapore’s Ministry of Health’s National Medical Research Council under its NMRC Research Training Fellowship (MOH-000595-01). P.M. receives funding support from the Programme Hospitalier de Recherche Clinique (French Minister for Health). H.C.-P. receives funding support from the FCT: Projectos De Investigação Científica E Desenvolvimento, Portugal.
Author information
Authors and Affiliations
Contributions
All authors contributed equally to all aspects of the manuscript.
Corresponding author
Ethics declarations
Competing interests
R.L. serves as a consultant or advisory board member for Anylam/Regeneron, Arrowhead Pharmaceuticals, AstraZeneca, Bristol-Myers Squibb, CohBar, Eli Lilly, Galmed, Gilead Sciences, Glympse Bio, Inipharm, Intercept, Ionis, Janssen, Merck, Metacrine, NGM Biopharmaceuticals, Novartis, Novo Nordisk, Pfizer, Promethera, Sagimet, 89bio and Viking Therapeutics. In addition, his institution has received grant support from Allergan, Boehringer-Ingelheim, Bristol-Myers Squibb, Cirius, Eli Lilly, Galectin Therapeutics, Galmed Pharmaceuticals, Genfit, Gilead, Intercept, Inventiva, Janssen, Madrigal Pharmaceuticals, Merck, NGM Biopharmaceuticals, Pfizer, pH Pharma and Siemens. He is also co-founder of Liponexus. D.Q.H. serves as an advisory board member for Eisai. P.M. serves as a consultant or advisory member for Ipsen, Eisai, Abbvie, Sanofi, Gilead Sciences, Evive Biotech, Novo Nordisk, Bayer Healthcare, Intercept, Surrozen and Pfizer. H.C.-P. lectures and receives advisory board fees from Intercept, Genfit, Promethera Bioscience, Orphalan, Novo Nordisk and Roche Portugal.
Peer review
Peer review information
Nature Reviews Gastroenterology & Hepatology thanks Ramon Bataller, Shiv Sarin and the other, anonymous, reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Review criteria
PubMed was searched using the terms ‘alcoholic liver disease’, ‘alcohol-associated cirrhosis’, ‘alcoholic cirrhosis’, ‘alcohol-related liver disease’, ‘alcohol-associated hepatocellular carcinoma’ and ‘alcohol-associated liver cancer’ without language restrictions. Guidelines, original articles and reviews were evaluated. The literature search was performed in February 2021.
Glossary
- Heavy alcohol consumption
-
The consumption of >40 g of pure alcohol per day over a sustained period of time.
- Current alcohol drinkers
-
Individuals who have consumed alcoholic beverages in the previous 12-month period.
- Age-standardized death rate
-
(ASDR). A weighted average of the age-specific death rates, where the weights are the proportions of a standard population in the corresponding age groups.
- Alcohol-associated
-
A disease state that is attributed to heavy consumption of alcohol.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Huang, D.Q., Mathurin, P., Cortez-Pinto, H. et al. Global epidemiology of alcohol-associated cirrhosis and HCC: trends, projections and risk factors. Nat Rev Gastroenterol Hepatol 20, 37–49 (2023). https://doi.org/10.1038/s41575-022-00688-6
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41575-022-00688-6
This article is cited by
-
Factors associated with hazardous alcohol use in patients with psychiatric disorders: a cross-sectional study in the Neuro-Psycho-Pathological Centre of Kinshasa
BMC Psychiatry (2026)
-
Deep learning-based system to predict hepatocellular carcinoma resection volume using contrast-enhanced CT
Scientific Reports (2026)
-
Demographic variation in weekly alcohol use across countries in the Global Flourishing Study
Communications Medicine (2026)
-
Construction of a prognostic model and multidimensional analysis of hepatocellular carcinoma based on palmitoylation-related genes
Discover Oncology (2026)
-
Functional roles and regulatory mechanisms of paeonol in the treatment of liver disease
Natural Products and Bioprospecting (2026)