Abstract
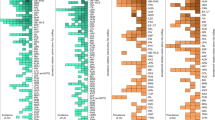
Inflammatory bowel disease (IBD) is a global condition that progresses through four epidemiologic stages: emergence, acceleration in incidence, compounding prevalence and prevalence equilibrium. Early industrialized countries are currently in the compounding prevalence stage before transitioning to the prevalence equilibrium stage, with >1% of their populations expected to live with IBD within the next decade. Prevalence equilibrium can be modelled using a health–illness–death compartment framework and partial differential equations to predict prevalence to 2045. Meanwhile, newly industrialized countries are projected to shift from accelerated incidence with low prevalence to compounding prevalence over the next two decades. This Perspective explores the global evolution of IBD through these epidemiologic stages, presenting a framework for disease prevention and innovative health-care strategies to address the critical challenges the global IBD community will face over the next 20 years.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Global IBD epidemiologic data depicted in the figures are provided in an open-access, downloadable, online interactive source, the ShinyApp: https://kaplan-gi.shinyapps.io/GIVES21.
References
Coward, S. et al. Past and future burden of inflammatory bowel diseases based on modeling of population-based data. Gastroenterology 156, 1345–1353.e4 (2019).
Coward, S. et al. Forecasting the incidence and prevalence of inflammatory bowel disease: a Canadian nationwide analysis. Am. J. Gastroenterol. 119, 1563–1570 (2024).
Lewis, J. D. et al. Incidence, prevalence, and racial and ethnic distribution of inflammatory bowel disease in the United States. Gastroenterology 165, 1197–1205.e2 (2023).
Hracs, L. et al. Global evolution of inflammatory bowel disease across epidemiologic stages. Nature 642, 458–466 (2025).
Kaplan, G. G. & Windsor, J. W. The four epidemiological stages in the global evolution of inflammatory bowel disease. Nat. Rev. Gastroenterol. Hepatol. 18, 56–66 (2021).
Ng, S. C. et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet 390, 2769–2778 (2017).
Kaplan, G. G. & Ng, S. C. Understanding and preventing the global increase of inflammatory bowel disease. Gastroenterology 152, 313–321.e2 (2017).
Kaplan, G. G. & Ng, S. C. Globalisation of inflammatory bowel disease: perspectives from the evolution of inflammatory bowel disease in the UK and China. Lancet Gastroenterol. Hepatol. 1, 307–316 (2016).
Ananthakrishnan, A. N., Kaplan, G. G. & Ng, S. C. Changing global epidemiology of inflammatory bowel diseases: sustaining health care delivery into the 21st century. Clin. Gastroenterol. Hepatol. 18, 1252–1260 (2020).
Burisch, J., Claytor, J., Hernandez, I., Hou, J. K. & Kaplan, G. G. The cost of inflammatory bowel disease care — how to make it sustainable. Clin. Gastroenterol. Hepatol. 23, 386–395 (2025).
Kaplan, G. G. The global burden of IBD: from 2015 to 2025. Nat. Rev. Gastroenterol. Hepatol. 12, 720–727 (2015).
Windsor, J. W. et al. The 2023 impact of inflammatory bowel disease in Canada: executive summary. J. Can. Assoc. Gastroenterol. 6, S1–S8 (2023).
Herauf, M. et al. Commentary on the epidemiology of inflammatory bowel disease in compounding prevalence nations: toward sustaining healthcare delivery. Gastroenterology 166, 949–956 (2024).
Chhibba, T. et al. Environmental risk factors of inflammatory bowel disease: toward a strategy of preventative health. J. Crohns Colitis 19, jjaf042 (2025).
Molodecky, N. A. et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology 142, 46–54.e42 (2012).
Bakhshi, Z., Harmsen, W. S., Tremaine, W. J. & Loftus, E. V. An update on inflammatory bowel disease epidemiology in Olmsted County, Minnesota from 1970 through 2019 [abstract 403]. Gastroenterology 162, S88–S89 (2022).
Shivashankar, R., Tremaine, W. J., Harmsen, W. S. & Loftus, E. V. Jr. Incidence and prevalence of Crohn’s disease and ulcerative colitis in Olmsted County, Minnesota from 1970 through 2010. Clin. Gastroenterol. Hepatol. 15, 857–863 (2017).
Rönnblom, A., Samuelsson, S. M. & Ekbom, A. Ulcerative colitis in the county of Uppsala 1945–2007: incidence and clinical characteristics. J. Crohns Colitis 4, 532–536 (2010).
Lapidus, A., Bernell, O., Hellers, G., Persson, P. G. & Löfberg, R. Incidence of Crohn’s disease in Stockholm County 1955–1989. Gut 41, 480–486 (1997).
Chiba, M., Morita, N., Nakamura, A., Tsuji, K. & Harashima, E. Increased incidence of inflammatory bowel disease in association with dietary transition (Westernization) in Japan. JMA J. 4, 347–357 (2021).
He, B. J. et al. Epidemiological study on the incidence of inflammatory bowel disease in Yinzhou District, Ningbo City from 2011 to 2020 [Chinese]. Beijing Da Xue Xue Bao Yi Xue Ban. 54, 511–519 (2022).
Mokhtar, N. M. et al. A four-decade analysis of the incidence trends, sociodemographic and clinical characteristics of inflammatory bowel disease patients at single tertiary centre, Kuala Lumpur, Malaysia. BMC Public Health 19, 550 (2019).
Hammada, T., Lemdaoui, M.-C., Boutra, F., Zoughailech, D. & Asselah, H. Epidemiological aspects of inflammatory bowel disease in an Algerian population. J. Afr. Hepato. Gastroenterol. 5, 293–302 (2011).
Ho, A. H. et al. Incidence and phenotype of inflammatory bowel disease in 16 regions across Asia, Africa, Latin America and Middle East from GIVES-21 consortium [abstract Tu1863]. Gastroenterology 166, S1443–S1444 (2024).
Qu, L. S. & Gubi, M. M. Clinical characteristics of colonoscopy in 448 patients in the Zanzibar Archipelago: a cross-sectional study. Pan Afr. Med. J. 41, 310 (2022).
Elbadry, M. et al. Clinico-epidemiological characteristics of patients with inflammatory bowel disease in Egypt: a nationwide multicenter study. Front. Med. 9, 867293 (2022).
Archampong, T. N. & Nkrumah, K. N. Inflammatory bowel disease in Accra: what new trends. West Afr. J. Med. 32, 40–44 (2013).
Khalifa, S. E., Mudawi, H. M. & Fedail, S. S. Presentation and management outcome of inflammatory bowel disease in Sudan. Trop. Gastroenterol. 26, 194–196 (2005).
Kotze, P. G. et al. Progression of inflammatory bowel diseases throughout Latin America and the Caribbean: a systematic review. Clin. Gastroenterol. Hepatol. 18, 304–312 (2020).
Banerjee, R. et al. Emerging inflammatory bowel disease demographics, phenotype, and treatment in South Asia, South-East Asia, and Middle East: preliminary findings from the Inflammatory Bowel Disease–Emerging Nations’ Consortium. J. Gastroenterol. Hepatol. 37, 1004–1015 (2022).
Balderramo, D. et al. Challenges in the diagnosis and treatment of inflammatory bowel disease in Latin America. Lancet Gastroenterol. Hepatol. 9, 263–272 (2024).
Ng, S. C. et al. Population density and risk of inflammatory bowel disease: a prospective population-based study in 13 countries or regions in Asia-Pacific. Am. J. Gastroenterol. 114, 107–115 (2019).
Lamb, C. A. et al. Inflammatory bowel disease has no borders: engaging patients as partners to deliver global, equitable and holistic health care. Lancet 404, 414–417 (2024).
Sood, A., Midha, V., Sood, N., Bhatia, A. S. & Avasthi, G. Incidence and prevalence of ulcerative colitis in Punjab, North India. Gut 52, 1587–1590 (2003).
Banerjee, R. et al. Inflammatory bowel disease (IBD) in rural and urban India: results from community colonoscopic evaluation of more than 30,000 symptomatic patients. Lancet Reg. Health Southeast Asia 19, 100259 (2023).
Molodecky, N. A. et al. Challenges associated with identifying the environmental determinants of the inflammatory bowel diseases. Inflamm. Bowel Dis. 17, 1792–1799 (2011).
Pavlovic-Calic, N., Salkic, N. N., Gegic, A., Smajic, M. & Alibegovic, E. Crohn’s disease in Tuzla region of Bosnia and Herzegovina: a 12-year study (1995-2006). Int. J. Colorectal Dis. 23, 957–964 (2008).
Piovani, D. et al. Environmental risk factors for inflammatory bowel diseases: an umbrella review of meta-analyses. Gastroenterology 157, 647–659.e4 (2019).
Sasson, A. N. et al. The role of precision nutrition in the modulation of microbial composition and function in people with inflammatory bowel disease. Lancet Gastroenterol. Hepatol. 6, 754–769 (2021).
Ueno, A. et al. Opposing effects of smoking in ulcerative colitis and Crohn’s disease may be explained by differential effects on dendritic cells. Inflamm. Bowel Dis. 20, 800–810 (2014).
Narula, N. et al. Food processing and risk of inflammatory bowel disease: a systematic review and meta-analysis. Clin. Gastroenterol. Hepatol. 21, 2483–2495.e1 (2023).
Dar, S. H. et al. The association of antibiotic exposure with new-onset inflammatory bowel disease: a systematic review and meta-analysis. Clin. Res. Hepatol. Gastroenterol. 47, 102129 (2023).
Xu, L. et al. Systematic review with meta-analysis: breastfeeding and the risk of Crohn’s disease and ulcerative colitis. Aliment. Pharmacol. Ther. 46, 780–789 (2017).
Turpin, W. et al. Mediterranean-like dietary pattern associations with gut microbiome composition and subclinical gastrointestinal inflammation. Gastroenterology 163, 685–698 (2022).
Lopes, E. W. et al. Lifestyle factors for the prevention of inflammatory bowel disease. Gut 72, 1093–1100 (2023).
Estevinho, M. M. et al. Emerging role of environmental pollutants in inflammatory bowel disease risk, outcomes and underlying mechanisms. Gut 74, 477–486 (2025).
Agrawal, M., Hansen, A. V., Colombel, J. F., Jess, T. & Allin, K. H. Association between early life exposure to agriculture, biodiversity, and green space and risk of inflammatory bowel disease: a population-based cohort study. eClinicalMedicine 70, 102514 (2024).
Kayali, S. et al. NOD2 and Crohn’s disease clinical practice: from epidemiology to diagnosis and therapy, rewired. Inflamm. Bowel Dis. 31, 552–562 (2024).
Kaplan, G. G. IBD: Global variations in environmental risk factors for IBD. Nat. Rev. Gastroenterol. Hepatol. 11, 708–709 (2014).
Banerjee, R., Pal, P., Mak, J. W. Y. & Ng, S. C. Challenges in the diagnosis and management of inflammatory bowel disease in resource-limited settings in Asia. Lancet Gastroenterol. Hepatol. 5, 1076–1088 (2020).
Schaffer, A. L., Dobbins, T. A. & Pearson, S. A. Interrupted time series analysis using autoregressive integrated moving average (ARIMA) models: a guide for evaluating large-scale health interventions. BMC Med. Res. Methodol. 21, 58 (2021).
Shah, S. C. et al. Sex-based differences in incidence of inflammatory bowel diseases — pooled analysis of population-based studies from western countries. Gastroenterology 155, 1079–1089.e3 (2018).
Jones, G. R. et al. IBD prevalence in Lothian, Scotland, derived by capture–recapture methodology. Gut 68, 1953–1960 (2019).
Ananthakrishnan, A. N. et al. Lifestyle, behaviour, and environmental modification for the management of patients with inflammatory bowel diseases: an International Organization for Study of Inflammatory Bowel Diseases consensus. Lancet Gastroenterol. Hepatol. 7, 666–678 (2022).
Brinks, R. & Landwehr, S. A new relation between prevalence and incidence of a chronic disease. Math. Med. Biol. 32, 425–435 (2015).
Keiding, N. Age-specific incidence and prevalence — a statistical perspective. J. R. Stat. Soc. A Stat. 154, 371–412 (1991).
Brinks, R. & Landwehr, S. Change rates and prevalence of a dichotomous variable: simulations and applications. PLoS ONE 10, e0118955 (2015).
Brinks, R. & Landwehr, S. Age- and time-dependent model of the prevalence of non-communicable diseases and application to dementia in Germany. Theor. Popul. Biol. 92, 62–68 (2014).
Brinks, R. Illness-death model in chronic disease epidemiology: characteristics of a related, differential equation and an inverse problem. Comput. Math. Methods Med. 2018, 5091096 (2018).
Brinks, R. & Hoyer, A. Illness-death model: statistical perspective and differential equations. Lifetime Data Anal. 24, 743–754 (2018).
Voeltz, D., Tönnies, T., Brinks, R. & Hoyer, A. Future prevalence of type 2 diabetes — a comparative analysis of chronic disease projection methods. PLoS ONE 17, e0264739 (2022).
Wang, J., Vordenbäumen, S., Schneider, M. & Brinks, R. Population-based epidemiological projections of rheumatoid arthritis in Germany until 2040. Scand. J. Rheumatol. 53, 161–172 (2024).
Wang, J. et al. A population-based projection of psoriatic arthritis in Germany until 2050: analysis of national statutory health insurance data of 65 million German population. Rheumatol. Int. 43, 2037–2047 (2023).
Bernstein, C. N., Blanchard, J. F., Rawsthorne, P. & Wajda, A. Epidemiology of Crohn’s disease and ulcerative colitis in a central Canadian province: a population-based study. Am. J. Epidemiol. 149, 916–924 (1999).
Quaresma, A. B. et al. Temporal trends in the epidemiology of inflammatory bowel diseases in the public healthcare system in Brazil: a large population-based study. Lancet Reg. Health Am. 13, 100298 (2022).
Kuenzig, M. E. et al. Twenty-first century trends in the global epidemiology of pediatric-onset inflammatory bowel disease: systematic review. Gastroenterology 162, 1147–1159.e4 (2022).
Kuenzig, M. E. et al. The NOD2-smoking interaction in Crohn’s disease is likely specific to the 1007fs mutation and may be explained by age at diagnosis: a meta-analysis and case-only study. eBioMedicine 21, 188–196 (2017).
Calkins, B. M. A meta-analysis of the role of smoking in inflammatory bowel disease. Dig. Dis. Sci. 34, 1841–1854 (1989).
Frolkis, A. D. et al. The association of smoking and surgery in inflammatory bowel disease is modified by age at diagnosis. Clin. Transl. Gastroenterol. 7, e165 (2016).
Hoyer, A., Kaufmann, S. & Brinks, R. Risk factors in the illness-death model: simulation study and the partial differential equation about incidence and prevalence. PLoS ONE 14, e0226554 (2019).
Duricova, D. et al. Overall and cause-specific mortality in Crohn’s disease: a meta-analysis of population-based studies. Inflamm. Bowel Dis. 16, 347–353 (2010).
Jess, T., Gamborg, M., Munkholm, P. & Sørensen, T. I. Overall and cause-specific mortality in ulcerative colitis: meta-analysis of population-based inception cohort studies. Am. J. Gastroenterol. 102, 609–617 (2007).
Kuenzig, M. E., Manuel, D. G., Donelle, J. & Benchimol, E. I. Life expectancy and health-adjusted life expectancy in people with inflammatory bowel disease. CMAJ 192, E1394–E1402 (2020).
Bernstein, C. N. et al. The impact of inflammatory bowel disease in Canada 2018: extra-intestinal diseases in IBD. J. Can. Assoc. Gastroenterol. 2, S73–S80 (2019).
Singh, S. et al. Postoperative mortality among patients with inflammatory bowel diseases: a systematic review and meta-analysis of population-based studies. Gastroenterology 149, 928–937 (2015).
Benchimol, E. I. et al. Inflammatory bowel disease in immigrants to Canada and their children: a population-based cohort study. Am. J. Gastroenterol. 110, 553–563 (2015).
Agrawal, M. et al. Inflammatory bowel diseases among first-generation and second-generation immigrants in Denmark: a population-based cohort study. Gut 70, 1037–1043 (2021).
Raftery, A. E. & Sevcikova, H. Probabilistic population forecasting: short to very long-term. Int. J. Forecast. 39, 73–97 (2023).
Ritchie, H. & Rodes-Guirao, L. Peak global population and other key findings from the 2024 UN World Population Prospects: falling fertility rates, migration movements, and China’s population decline. Our World in Data https://ourworldindata.org/un-population-2024-revision (2024).
Agrawal, M. & Jess, T. Implications of the changing epidemiology of inflammatory bowel disease in a changing world. United European Gastroenterol. J. 10, 1113–1120 (2022).
Mak, J. W. Y. et al. Development of the global inflammatory bowel disease visualization of epidemiology studies in the 21st century (GIVES-21). BMC Med. Res. Methodol. 23, 129 (2023).
GBD 2017 Inflammatory Bowel Disease Collaborators The global, regional, andnational burden of inflammatory bowel disease in 195 countries and territories,1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol. 5, 17–30 (2020).
Bernstein, C. N. et al. The epidemiology of inflammatory bowel disease in Canada: a population-based study. Am. J. Gastroenterol. 101, 1559–1568 (2006).
Wang, R., Li, Z., Liu, S. & Zhang, D. Global, regional and national burden of inflammatory bowel disease in 204 countries and territories from 1990 to 2019: a systematic analysis based on the Global Burden of Disease Study 2019. BMJ Open 13, e065186 (2023).
GBD 2021 Diseases and Injuries Collaborators Global incidence, prevalence, years lived with disability (YLDs), disability-adjusted life-years (DALYs), and healthy life expectancy (HALE) for 371 diseases and injuries in 204 countries and territories and 811 subnational locations, 1990–2021: a systematic analysis for the Global Burden of Disease Study 2021. Lancet 403, 2133–2161 (2024).
Ng, W. K., Wong, S. H. & Ng, S. C. Changing epidemiological trends of inflammatory bowel disease in Asia. Intest. Res. 14, 111–119 (2016).
Dorn-Rasmussen, M. et al. The incidence and prevalence of paediatric- and adult-onset inflammatory bowel disease in Denmark during a 37-year period: a nationwide cohort study (1980–2017). J. Crohns Colitis 17, 259–268 (2023).
Shah, S. C. et al. Sex-based differences in the incidence of inflammatory bowel diseases — pooled analysis of population-based studies from the Asia-Pacific region. Aliment. Pharmacol. Ther. 49, 904–911 (2019).
Burisch, J. et al. The cost of inflammatory bowel disease in high-income settings: a Lancet Gastroenterology & Hepatology Commission. Lancet Gastroenterol. Hepatol. 8, 458–492 (2023).
Kuenzig, M. E. et al. The 2023 impact of inflammatory bowel disease in Canada: indirect (individual and societal) and direct out-of-pocket costs. J. Can. Assoc. Gastroenterol. 6, S16–S22 (2023).
Agrawal, M. et al. The rising burden of inflammatory bowel disease in Denmark over two decades: a nationwide cohort study. Gastroenterology 163, 1547–1554.e5 (2022).
Larsen, L. et al. Has the Incidence of inflammatory bowel disease peaked? Evidence from the population-based NorDIBD cohort 1978-2020. Am. J. Gastroenterol. 118, 501–510 (2023).
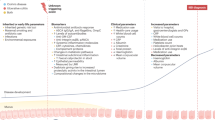
Bronze, S., Agrawal, M., Colombel, J. F., Torres, J. & Ungaro, R. C. Review article: prevention of inflammatory bowel disease — the path forward. Aliment. Pharmacol. Ther. 60, 1166–1175 (2024).
Lopes, E. W., Turpin, W., Croitoru, K., Colombel, J. F. & Torres, J. Prediction and prevention of inflammatory bowel disease. Clin. Gastroenterol. Hepatol. 23, 396–405.e1 (2025).
Rudbaek, J. J. et al. Deciphering the different phases of preclinical inflammatory bowel disease. Nat. Rev. Gastroenterol. Hepatol. 21, 86–100 (2024).
Israeli, E. et al. Anti-Saccharomyces cerevisiae and antineutrophil cytoplasmic antibodies as predictors of inflammatory bowel disease. Gut 54, 1232–1236 (2005).
Lee, S. H. et al. Anti-microbial antibody response is associated with future onset of Crohn’s disease independent of biomarkers of altered gut barrier function, subclinical inflammation, and genetic risk. Gastroenterology 161, 1540–1551 (2021).
Mortha, A. et al. Neutralizing anti-granulocyte macrophage-colony stimulating factor autoantibodies recognize post-translational glycosylations on granulocyte macrophage-colony stimulating factor years before diagnosis and predict complicated Crohn’s disease. Gastroenterology 163, 659–670 (2022).
Torres, J. et al. Serum biomarkers identify patients who will develop inflammatory bowel diseases up to 5 years before diagnosis. Gastroenterology 159, 96–104 (2020).
Bergemalm, D. et al. Systemic inflammation in preclinical ulcerative colitis. Gastroenterology 161, 1526–1539.e9 (2021).
Livanos, A. E. et al. Anti-integrin αvβ6 autoantibodies are a novel biomarker that antedate ulcerative colitis. Gastroenterology 164, 619–629 (2023).
Grännö, O. et al. Preclinical protein signatures of Crohn’s disease and ulcerative colitis: a nested case-control study within large population-based cohorts. Gastroenterology 168, 741–753 (2025).
Raygoza Garay, J. A. et al. Gut microbiome composition is associated with future onset of Crohn’s disease in healthy first-degree relatives. Gastroenterology 165, 670–681 (2023).
Galipeau, H. J. et al. Novel fecal biomarkers that precede clinical diagnosis of ulcerative colitis. Gastroenterology 160, 1532–1545 (2021).
Lee, S. H. et al. Development and validation of an integrative risk score for future risk of Crohn’s disease in healthy first-degree relatives: a multicenter prospective cohort study. Gastroenterology 168, 150–153.e4 (2025).
Zhang, L., Agrawal, M., Ng, S. C. & Jess, T. Early-life exposures and the microbiome: implications for IBD prevention. Gut 73, 541–549 (2024).
D’Haens, G. & Simsek, M. Early Crohn’s disease: can we change the disease course? Nat. Rev. Gastroenterol. Hepatol. 22, 367–368 (2025).
Herold, K. C. et al. An anti-CD3 antibody, teplizumab, in relatives at risk for type 1 diabetes. N. Engl. J. Med. 381, 603–613 (2019).
Cope, A. P. et al. Abatacept in individuals at high risk of rheumatoid arthritis (APIPPRA): a randomised, double-blind, multicentre, parallel, placebo-controlled, phase 2b clinical trial. Lancet 403, 838–849 (2024).
Sanderson, J. et al. A phase 1b clinical trial to determine the safety, tolerability and immunogenicity of simian adenovirus and poxvirus vectored vaccines against a Mycobacterium avium complex subspecies in patients with active Crohn’s disease. eBioMedicine 113, 105570 (2025).
Krienke, C. et al. A noninflammatory mRNA vaccine for treatment of experimental autoimmune encephalomyelitis. Science 371, 145–153 (2021).
Mohammadzamani, M. et al. Insights into the interplay between Epstein-Barr virus (EBV) and multiple sclerosis (MS): a state-of-the-art review and implications for vaccine development. Health Sci. Rep. 7, e1898 (2024).
Bjornevik, K. et al. Longitudinal analysis reveals high prevalence of Epstein-Barr virus associated with multiple sclerosis. Science 375, 296–301 (2022).
Levine, A. et al. Dietary guidance from the International Organization for the Study of Inflammatory Bowel Diseases. Clin. Gastroenterol. Hepatol. 18, 1381–1392 (2020).
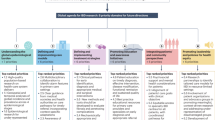
Solitano, V. et al. Shaping the future of inflammatory bowel disease: a global research agenda for better management and public health response. Nat. Rev. Gastroenterol. Hepatol. 22, 438–452 (2025).
Hazlewood, G. S. et al. Patient preferences for maintenance therapy in Crohn’s disease: a discrete-choice experiment. PLoS ONE 15, e0227635 (2020).
Vutcovici, M. et al. Patient perspectives of IBD care and services: an integral part of a pan-Canadian quality improvement initiative. J. Can. Assoc. Gastroenterol. 4, 229–233 (2021).
Mathias, H. et al. The 2023 impact of inflammatory bowel disease in Canada: access to and models of care. J. Can. Assoc. Gastroenterol. 6, S111–S121 (2023).
Zhen, J., Marshall, J. K., Nguyen, G. C., Atreja, A. & Narula, N. Impact of digital health monitoring in the management of inflammatory bowel disease. J. Med. Syst. 45, 23 (2021).
Habashi, P., Bouchard, S. & Nguyen, G. C. Transforming access to specialist care for inflammatory bowel disease: the PACE telemedicine program. J. Can. Assoc. Gastroenterol. 2, 186–194 (2019).
Novak, K. et al. Clinic-based point of care transabdominal ultrasound for monitoring Crohn’s disease: impact on clinical decision making. J. Crohns Colitis 9, 795–801 (2015).
Shaffer, S. R. et al. The 2023 impact of inflammatory bowel disease in Canada: special populations — IBD in seniors. J. Can. Assoc. Gastroenterol. 6, S45–S54 (2023).
Crosby, M., Tadrous, M. & Gomes, T. Potential cost implications of mandatory non-medical switching policies for biologics for rheumatic conditions and inflammatory bowel disease in Canada. Clin. Pharmacol. Ther. 109, 739–745 (2021).
Murthy, S. K. et al. The 2023 impact of inflammatory bowel disease in Canada: treatment landscape. J. Can. Assoc. Gastroenterol. 6, S97–S110 (2023).
Silverman, A. L., Shung, D., Stidham, R. W., Kochhar, G. S. & Iacucci, M. How artificial intelligence will transform clinical care, research, and trials for inflammatory bowel disease. Clin. Gastroenterol. Hepatol. 23, 428–439 (2025).
Acknowledgements
The author acknowledges S. Coward, M. Cummings, L. Hracs and J. Windsor for their expertise, contextual insights and editorial contributions to the article. In particular, the author thanks J. Gorospe for her assistance in drafting the figures and J. Windsor for developing the visual representation and guitar-string analogy in Fig. 1. The author’s research is funded by operating grants from the Canadian Institutes of Health Research and the Leona M. and Harry B. Helmsley Charitable Trust.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
G.G.K. has received honoraria for speaking or consultancy from AbbVie, Amgen, Janssen, Pfizer and Takeda; and has received grants for research from Ferring and for educational activities from AbbVie, Bristol Myers Squibb, Ferring, Fresenius-Kabi, Janssen, Pfizer and Takeda. He shares ownership of a patent: Use of mirtazapine in the treatment of inflammatory disorders, autoimmune disease and PBC (patent WO/2019/046959A1, PCT/CA2018/051098).
Peer review
Peer review information
Nature Reviews Gastroenterology & Hepatology thanks Manasi Agrawal, Stefanos Bonovas and Zhihua Ran for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kaplan, G.G. The global burden of inflammatory bowel disease: from 2025 to 2045. Nat Rev Gastroenterol Hepatol 22, 708–720 (2025). https://doi.org/10.1038/s41575-025-01097-1
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41575-025-01097-1
This article is cited by
-
Lacticaseibacillus rhamnosus LRa05 mitigates DSS-Induced colitis via boosting beneficial gut microbiota and facilitating CD4⁺Foxp3⁺ Treg differentiation
BMC Microbiology (2026)
-
Mucosal glycans: key drivers of the development of inflammatory bowel disease and a potential new therapeutic target
Nature Reviews Gastroenterology & Hepatology (2026)
-
Hyperglycemia impairs the expression of inflammatory mediators in rat intestine: an implication for intestinal inflammation and inflammatory bowel disease
Molecular and Cellular Biochemistry (2026)
-
Trends in the disease burden of inflammatory bowel disease among the working-age population (20–64 years) from 1990 to 2021: a population-based study
BMC Gastroenterology (2025)
-
Incidental Inflammatory Bowel Disease: Is Surveillance and Treatment Needed?
Digestive Diseases and Sciences (2025)