Abstract
Inflammatory bowel disease (IBD) is a global condition that progresses through four epidemiologic stages: emergence, acceleration in incidence, compounding prevalence and prevalence equilibrium. Early industrialized countries are currently in the compounding prevalence stage before transitioning to the prevalence equilibrium stage, with >1% of their populations expected to live with IBD within the next decade. Prevalence equilibrium can be modelled using a health–illness–death compartment framework and partial differential equations to predict prevalence to 2045. Meanwhile, newly industrialized countries are projected to shift from accelerated incidence with low prevalence to compounding prevalence over the next two decades. This Perspective explores the global evolution of IBD through these epidemiologic stages, presenting a framework for disease prevention and innovative health-care strategies to address the critical challenges the global IBD community will face over the next 20 years.
Similar content being viewed by others
Introduction
Total solar eclipses are a global phenomenon — a shared experience across time and geography — marked by human civilizations throughout history. Initially explained through divinity and mysticism, they were later predicted using the tools of astronomy and mathematics. In 1919, as inflammatory bowel disease (IBD) sporadically emerged with a handful of documented cases in Western countries, Sir Arthur Eddington used a total solar eclipse to experimentally observe gravitational lensing, confirming Einstein’s theory of general relativity, which predicted the bending of light by the Sun’s gravitational field. Over a century later, in April 2024, people in North America had the opportunity to view another total solar eclipse, including over 2.5 million individuals — approximately 0.8% of the population of North America — living with IBD at that time1,2,3. The next total eclipse visible in North America will occur in August 2045, a prediction made possible through temporospatial astronomical calculations of the cyclical orbits of the Sun, Earth and Moon. Similarly, could modelling epidemiologic trends of IBD enable us to predict the number of people living with IBD and their demographic characteristics in 2045 (ref. 4)?
The epidemiologic evolution of IBD has followed distinct patterns across time and geography, enabling predictions about changing prevalence and demographics in the coming decades4,5,6. Predicting future epidemiologic patterns of IBD relies on several fundamental assumptions: IBD is a chronic and incurable disease; although IBD can be diagnosed at any age, it is most commonly diagnosed in young adults; the life expectancy of individuals with IBD is comparable to, or only slightly lower than, that of the general population; all humans are susceptible to developing IBD, regardless of ethnicity/race or geography; and the rate of new diagnoses in each society depends on genetic predisposition and exposure to environmental factors linked to industrialization, urbanization and westernization5,7,8. Given these assumptions, it was hypothesized that the evolution of IBD could be classified into distinct epidemiologic stages, which could then be modelled mathematically4,5.
A theoretical framework was proposed to stratify the evolution of IBD into four epidemiologic stages4,5. Emergence (stage 1) is characterized by a low incidence and prevalence of IBD, with initial sporadic cases appearing in low-income regions. Acceleration in incidence (stage 2) marks a rapid rise in the incidence of IBD, coinciding with socioeconomic advances that alter health-care infrastructure, diet, urbanization and hygiene. During this stage, although new diagnoses of IBD appear more widely, overall prevalence remains low. Compounding prevalence (stage 3) involves a steady increase in the number of people living with IBD due to its chronic nature and low mortality rate. The rapid growth in incidence seen in stage 2 slows, but the cumulative effect of new diagnoses over decades leads to a substantial rise in prevalence over time. Prevalence equilibrium (stage 4) represents the point at which the growth in prevalence slows owing to the natural increase in mortality associated with ageing of the IBD population4,5. The time to reach prevalence equilibrium and the prevalence level at this point depend on the value at which incidence stabilizes and the rate of change in incidence over time4,5 (Fig. 1). Recognizing the progression of IBD through these epidemiologic stages enables health-care systems to predict and prepare for the future burden of disease9,10.
A guitar-string analogy demonstrates the four epidemiologic stages of inflammatory bowel disease (IBD) evolution, in which incidence is represented by the vibration of the guitar string and prevalence would be the pitch we hear. In stage 1 (emergence), the guitar string is slack; few vibrations pass down the neck. Low-frequency waves symbolize the rare, sporadic appearance of IBD cases, and the ear hears only a faint, low pitch (prevalence is minimal). In stage 2 (acceleration in incidence), environmental forces tighten the tuning peg, raising string tension. Wave frequency climbs, representing the accelerating influx of new cases. The perceived pitch rises, and prevalence begins its upward trajectory. In stage 3 (compounding prevalence), the string is now at maximal tension. Wave frequency plateaus at its highest level with the continuous stream of vibrations causing more crests to fill the neck of the guitar. Prevalence keeps climbing even though incidence is no longer increasing. In stage 4 (prevalence equilibrium), with no further tightening, the propagating wave gradually loses amplitude, illustrating how the annual addition of new cases is offset by the mortality of an ageing IBD population. The pitched sound stabilizes and softens, mirroring a steady-state prevalence.
This Perspective explains the mathematical modelling required to examine the transitions between the four epidemiologic stages of IBD over a 20-year period, anticipating the challenges that health-care systems, governments and the IBD community will face in the years to come. The goal of this article is to equip clinicians and health-care systems worldwide with an understanding of the methods used to predict IBD trends, enabling them to address the challenges posed by changing demographics and the increasing number of people living with Crohn’s disease and ulcerative colitis into the mid-twenty-first century.
The global burden of IBD in 2025
A 2015 Perspective paper examined the epidemiology of IBD and the challenges the IBD community would face between 2015 and 2025 (ref. 11). The key takeaway was that by 2025, IBD would be firmly established as a global disease, with the Western world facing a growing prevalence of IBD in an ageing population, and newly industrialized countries in Asia and Latin America preparing for a sharp rise in incidence11. That article concluded that each region must customize its health-care response to ensure adequate resources, infrastructure and personnel to manage the burden of IBD equitably and cost-effectively, in alignment with the unique needs of its society11,12,13.
Global epidemiology in 2025
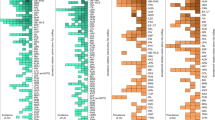
What proportion of the 8.2 billion people living on Earth have IBD in 2025? A study co-led by the author analysing over 500 population-based studies on the incidence and prevalence of IBD examined cohorts from 1920 to 2024 across more than 80 global regions4. These data were used to train a machine learning model that classified regions with available population-based data into stage 1, 2 or 3 with an overall 95% accuracy. Using the machine learning classifier, coalescing ranges (CR) for incidence (CR-I) and prevalence (CR-P) per 100,000 population were calculated as the 25th to the 75th percentiles for IBD across the first three epidemiologic stages: stage 1 (CR-I 0.2–1.2, CR-P 1.1–10.4), stage 2 (CR-I 3.3–10.6, CR-P 31.2–100.5) and stage 3 (CR-I 18.1–34.1, CR-P 362.9–660.1)4 (Fig. 2). The CR integrated epidemiologic data across time and geography for the first three stages — for example, by including stage 2 data from the twentieth century for early industrialized countries and stage 2 data from the twenty-first century for newly industrialized countries. Although regional variability exists within each stage, values that fall between stages could indicate regions undergoing an epidemiologic transition4. The timing of these transitions varies by region and is influenced by localized factors such as genetic susceptibility, environmental exposures, health-care access and cultural practices14.
A machine learning model classified epidemiologic stages across global regions, defining coalescing incidence ranges (25th–75th percentiles) (part a) and coalescing prevalence ranges (25th–75th percentiles) for IBD (part b) across stage 1, stage 2 and stage 3 (ref. 4). Data source and availability: https://kaplan-gi.shinyapps.io/GIVES21.
During the twentieth century, IBD was characterized as a disease of the Western world15. However, twenty-first century epidemiologic data revealed that IBD is a global disease capable of manifesting anywhere6. A review of a century’s worth of historical epidemiologic data highlighted transition zones between stages, reflecting patterns not confined to specific geographical regions but inherently predictable4. Long-standing stage 1 (low incidence and prevalence) is societally triggered to transition into stage 2 (accelerating incidence with low prevalence), followed by a mathematically anticipated shift to compounding prevalence in stage 3 (refs. 4,5). Societal triggers initiate a predictable wave of rising incidence that eventually stabilizes, followed by increasing prevalence, with the rate of increase in prevalence gradually slowing over time4 (Fig. 1). Although the timing of these points differs geographically, the progression of IBD is expected to follow similar patterns globally, albeit shifted across time.
Early industrialized countries in North America, Europe and Oceania transitioned into stage 2 following World War II, with the greatest rise in incidence during the latter half of the twentieth century6,15. For example, analysis of data from the USA spanning a century using a machine learning classifier indicated a transition from stage 1 to stage 2 in the 1950s, followed by stage 3 in the 1970s4,16,17 (Fig. 3). Similarly, the model identified a transition from stage 2 to stage 3 in Sweden during the 1970s, and confirmed stage 3 in Canada, with its earliest data from the 1980s2,4,18,19. By 2025, early industrialized countries are firmly entrenched in the third epidemiologic stage4.
Shifts in disease burden across four example countries are shown as they transition through the epidemiologic stages (stage 1, emergence; stage 2, acceleration in incidence; stage 3, compounding prevalence; and stage 4, prevalence equilibrium). The USA is a country with epidemiologic data supporting its progression through the first three stages and is expected to enter stage 4 in the following decades. Japan is a stage 2 country that is on the verge of transitioning to stage 3. Malaysia is a country that only entered stage 2 within the past decade. Zambia’s epidemiologic data suggest it is currently in stage 1, with a projected transition to stage 2 as the country undergoes greater industrialization, urbanization, westernization, and associated lifestyle and dietary changes. Representative burden data were derived from ref. 4.
At the turn of the twenty-first century, countries in Africa, Asia and Latin America demonstrated susceptibility to IBD6. Industrialization, urbanization and westernization triggered a wave of IBD in these regions, only delayed in time compared with countries that industrialized early7. Temporal shifts in the epidemiology of IBD occur across newly industrialized nations (Fig. 3). For example, Japan experienced rapid industrialization after World War II in the 1960s, whereas China underwent economic reform in the 1990s. The machine learning classifier showed that Japan transitioned from stage 1 to stage 2 in the 1990s, whereas China transitioned in the 2010s — a 30-year difference paralleling the timing of societal economic advances4,20,21. By contrast, the epidemiology of IBD in economically less advanced nations in Asia is further delayed. Malaysia, with continuous epidemiologic data from the 1980s to 2020s, remained in stage 1 for decades until the model classified the country as in stage 2 with newly available data from the 2020s4,22. By 2025, most countries in Asia and Latin America with available epidemiologic data were either in stage 2 or transitioning from stage 1 to stage 2 (ref. 4) (Fig. 3).
Notably, IBD epidemiologic data for Africa are sparse6. Algeria was classified as in stage 2 in the 2000s23, whereas data from the 2020s classify Tanzania, Zambia and Egypt as in stage 1 (refs. 4,24,25,26) (Fig. 3). Non-population-based studies document IBD in Ghana and Sudan, with rates estimated to be very low27,28. Collectively, these limited data suggest that most of Africa remains in stage 1 in 2025, underscoring the urgent need for population-based epidemiologic surveillance.
The transition from stage 1 to 2
The rapid rise in the incidence of IBD during stage 2 is thought to result from a combination of unmasking previously undiagnosed cases and a true increase in incidence driven by environmental risk factors5.
Health-care infrastructure, resources and personnel develop in tandem with the economic progress accompanying the industrialization and urbanization of society9,29. With increased national wealth, the number of gastroenterologists and their expertise in IBD grow, availability of diagnostic tools such as colonoscopy expands, and awareness of previously uncommon diseases such as IBD broadens within the medical community30,31. Urbanization involves the migration of people from rural areas to densely populated urban areas with better access to gastroenterologists32. Over time, expanded medical care for individuals of lower socioeconomic status in rural areas enhances equity in health-care delivery33. Collectively, these transformations in health-care access result in the unmasking of previously unrecognized cases of IBD, contributing to an apparent increase in incidence over time5,33.
A study from the Punjab state, India, effectively demonstrated the concept of unmasking34. In 1999, investigators conducted over 50,000 home surveys to identify individuals with symptoms consistent with ulcerative colitis, who were then transported to an endoscopy unit for diagnosis. This systematic approach established that the prevalence of ulcerative colitis was 44 per 100,000 population in 1999. A follow-up survey a year later identified new cases within the same communities, yielding an incidence of 6 per 100,000 person-years in 2000 (ref. 34). This study demonstrated the effects of enhanced health-care infrastructure on identifying new cases that would have otherwise remained undetected, contributing to the observed increase in incidence of IBD34.
However, the unmasking of IBD incidence alone does not fully explain the transition from stage 1 to stage 2, nor the subsequent acceleration in incidence5, as demonstrated by a study from Telangana state, India35. Between 2020 and 2022, investigators conducted a rural outreach programme, bringing gastroenterologists and a mobile endoscopy unit to underserved villages35. They identified IBD in 5.6% of symptomatic individuals, a rate comparable to that observed in urban centres and substantially higher than found in the 2006 survey of over 4,800 villages, which found only two cases of IBD (0.1%) out of 1,500 colonoscopies performed in symptomatic individuals35. Although some discrepancies might be attributed to methodological heterogeneity36, the data collectively highlight the progression of IBD in India, even when accounting for improved access to health-care infrastructure35.
As IBD evolves within a society, ulcerative colitis is initially diagnosed more frequently than Crohn’s disease; however, this ratio narrows over time29. Collectively, in stage 1 regions, the incidence ratio of ulcerative colitis to Crohn’s disease is 3.2:1, compared with 1.5:1 in stage 3 regions4. This shift could be explained by improved health-care resources as increased access to colonoscopy facilitates the diagnosis of mild Crohn’s disease that might otherwise go undetected37.
Although health-care advances play a critical part in unmasking hidden cases, environmental and societal factors drive the genuine increase in IBD incidence7. Westernization of society — characterized by changes in lifestyle and diet — alters the gut microbiota in genetically susceptible individuals, contributing to the development of IBD7. Environmental determinants of IBD have been extensively reviewed in the literature38,39, highlighting risk factors such as smoking40, consumption of ultra-processed foods41 and childhood antibiotic use42, which are associated with heightened risk. Conversely, protective factors including breastfeeding43, adhering to a Mediterranean diet44 and maintaining healthy lifestyle habits (such as adequate sleep, physical activity and a healthy weight)45 could reduce the likelihood of developing IBD. Moreover, studies published in the past couple of years suggest that population-level shifts in environmental exposures — such as air and ingestible pollutants, agricultural practices, and the alteration of food composition through additives and emulsifiers — might contribute to the milieu that fosters the development of IBD46,47.
After transitioning to stage 2, the trajectory of rising and peak incidence varies across regions4. This regional heterogeneity in incidence values and trends is influenced by several factors, including geographical differences in genetic susceptibility (for example, population-level variability in the NOD2 gene)48 and variations in how environmental exposures affect different populations (for example, the absence of an association between smoking and Crohn’s disease in Asia)49. Additionally, disparities in clinical access and health-care resources can influence the likelihood of IBD diagnosis50, whereas the methodological rigour and completeness of disease surveillance systems — particularly in stage 1 and stage 2 regions — affect the reliability of incidence and prevalence estimates36. Collectively, these factors shape the timeline for incidence stabilization, the plateau level it reaches, and the long-term growth in prevalence over subsequent decades.
Forecasting the future prevalence of IBD
Understanding shifts in disease prevalence, demographic changes and the evolving burden of disease enables health systems to strategically allocate resources and plan for future demands. Accurate forecasting enables policymakers and health-care systems to implement informed changes to positively shape societal and individual health outcomes10. Epidemiologic studies in IBD from the past few years have forecast prevalence using multiple methodologies to combine short-term accuracy with long-term adaptability1,2,4. This dual approach is particularly effective for modelling dynamic trends influenced by changing demographics, incidence rates and unforeseen events. However, each modelling approach must be interpreted in the context of its inherent limitations (Box 1).
Short-term prediction of prevalence: ARIMA models
Autoregressive integrated moving average (ARIMA) models are a popular tool for forecasting future values based on past data. The AR (autoregressive) component identifies temporal patterns by assuming that prior observations influence future values. The MA (moving average) component uses past forecasting errors, or residuals, to improve predictions. The I (integration) component ensures stationarity, stabilizing data with processes such as differencing to account for long-term trends or repeating patterns51.
ARIMA’s ability to handle complex time-series data makes it well-suited for short-term IBD prevalence forecasting. In all regions with population-based longitudinal epidemiologic data, IBD prevalence has shown a steady increase. For example, in Olmsted County, MN, USA, prevalence more than doubled from 0.15% in 1965 to 0.36% in 1991 and nearly doubled again to 0.63% by 2019 (refs. 16,17). National data from the USA, which includes a more racially diverse population than Olmsted County, show similar trends, with the overall prevalence of IBD estimated at approximately 0.7%3. This consistent rise in prevalence is primarily driven by incidence exceeding mortality, a pattern characteristic of IBD, as most new diagnoses occur in young adults with a high likelihood of survival over a limited forecasting horizon (for example, 10 years)52. By analysing historical epidemiologic trends, ARIMA models have been applied to predict future IBD prevalence in countries such as Canada and Scotland, with estimates suggesting that the prevalence of IBD is approximately 0.85% in 2025 and will exceed 1% of the population before 2035 (refs. 1,2,53).
Validation studies have confirmed that ARIMA models can accurately forecast the prevalence of IBD over a short time horizon2. A national Canadian study used historical prevalence data from 2002 to 2008 to project future prevalence1, with an updated study incorporating data through 2014 for comparison2. This step enabled direct comparison between the forecast prevalence for 2009 to 2014 in the original study (0.64% in 2014) and the actual measured prevalence (0.65% in 2014)2.
Limitations of ARIMA models arise when unexpected changes in future incidence or mortality rates challenge the model’s accuracy (Box 1). For example, the emergence of a cure for IBD or a new environmental exposure that markedly increases incidence could disrupt historical trends and compromise forecast validity9,54. Over longer time horizons, forecast uncertainty increases, reflected by widening prediction intervals around prevalence estimates. Moreover, a gradual shift occurs in epidemiologic stage 3 that influences mortality over time: the changing demographics of the ageing IBD population5. To explore the transition from stage 3 to stage 4, prevalence equilibrium, a different modelling approach is considered: partial differential equations (PDEs) derived from compartment models55.
Long-term predictions of prevalence: PDE
In 1991, Keiding proposed a compartment model framework for chronic diseases that transition between health, chronic illness and death56 (Fig. 4). Transitions between these compartments were governed by age-specific and time-specific incidence rates, as well as the mortality rate in the general population relative to that among individuals with the chronic disease56. Brinks, Landwehr and Hoyer, in a series of groundbreaking papers, explored the use of a PDE derived from a health–illness–death compartment model to predict changes in age-specific prevalence over time55,57,58,59,60 (Fig. 4). PDE models have been used to project future prevalence in chronic conditions ranging from diabetes to dementia58,61, with estimates derived for chronic immune-mediated diseases including rheumatoid arthritis, psoriatic arthritis and IBD4,62,63.
The compartment model for a chronic disease illustrates how individuals transition between three states: healthy, ill and deceased. These transitions are influenced by the individual’s age (a), disease duration (d) and calendar time (t). New cases of illness arise at the incidence rate (incidence (t,a)), whereas the recovery rate (recovery (t,a,d)) returns individuals from the ill to the healthy state. Two separate mortality rates are shown: mortality (t,a) for the healthy state and mortality (t,a,d) for the ill state, representing age-dependent and disease duration-dependent mortality (part A). The equation in part B captures this model mathematically, demonstrating how changes in age-specific prevalence over time (left) are influenced by the incidence rate, adjusted for the differential mortality between the general population and individuals with IBD, and accounting for migration from regions at different epidemiologic stages (right). Part C presents representative scenario analyses illustrating how incidence patterns affect the age structure of prevalence. A stable incidence rate from 2015 to 2045 (panel Ca) results in an upward and rightward shift of age-specific prevalence, with prevalence equilibrium occurring in the 2040s (panel Cc). By contrast, a scenario with slowly declining incidence (panel Cb) achieves a lower prevalence and an earlier transition to prevalence equilibrium (panel Cd). Representative data and model are based on methodology presented in refs. 4,55. PDE, partial differential equation.
In countries in stage 1, 2 or 3, the main driver of prevalence increase is the time-dependent, age-specific incidence rate. Although IBD can be newly diagnosed at any age — from infancy to old ages — most epidemiologic studies report annual age-specific incidence with a classic left-skewed distribution in which incidence rises with age, often peaking in the fourth decade of life and then gradually declining thereafter52. Some studies have suggested a double peak, with a second smaller rise in incidence during mid-adulthood to late adulthood, particularly for ulcerative colitis. However, the single-peaked, left-skewed pattern has largely remained consistent across geographical regions and time, as demonstrated in studies from Canada in the 1990s to Brazil in the 2020s64,65. This age stratification ensures that each year, new cases are added to the PDE model in proportion to the age-specific incidence distribution, resulting in more 20-year-olds than 60-year-olds entering the projected IBD population annually.
In a PDE model, scenario analyses can incorporate time and age-dynamic variations in incidence (Fig. 4). If incidence remains stable, as observed in countries such as Canada and the USA2,17, a time-constant but age-dependent incidence rate can be applied across each calendar year to forecast future prevalence. PDE models can also account for variations in incidence across subpopulations, such as demographic differences. For instance, epidemiologic studies have indicated that the incidence of paediatric-onset IBD might be rising in stage 3 countries66, whereas the incidence of adult-onset IBD is declining6.
The reasons underlying the differing incidence patterns between paediatric-onset and adult-onset IBD remain unclear but could be driven by gene–environment interactions. For instance, one study found that children with Crohn’s disease show enrichment in NOD2 mutations, whereas adults diagnosed after 40 years of age have low penetrance of NOD2 mutations67. By contrast, a history of smoking was common among adults diagnosed after 40 years of age, but absent among children diagnosed with IBD67. In adults, active smoking raises the risk of Crohn’s disease, whereas smoking cessation increases the risk of ulcerative colitis in adults68. The steady decline in smoking rates in stage 3 countries from the 1980s to the 2020s could have contributed to reducing the incidence of adult-onset IBD, with little effect on paediatric IBD rates69. Accordingly, PDE models can apply a fixed increase in paediatric incidence (for example, +1% per year) and simultaneously adjust adult-onset incidence downward (for example, −1% per year)70.
Important limitations in PDE models should be considered, such as the differential mortality between individuals with the chronic disease and the general population. Mortality acts as a counterbalance to incidence, gradually reducing the size of the IBD population over time. When mortality rates in people with chronic disease approximate those in the general population, age-related mortality roughly follows Gompertz law, which describes a low baseline mortality in youth that rises exponentially with age59. However, the accuracy of PDE models depends on access to reliable, age-specific mortality rates for both individuals with IBD and the general population. These data are often unavailable, and when they are, the model typically assumes that historical differences in mortality will remain constant over time — a limitation that might not hold true in future scenarios.
Meta-analyses published in 2007 and 2010 indicated that mortality rates are slightly higher among individuals with Crohn’s disease, but not those with ulcerative colitis, than among those without IBD71,72. In 2011, life expectancy for individuals with IBD in Canada was 76 years for men and 78.4 years for women, which was 6 and 7 years shorter than for men and women without IBD, respectively73. This reduced life expectancy associated with IBD can be attributed to higher rates of comorbidities, such as venous thromboembolism and cardiovascular disease, colon cancer, and cirrhosis in individuals with primary sclerosing cholangitis, as well as complications directly related to IBD such as bowel obstruction74. The primary driver of these comorbidities and complications is disease activity; for instance, postoperative mortality is over five times higher following emergent surgery for IBD than following elective surgery75. Owing to this differential mortality, scenario analyses can be integrated into PDE models to account for potential age-specific changes in mortality over time. For example, a scenario could model reduced mortality rates with the advent of safer and more effective medical treatments with lower complication rates.
Another factor that can be incorporated into PDE models of IBD prevalence is the rate of in-region and out-of-region population migration. For countries with low migration rates or where the migrating population has similar IBD rates to the modelled country (for example, migration between two stage 3 countries), the migration term is relatively insignificant and can be omitted. However, in countries with high immigration from regions with lower IBD prevalence (for example, migration from a stage 1 to a stage 3 country), the initial effect is a dilution of the IBD population, reducing the overall prevalence. Over time, this effect diminishes, as studies have shown that the IBD risk in individuals migrating from stage 1 or stage 2 regions to stage 3 regions eventually increases to that in their new region, particularly among first-degree offspring76,77.
Policy decisions in health care made today — such as health-care workforce planning, resource allocation and research investment — rely on accurate predictions of how disease burden will evolve in coming decades10. For IBD, understanding the number of individuals living with the disease and its shifting demographics is crucial, as the needs of an ageing IBD population over the next 20 years will differ greatly from those of a younger population shaped by age-specific incidence patterns from 20 years ago5,11. By combining the high accuracy of short-term projections from ARIMA models with the long-term horizon projections from PDE models, societies can develop plausible forecasts of age-specific prevalence from 2025 to 2045 (Fig. 4). Running these models today provides the lead time needed to adapt to the evolving landscape of IBD in the decades ahead55,57.
The global burden of IBD in 2045
In July 2024, the United Nations published the 2024 revision of ‘World Population Prospects’, a biennial report summarizing global population size and demographics from 1950 to 2023, and projecting changes in population size and age distribution through 2100 using Bayesian probabilistic hierarchical models78,79. In 1950, the global population was 2.5 billion, doubling to 5 billion by the mid-1980s, reaching 6.2 billion at the turn of the twenty-first century, and growing to 8.2 billion by 2025 (ref. 79). Although the population will continue to expand throughout the twenty-first century, the growth rate has been slowing since the 1990s — from 1.75% per year to 1.35% in 2000, 1% in 2019, 0.84% in 2025 and an anticipated 0.5% in 2045. This deceleration is attributed to an ageing population, increasing age-related mortality and a decline in global fertility rates, which dropped from approximately five live births per woman during the 1950s and 1960s to 2.24 in 2025, and are projected to remain stable to 2045 at 2.21 per woman79. As a result, the global population is expected to gradually rise to 9.44 billion by 2045 (ref. 79).
As populations age and demographic growth slows, the epidemiologic profile of chronic conditions such as IBD is also expected to shift, influencing future health-care demands. What proportion of the 9.44 billion people living on Earth will have IBD in 2045? Answering this question requires understanding how regions worldwide will transition across epidemiologic stages during the period to 2045. A dual approach is necessary: global studies provide the ‘forest’, capturing overarching trends, whereas local analyses focus on individual ‘trees’, addressing the unique nuances of each society’s data, environment and projections. Collecting data and forecasting outcomes provides the crucial lead time needed to prepare for the rising prevalence and ageing in the IBD population in 2045. This foresight enables the IBD community to prioritize research efforts on disease prevention while innovating care delivery to meet the evolving needs of the IBD population in 2045 (ref. 33).
Global epidemiology in 2045
In 2025, stage 1 countries in Africa, Asia and Latin America are expected to maintain low incidence and prevalence of IBD until societal, economic and environmental factors trigger a rapid rise in incidence5. The rate of increase and the peak incidence during transitions across stages will vary by region, shaped by the interplay of environmental exposures, genetic backgrounds and health-care infrastructure80.
The timing of societal triggers that drive the transition from stage 1 to stage 2 remains uncertain. Identifying these transitions is further complicated in stage 1 regions due to the lack of robust epidemiologic surveillance tools required to track population-based incidence and prevalence. However, multi-country prospective cohort studies, such as the Global IBD Visualization of Epidemiology Studies in the twenty-first century (GIVES-21) and Inflammatory Bowel Disease–Emerging Nations Consortium, are generating high-quality research in regions with scarce epidemiologic data on IBD30,81. Through longitudinal data, annualized trends can be integrated into the machine learning epidemiologic stage classifier to better understand transitions from stage 1 to stage 2 (ref. 4).
Despite growing research in stage 1 and stage 2 regions, many countries still lack the infrastructure and resources to conduct epidemiologic studies. Extrapolating data from regions with available information to those without enables modelling estimates of IBD epidemiology. For example, the Global Burden of Disease (GBD) project provides incidence and prevalence estimates for every country by modelling data from regions with available information and extrapolating to nearby regions with no data. However, GBD models do not account for epidemiologic differences across stages, leading to underestimation of prevalence in stage 3 regions and overestimation of incidence in stage 1 regions82. For instance, despite several high-quality population-based prevalence estimates from Canada1,83, the 2017 GBD model provided a tenfold lower prevalence for Canada (~50 per 100,000 population) than the actual, non-modelled prevalence estimates82. In the 2019 revision, this value was adjusted upward but remained substantially underestimated at ~350 per 100,000 population84. These discrepancies, despite the success of the GBD in generating high-quality global estimates for numerous other conditions85, highlight the complex and nuanced nature of IBD epidemiologic data. To accurately estimate the global burden of IBD, modelling efforts must integrate epidemiologic stage frameworks and incorporate precise local data to minimize underestimations or overestimations36.
Stage 2 regions such as Japan, Brazil and South Korea, which are likely to transition to stage 3 over the next decade, might not experience the same magnitude of IBD incidence over time as observed in current stage 3 countries20,29,86,87. Consequently, they might not reach the same prevalence peak during stage 3. However, even if the prevalence of IBD in Asia and Latin America remains a fraction of that in 2025 in early industrialized countries, the sheer population size in these regions will result in an absolute number of IBD cases that far exceeds those managed in North America and Europe by 2045 (refs. 31,50). Thus, a concerted effort to address the rising global burden of IBD in 2025 is crucial to effectively manage the anticipated challenges of 2045 (refs. 9,10).
Other regions with delayed economic advance that only recently transitioned into stage 2, such as China, might remain in stage 2 over the next two decades21. However, the continuous rise in IBD incidence in these regions means that new cases will predominantly affect younger individuals88. Consequently, those primarily affected by IBD in stage 2 regions are working-age adults with IBD or parents caring for children with IBD. This particular age demographic faces both the increased direct cost of accessing expensive therapies and the indirect cost of managing a chronic illness, such as loss of educational opportunity and reduced work productivity89,90.
The fundamental question for stage 3 regions is how long the transition to stage 4 will take and at what level prevalence will peak during this process. In Canada, incidence has stabilized at 30 per 100,000 person-years2. PDE modelling, which incorporates the changing demographics of an ageing IBD population, predicts a slowing rate of increase in prevalence throughout the 2030s. By the 2040s, the model estimates that the rate of change in prevalence will approach 0% over a 5-year period, suggesting that Canada will achieve prevalence equilibrium (stage 4) by 2043, with a prevalence of 1.05%4. By contrast, PDE models for Denmark predict substantially higher prevalence of IBD, rising from 1.19% in 2025 to 1.44% in 2035, and reaching 1.59% by 2043 (ref. 4). Scandinavian countries have consistently reported higher incidence and prevalence of IBD than other early industrialized countries87. Although most stage 3 countries in North America and Europe showed stabilization of incidence during the first decade of the twenty-first century6, incidence in Denmark continued to climb91, with only data published in 2023 indicating stabilization since 2020 (ref. 92). As a result, modelling for Denmark suggests that prevalence will peak higher than in Canada and that it will take longer for Denmark to achieve prevalence equilibrium4.
Scenario analyses that adjust age-specific incidence projections have important implications for future prevalence estimates. For example, if incidence in Denmark continues to rise by 2% per year over the next two decades, the prevalence of IBD is projected to reach 1.76% by 2043 (ref. 4). Conversely, a 2% annual decline in incidence would result in a prevalence of 1.45%, with the PDE model predicting a slowing growth rate, achieving prevalence equilibrium in the 2040s4. Similarly, in Canada, a scenario analysis modelling a modest 2% annual decline in age-stratified incidence projected that prevalence would actually decrease, falling below 1% by 2045 (ref. 4) (Fig. 4). These projections underscore that future prevalence estimates for stage 3 regions depend heavily on the ability of local regions to influence incidence trends over the next two decades.
Addressing the global burden of IBD in 2045
By 2045, most countries will be in stage 2 or stage 3 of IBD evolution, with fewer countries remaining in stage 1 and some potentially achieving stage 4 (refs. 4,5). Globally, all nations must anticipate and prepare for the rising burden and shifting demographics of IBD within their regions. Insights from PDE modelling of stage 3 countries transitioning to stage 4 suggest that even modest changes in annual incidence can influence prevalence estimates for the 2040s. Preventive health strategies aimed at reducing incidence over time have the potential to alter prevalence trajectories and, in some cases, reverse the trend. In the interim, every health-care jurisdiction must focus on optimizing disease management, innovating care delivery, and implementing cost-effective strategies to mitigate the burden of IBD (Fig. 5).
Reducing the incidence of IBD — an achievable goal?
The discovery of novel biomarkers of disease risk, combined with advances in predictive modelling, presents an opportunity to identify individuals at high risk of developing IBD93,94,95. Identifying these high-risk individuals could enable the design of interventional studies aimed at delaying or preventing IBD onset93,94,95. Potential interventions might include pharmacological therapies, preventive microbiome-targeted strategies, dietary modifications or vaccination. Proven preventive strategies targeting individuals with high-risk biomarkers could modify disease risk and reduce IBD incidence in the future93,94,95.
Work in pre-disease cohorts has identified promising blood and faecal biomarkers predictive of IBD development. Antibodies such as perinuclear anti-neutrophil cytoplasmic antibodies (pANCA) and anti-Saccharomyces cerevisiae mannan antibodies (ASCA) were detected prior to the diagnosis of ulcerative colitis and Crohn’s disease, respectively96. Pre-disease antimicrobial serum antibody responses97 and autoantibodies to granulocyte–macrophage colony-stimulating factor (GM-CSF)98 were observed years before the diagnosis of Crohn’s disease. Beyond serology, protein signatures such as those related to intestinal barrier integrity and macrophage function have also demonstrated predictive value years before clinical diagnosis99,100,101,102.
Alterations in gut microbial communities were detected up to 5 years before a Crohn’s disease diagnosis103, whereas elevated faecal proteolytic activity was observed prior to ulcerative colitis onset104. The Crohn’s and Colitis Canada Genetic, Environmental, Microbial (CCC-GEM) project, which tracked over 5,000 healthy first-degree relatives of people with Crohn’s disease, developed the GEM-Integrated Risk Score, a machine learning model that integrates clinical data, stool biomarkers (such as calprotectin levels) and microbiome profiles, and demonstrates high accuracy in predicting Crohn’s disease risk105. Moreover, ongoing research is evaluating simple serum-based risk scores that could serve as more feasible alternatives or complements to stool-based predictive tools93.
The ultimate objective of identifying pre-disease biomarkers is to differentiate individuals at lower risk from those at higher risk, enabling targeted interventions. For high-risk individuals, strategies such as dietary modifications or faecal microbial transplantation to improve microbiome composition could be explored to determine whether these approaches reduce the future risk of developing IBD93,94,95,106.
Certain pre-disease biomarkers such as neutralizing anti-GM-CSF autoantibodies are elevated in individuals who later develop a more complicated disease course of Crohn’s disease98. Those at higher risk of developing severe Crohn’s disease with complications could be considered for proactive pharmaceutical therapies aimed at delaying the onset and reducing the severity of IBD95,107. This concept is analogous to randomized controlled trials involving teplizumab, which slowed the onset of type 1 diabetes in relatives at high risk of disease development108, and abatacept, which curtailed the progression of rheumatoid arthritis in those with serological markers of emerging disease109.
A more ambitious preventive strategy involves developing a vaccine to prevent the onset of IBD. Advances in microbiome analysis may eventually lead to the identification of pathogenic microorganisms that contribute to disease onset, paving the way for vaccines that target these microorganisms for disease prevention or modulation106 — a strategy recently piloted against Mycobacterium avium complex in those with active Crohn’s disease110. The advent of mRNA technology has created new opportunities to modulate immune responses, as demonstrated in multiple sclerosis111,112,113. In animal models, mRNA vaccines delivering multiple sclerosis autoantigens to dendritic cells have shown the ability to reduce disease severity and progression111,112,113. Although vaccine research in IBD is still in its infancy, future studies could explore targeting specific microorganisms or modulating the immune response to reduce disease risk.
Although targeting individuals at high risk of disease with proven preventive interventions is the ideal scenario, implementing this approach into clinical practice remains a long-term goal. In the meantime, population-level strategies to modify environmental exposures offer a practical approach the clinical community can adopt today93,94,95. Initiatives such as smoking reduction policies, promoting breastfeeding, judicious antibiotic use, minimizing exposure to environmental pollutants in air and food and encouraging healthy diets (for example, Mediterranean diet) can generate cohort-wide effects, lowering exposure risks and potentially preventing future cases14,45,54,114. Advocacy for health-promoting policies, such as fostering balanced diets and healthy lifestyles, could further contribute to reducing IBD incidence on a societal level39,45,54,114. Moreover, emphasizing population-level interventions targeting lifestyle and dietary habits in low-income and middle-income countries is crucial to curbing the rise of IBD as these regions undergo economic development and adopt characteristics of Western societies50.
Although controlled interventional trials are currently lacking, cumulative evidence supports the integration of environmental health strategies into preventive care54. Lifestyle factors present a promising avenue for reducing IBD risk. Observational studies suggest that dietary and behavioural modifications can markedly influence disease risk7. Specifically, adhering to a combination of healthy lifestyle practices — including minimal alcohol intake; maintaining a healthy weight; engaging in regular physical activity; avoiding smoking; consuming a diet rich in fruits, vegetables, fibre, nuts, seeds and fish; and limiting red meat — could lower the incidence of IBD45. Estimated prevention rates range from 48.8% to 60.4% for Crohn’s disease and from 46.8% to 56.3% for ulcerative colitis45. By emphasizing environmental and lifestyle modifications, IBD prevention might be possible while simultaneously promoting overall health and reducing other age-related comorbidities, such as diabetes, cardiovascular disease, cancer and dementia14,94.
Governmental and non-governmental organizations will need to fund the translational, clinical and public health research necessary to build the evidence base for preventive health12,13. However, these investments hold the potential to not only enhance patient well-being but also alleviate broader societal and economic pressures10. Reducing the incidence of IBD and achieving prevalence equilibrium more rapidly would enable health-care resources to be redirected towards enhancing the management of individuals living with IBD10,14.
Innovating health-care delivery to needs
Societies might be ill-equipped to address the growing global IBD population while maintaining equitable, accessible and high-quality health care without a concerted effort to innovate health-care delivery and models of care. Over the next two decades, collaboration among patients, providers and policymakers will be essential to developing systems that optimize health outcomes, quality of life and cost-efficiency for individuals living with IBD115 (Fig. 5).
Patients play a critical part by advocating for IBD prioritization within health-care systems, adhering to disease management plans, participating in surveillance monitoring and adopting healthy lifestyle habits that improve outcomes116,117. The International Organization for the Study of Inflammatory Bowel Diseases has published guidelines recommending lifestyle and environmental modifications for people living with IBD, including smoking cessation, adherence to a Mediterranean-type diet, regular physical activity and managing mental health conditions54. Research should focus on understanding patient preferences and improving engagement to ensure that individuals with IBD and their care-givers are supported in actively participating in their own care116,117.
Multidisciplinary care teams are essential for managing the multifaceted needs of people with IBD, including gastroenterologists, colorectal surgeons, non-gastrointestinal specialists, primary care physicians, nurses, nurse practitioners, pharmacists, dietitians and counsellors118. Multidisciplinary teams can address diverse patient needs across age, gender, socioeconomic status and geographical location, while considering variations in disease phenotype, preferences and acceptance of care13. Although multidisciplinary, personalized care adds complexity, strategies can enhance efficiency today and guide future research. Shifting to home-based care models, such as telemedicine and remote monitoring (for example, home faecal calprotectin testing), can alleviate clinic constraints for expanding patient populations118,119,120. Similarly, clinic-based surveillance tools, such as bedside intestinal ultrasonography, enable real-time intervention, reducing reliance on invasive and costly endoscopic monitoring121. Home-centric care models can improve access for individuals facing barriers such as logistical challenges for older individuals or geographical limitations in rural areas120,122.
The increasing burden of IBD on patients, care-givers, health-care systems and society — marked by rising prevalence and escalating health-care costs5 — places further strain on systems already challenged to deliver efficient, accessible and equitable care9,11. The reliance on costly advanced therapies such as biologic agents is a major driver of IBD-related expenses89. However, these costs can be mitigated through the integration of biosimilars and generic medications, alongside government-led efforts to negotiate reduced drug prices123,124. Savings from these strategies could be reinvested to improve health care for individuals living with IBD89.
Although IBD is currently considered an irreversible chronic disease, compartment models include a recovery term, allowing for the possibility of a return to a non-diseased state57 (Fig. 4). Although PDE models typically exclude recovery as a factor influencing future prevalence (in the absence of a transformative discovery that cures IBD), recovery need not imply complete disease eradication. Instead, recovery might encompass achieving deep, sustainable transmural and histological remission. Within the PDE framework, incorporating a recovery component could allow researchers and health-care planners to define strata of patient outcomes (for example, sustained remission off therapy) and generate forecasting projections that stratify the IBD population by varying levels of disease activity or remission.
Over the next two decades, advances in understanding IBD pathogenesis could enable strategies such as microbiome modulation or immune system reprogramming to redefine recovery. Although these approaches might not cure IBD in the traditional sense (as with infectious diseases or early-stage cancers), they could substantially reduce the burden of the disease to the extent that its effects on the individual, health-care provider and society become negligible. In such cases, individuals in deep remission might be considered recovered and excluded from the prevalent IBD population.
Artificial intelligence holds substantial potential to enhance efficiencies in care delivery and advance research into disease prevention125. As an underlying, unifying thread, artificial intelligence can support collective efforts to reduce IBD incidence through preventive strategies and drive innovation in health-care delivery models to address the growing global burden of IBD125 (Fig. 5).
Conclusions
In the twentieth century, IBD was often described as a Western disease. However, by the turn of the twenty-first century, its global reach had expanded to include early industrialized countries in North America, Europe and Oceania and newly industrialized and developing regions in Africa, Asia and Latin America. A century’s worth of incidence and prevalence data have revealed predictable temporal shifts in epidemiologic patterns across geography and time. As such, future definitions of IBD epidemiology should move away from arbitrary geographical classifications and adopt the framework of the four epidemiologic stages as the gold standard. In 2025, every country falls into one of the first three stages, with transitions through the stages expected over the next two decades. By using accurate short-term modelling (for example, ARIMA) and flexible long-term modelling (for example, PDE), we can forecast these transitions, enabling countries to prepare for the anticipated rise in prevalence and shifts in the demographics of their IBD populations. Armed with these predictive tools, we can collectively prioritize research aimed at preventing IBD and innovate health-care delivery to meet the challenges posed by the rising global burden of the disease.
Data availability
Global IBD epidemiologic data depicted in the figures are provided in an open-access, downloadable, online interactive source, the ShinyApp: https://kaplan-gi.shinyapps.io/GIVES21.
References
Coward, S. et al. Past and future burden of inflammatory bowel diseases based on modeling of population-based data. Gastroenterology 156, 1345–1353.e4 (2019).
Coward, S. et al. Forecasting the incidence and prevalence of inflammatory bowel disease: a Canadian nationwide analysis. Am. J. Gastroenterol. 119, 1563–1570 (2024).
Lewis, J. D. et al. Incidence, prevalence, and racial and ethnic distribution of inflammatory bowel disease in the United States. Gastroenterology 165, 1197–1205.e2 (2023).
Hracs, L. et al. Global evolution of inflammatory bowel disease across epidemiologic stages. Nature 642, 458–466 (2025).
Kaplan, G. G. & Windsor, J. W. The four epidemiological stages in the global evolution of inflammatory bowel disease. Nat. Rev. Gastroenterol. Hepatol. 18, 56–66 (2021).
Ng, S. C. et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet 390, 2769–2778 (2017).
Kaplan, G. G. & Ng, S. C. Understanding and preventing the global increase of inflammatory bowel disease. Gastroenterology 152, 313–321.e2 (2017).
Kaplan, G. G. & Ng, S. C. Globalisation of inflammatory bowel disease: perspectives from the evolution of inflammatory bowel disease in the UK and China. Lancet Gastroenterol. Hepatol. 1, 307–316 (2016).
Ananthakrishnan, A. N., Kaplan, G. G. & Ng, S. C. Changing global epidemiology of inflammatory bowel diseases: sustaining health care delivery into the 21st century. Clin. Gastroenterol. Hepatol. 18, 1252–1260 (2020).
Burisch, J., Claytor, J., Hernandez, I., Hou, J. K. & Kaplan, G. G. The cost of inflammatory bowel disease care — how to make it sustainable. Clin. Gastroenterol. Hepatol. 23, 386–395 (2025).
Kaplan, G. G. The global burden of IBD: from 2015 to 2025. Nat. Rev. Gastroenterol. Hepatol. 12, 720–727 (2015).
Windsor, J. W. et al. The 2023 impact of inflammatory bowel disease in Canada: executive summary. J. Can. Assoc. Gastroenterol. 6, S1–S8 (2023).
Herauf, M. et al. Commentary on the epidemiology of inflammatory bowel disease in compounding prevalence nations: toward sustaining healthcare delivery. Gastroenterology 166, 949–956 (2024).
Chhibba, T. et al. Environmental risk factors of inflammatory bowel disease: toward a strategy of preventative health. J. Crohns Colitis 19, jjaf042 (2025).
Molodecky, N. A. et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology 142, 46–54.e42 (2012).
Bakhshi, Z., Harmsen, W. S., Tremaine, W. J. & Loftus, E. V. An update on inflammatory bowel disease epidemiology in Olmsted County, Minnesota from 1970 through 2019 [abstract 403]. Gastroenterology 162, S88–S89 (2022).
Shivashankar, R., Tremaine, W. J., Harmsen, W. S. & Loftus, E. V. Jr. Incidence and prevalence of Crohn’s disease and ulcerative colitis in Olmsted County, Minnesota from 1970 through 2010. Clin. Gastroenterol. Hepatol. 15, 857–863 (2017).
Rönnblom, A., Samuelsson, S. M. & Ekbom, A. Ulcerative colitis in the county of Uppsala 1945–2007: incidence and clinical characteristics. J. Crohns Colitis 4, 532–536 (2010).
Lapidus, A., Bernell, O., Hellers, G., Persson, P. G. & Löfberg, R. Incidence of Crohn’s disease in Stockholm County 1955–1989. Gut 41, 480–486 (1997).
Chiba, M., Morita, N., Nakamura, A., Tsuji, K. & Harashima, E. Increased incidence of inflammatory bowel disease in association with dietary transition (Westernization) in Japan. JMA J. 4, 347–357 (2021).
He, B. J. et al. Epidemiological study on the incidence of inflammatory bowel disease in Yinzhou District, Ningbo City from 2011 to 2020 [Chinese]. Beijing Da Xue Xue Bao Yi Xue Ban. 54, 511–519 (2022).
Mokhtar, N. M. et al. A four-decade analysis of the incidence trends, sociodemographic and clinical characteristics of inflammatory bowel disease patients at single tertiary centre, Kuala Lumpur, Malaysia. BMC Public Health 19, 550 (2019).
Hammada, T., Lemdaoui, M.-C., Boutra, F., Zoughailech, D. & Asselah, H. Epidemiological aspects of inflammatory bowel disease in an Algerian population. J. Afr. Hepato. Gastroenterol. 5, 293–302 (2011).
Ho, A. H. et al. Incidence and phenotype of inflammatory bowel disease in 16 regions across Asia, Africa, Latin America and Middle East from GIVES-21 consortium [abstract Tu1863]. Gastroenterology 166, S1443–S1444 (2024).
Qu, L. S. & Gubi, M. M. Clinical characteristics of colonoscopy in 448 patients in the Zanzibar Archipelago: a cross-sectional study. Pan Afr. Med. J. 41, 310 (2022).
Elbadry, M. et al. Clinico-epidemiological characteristics of patients with inflammatory bowel disease in Egypt: a nationwide multicenter study. Front. Med. 9, 867293 (2022).
Archampong, T. N. & Nkrumah, K. N. Inflammatory bowel disease in Accra: what new trends. West Afr. J. Med. 32, 40–44 (2013).
Khalifa, S. E., Mudawi, H. M. & Fedail, S. S. Presentation and management outcome of inflammatory bowel disease in Sudan. Trop. Gastroenterol. 26, 194–196 (2005).
Kotze, P. G. et al. Progression of inflammatory bowel diseases throughout Latin America and the Caribbean: a systematic review. Clin. Gastroenterol. Hepatol. 18, 304–312 (2020).
Banerjee, R. et al. Emerging inflammatory bowel disease demographics, phenotype, and treatment in South Asia, South-East Asia, and Middle East: preliminary findings from the Inflammatory Bowel Disease–Emerging Nations’ Consortium. J. Gastroenterol. Hepatol. 37, 1004–1015 (2022).
Balderramo, D. et al. Challenges in the diagnosis and treatment of inflammatory bowel disease in Latin America. Lancet Gastroenterol. Hepatol. 9, 263–272 (2024).
Ng, S. C. et al. Population density and risk of inflammatory bowel disease: a prospective population-based study in 13 countries or regions in Asia-Pacific. Am. J. Gastroenterol. 114, 107–115 (2019).
Lamb, C. A. et al. Inflammatory bowel disease has no borders: engaging patients as partners to deliver global, equitable and holistic health care. Lancet 404, 414–417 (2024).
Sood, A., Midha, V., Sood, N., Bhatia, A. S. & Avasthi, G. Incidence and prevalence of ulcerative colitis in Punjab, North India. Gut 52, 1587–1590 (2003).
Banerjee, R. et al. Inflammatory bowel disease (IBD) in rural and urban India: results from community colonoscopic evaluation of more than 30,000 symptomatic patients. Lancet Reg. Health Southeast Asia 19, 100259 (2023).
Molodecky, N. A. et al. Challenges associated with identifying the environmental determinants of the inflammatory bowel diseases. Inflamm. Bowel Dis. 17, 1792–1799 (2011).
Pavlovic-Calic, N., Salkic, N. N., Gegic, A., Smajic, M. & Alibegovic, E. Crohn’s disease in Tuzla region of Bosnia and Herzegovina: a 12-year study (1995-2006). Int. J. Colorectal Dis. 23, 957–964 (2008).
Piovani, D. et al. Environmental risk factors for inflammatory bowel diseases: an umbrella review of meta-analyses. Gastroenterology 157, 647–659.e4 (2019).
Sasson, A. N. et al. The role of precision nutrition in the modulation of microbial composition and function in people with inflammatory bowel disease. Lancet Gastroenterol. Hepatol. 6, 754–769 (2021).
Ueno, A. et al. Opposing effects of smoking in ulcerative colitis and Crohn’s disease may be explained by differential effects on dendritic cells. Inflamm. Bowel Dis. 20, 800–810 (2014).
Narula, N. et al. Food processing and risk of inflammatory bowel disease: a systematic review and meta-analysis. Clin. Gastroenterol. Hepatol. 21, 2483–2495.e1 (2023).
Dar, S. H. et al. The association of antibiotic exposure with new-onset inflammatory bowel disease: a systematic review and meta-analysis. Clin. Res. Hepatol. Gastroenterol. 47, 102129 (2023).
Xu, L. et al. Systematic review with meta-analysis: breastfeeding and the risk of Crohn’s disease and ulcerative colitis. Aliment. Pharmacol. Ther. 46, 780–789 (2017).
Turpin, W. et al. Mediterranean-like dietary pattern associations with gut microbiome composition and subclinical gastrointestinal inflammation. Gastroenterology 163, 685–698 (2022).
Lopes, E. W. et al. Lifestyle factors for the prevention of inflammatory bowel disease. Gut 72, 1093–1100 (2023).
Estevinho, M. M. et al. Emerging role of environmental pollutants in inflammatory bowel disease risk, outcomes and underlying mechanisms. Gut 74, 477–486 (2025).
Agrawal, M., Hansen, A. V., Colombel, J. F., Jess, T. & Allin, K. H. Association between early life exposure to agriculture, biodiversity, and green space and risk of inflammatory bowel disease: a population-based cohort study. eClinicalMedicine 70, 102514 (2024).
Kayali, S. et al. NOD2 and Crohn’s disease clinical practice: from epidemiology to diagnosis and therapy, rewired. Inflamm. Bowel Dis. 31, 552–562 (2024).
Kaplan, G. G. IBD: Global variations in environmental risk factors for IBD. Nat. Rev. Gastroenterol. Hepatol. 11, 708–709 (2014).
Banerjee, R., Pal, P., Mak, J. W. Y. & Ng, S. C. Challenges in the diagnosis and management of inflammatory bowel disease in resource-limited settings in Asia. Lancet Gastroenterol. Hepatol. 5, 1076–1088 (2020).
Schaffer, A. L., Dobbins, T. A. & Pearson, S. A. Interrupted time series analysis using autoregressive integrated moving average (ARIMA) models: a guide for evaluating large-scale health interventions. BMC Med. Res. Methodol. 21, 58 (2021).
Shah, S. C. et al. Sex-based differences in incidence of inflammatory bowel diseases — pooled analysis of population-based studies from western countries. Gastroenterology 155, 1079–1089.e3 (2018).
Jones, G. R. et al. IBD prevalence in Lothian, Scotland, derived by capture–recapture methodology. Gut 68, 1953–1960 (2019).
Ananthakrishnan, A. N. et al. Lifestyle, behaviour, and environmental modification for the management of patients with inflammatory bowel diseases: an International Organization for Study of Inflammatory Bowel Diseases consensus. Lancet Gastroenterol. Hepatol. 7, 666–678 (2022).
Brinks, R. & Landwehr, S. A new relation between prevalence and incidence of a chronic disease. Math. Med. Biol. 32, 425–435 (2015).
Keiding, N. Age-specific incidence and prevalence — a statistical perspective. J. R. Stat. Soc. A Stat. 154, 371–412 (1991).
Brinks, R. & Landwehr, S. Change rates and prevalence of a dichotomous variable: simulations and applications. PLoS ONE 10, e0118955 (2015).
Brinks, R. & Landwehr, S. Age- and time-dependent model of the prevalence of non-communicable diseases and application to dementia in Germany. Theor. Popul. Biol. 92, 62–68 (2014).
Brinks, R. Illness-death model in chronic disease epidemiology: characteristics of a related, differential equation and an inverse problem. Comput. Math. Methods Med. 2018, 5091096 (2018).
Brinks, R. & Hoyer, A. Illness-death model: statistical perspective and differential equations. Lifetime Data Anal. 24, 743–754 (2018).
Voeltz, D., Tönnies, T., Brinks, R. & Hoyer, A. Future prevalence of type 2 diabetes — a comparative analysis of chronic disease projection methods. PLoS ONE 17, e0264739 (2022).
Wang, J., Vordenbäumen, S., Schneider, M. & Brinks, R. Population-based epidemiological projections of rheumatoid arthritis in Germany until 2040. Scand. J. Rheumatol. 53, 161–172 (2024).
Wang, J. et al. A population-based projection of psoriatic arthritis in Germany until 2050: analysis of national statutory health insurance data of 65 million German population. Rheumatol. Int. 43, 2037–2047 (2023).
Bernstein, C. N., Blanchard, J. F., Rawsthorne, P. & Wajda, A. Epidemiology of Crohn’s disease and ulcerative colitis in a central Canadian province: a population-based study. Am. J. Epidemiol. 149, 916–924 (1999).
Quaresma, A. B. et al. Temporal trends in the epidemiology of inflammatory bowel diseases in the public healthcare system in Brazil: a large population-based study. Lancet Reg. Health Am. 13, 100298 (2022).
Kuenzig, M. E. et al. Twenty-first century trends in the global epidemiology of pediatric-onset inflammatory bowel disease: systematic review. Gastroenterology 162, 1147–1159.e4 (2022).
Kuenzig, M. E. et al. The NOD2-smoking interaction in Crohn’s disease is likely specific to the 1007fs mutation and may be explained by age at diagnosis: a meta-analysis and case-only study. eBioMedicine 21, 188–196 (2017).
Calkins, B. M. A meta-analysis of the role of smoking in inflammatory bowel disease. Dig. Dis. Sci. 34, 1841–1854 (1989).
Frolkis, A. D. et al. The association of smoking and surgery in inflammatory bowel disease is modified by age at diagnosis. Clin. Transl. Gastroenterol. 7, e165 (2016).
Hoyer, A., Kaufmann, S. & Brinks, R. Risk factors in the illness-death model: simulation study and the partial differential equation about incidence and prevalence. PLoS ONE 14, e0226554 (2019).
Duricova, D. et al. Overall and cause-specific mortality in Crohn’s disease: a meta-analysis of population-based studies. Inflamm. Bowel Dis. 16, 347–353 (2010).
Jess, T., Gamborg, M., Munkholm, P. & Sørensen, T. I. Overall and cause-specific mortality in ulcerative colitis: meta-analysis of population-based inception cohort studies. Am. J. Gastroenterol. 102, 609–617 (2007).
Kuenzig, M. E., Manuel, D. G., Donelle, J. & Benchimol, E. I. Life expectancy and health-adjusted life expectancy in people with inflammatory bowel disease. CMAJ 192, E1394–E1402 (2020).
Bernstein, C. N. et al. The impact of inflammatory bowel disease in Canada 2018: extra-intestinal diseases in IBD. J. Can. Assoc. Gastroenterol. 2, S73–S80 (2019).
Singh, S. et al. Postoperative mortality among patients with inflammatory bowel diseases: a systematic review and meta-analysis of population-based studies. Gastroenterology 149, 928–937 (2015).
Benchimol, E. I. et al. Inflammatory bowel disease in immigrants to Canada and their children: a population-based cohort study. Am. J. Gastroenterol. 110, 553–563 (2015).
Agrawal, M. et al. Inflammatory bowel diseases among first-generation and second-generation immigrants in Denmark: a population-based cohort study. Gut 70, 1037–1043 (2021).
Raftery, A. E. & Sevcikova, H. Probabilistic population forecasting: short to very long-term. Int. J. Forecast. 39, 73–97 (2023).
Ritchie, H. & Rodes-Guirao, L. Peak global population and other key findings from the 2024 UN World Population Prospects: falling fertility rates, migration movements, and China’s population decline. Our World in Data https://ourworldindata.org/un-population-2024-revision (2024).
Agrawal, M. & Jess, T. Implications of the changing epidemiology of inflammatory bowel disease in a changing world. United European Gastroenterol. J. 10, 1113–1120 (2022).
Mak, J. W. Y. et al. Development of the global inflammatory bowel disease visualization of epidemiology studies in the 21st century (GIVES-21). BMC Med. Res. Methodol. 23, 129 (2023).
GBD 2017 Inflammatory Bowel Disease Collaborators The global, regional, andnational burden of inflammatory bowel disease in 195 countries and territories,1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol. 5, 17–30 (2020).
Bernstein, C. N. et al. The epidemiology of inflammatory bowel disease in Canada: a population-based study. Am. J. Gastroenterol. 101, 1559–1568 (2006).
Wang, R., Li, Z., Liu, S. & Zhang, D. Global, regional and national burden of inflammatory bowel disease in 204 countries and territories from 1990 to 2019: a systematic analysis based on the Global Burden of Disease Study 2019. BMJ Open 13, e065186 (2023).
GBD 2021 Diseases and Injuries Collaborators Global incidence, prevalence, years lived with disability (YLDs), disability-adjusted life-years (DALYs), and healthy life expectancy (HALE) for 371 diseases and injuries in 204 countries and territories and 811 subnational locations, 1990–2021: a systematic analysis for the Global Burden of Disease Study 2021. Lancet 403, 2133–2161 (2024).
Ng, W. K., Wong, S. H. & Ng, S. C. Changing epidemiological trends of inflammatory bowel disease in Asia. Intest. Res. 14, 111–119 (2016).
Dorn-Rasmussen, M. et al. The incidence and prevalence of paediatric- and adult-onset inflammatory bowel disease in Denmark during a 37-year period: a nationwide cohort study (1980–2017). J. Crohns Colitis 17, 259–268 (2023).
Shah, S. C. et al. Sex-based differences in the incidence of inflammatory bowel diseases — pooled analysis of population-based studies from the Asia-Pacific region. Aliment. Pharmacol. Ther. 49, 904–911 (2019).
Burisch, J. et al. The cost of inflammatory bowel disease in high-income settings: a Lancet Gastroenterology & Hepatology Commission. Lancet Gastroenterol. Hepatol. 8, 458–492 (2023).
Kuenzig, M. E. et al. The 2023 impact of inflammatory bowel disease in Canada: indirect (individual and societal) and direct out-of-pocket costs. J. Can. Assoc. Gastroenterol. 6, S16–S22 (2023).
Agrawal, M. et al. The rising burden of inflammatory bowel disease in Denmark over two decades: a nationwide cohort study. Gastroenterology 163, 1547–1554.e5 (2022).
Larsen, L. et al. Has the Incidence of inflammatory bowel disease peaked? Evidence from the population-based NorDIBD cohort 1978-2020. Am. J. Gastroenterol. 118, 501–510 (2023).
Bronze, S., Agrawal, M., Colombel, J. F., Torres, J. & Ungaro, R. C. Review article: prevention of inflammatory bowel disease — the path forward. Aliment. Pharmacol. Ther. 60, 1166–1175 (2024).
Lopes, E. W., Turpin, W., Croitoru, K., Colombel, J. F. & Torres, J. Prediction and prevention of inflammatory bowel disease. Clin. Gastroenterol. Hepatol. 23, 396–405.e1 (2025).
Rudbaek, J. J. et al. Deciphering the different phases of preclinical inflammatory bowel disease. Nat. Rev. Gastroenterol. Hepatol. 21, 86–100 (2024).
Israeli, E. et al. Anti-Saccharomyces cerevisiae and antineutrophil cytoplasmic antibodies as predictors of inflammatory bowel disease. Gut 54, 1232–1236 (2005).
Lee, S. H. et al. Anti-microbial antibody response is associated with future onset of Crohn’s disease independent of biomarkers of altered gut barrier function, subclinical inflammation, and genetic risk. Gastroenterology 161, 1540–1551 (2021).
Mortha, A. et al. Neutralizing anti-granulocyte macrophage-colony stimulating factor autoantibodies recognize post-translational glycosylations on granulocyte macrophage-colony stimulating factor years before diagnosis and predict complicated Crohn’s disease. Gastroenterology 163, 659–670 (2022).
Torres, J. et al. Serum biomarkers identify patients who will develop inflammatory bowel diseases up to 5 years before diagnosis. Gastroenterology 159, 96–104 (2020).
Bergemalm, D. et al. Systemic inflammation in preclinical ulcerative colitis. Gastroenterology 161, 1526–1539.e9 (2021).
Livanos, A. E. et al. Anti-integrin αvβ6 autoantibodies are a novel biomarker that antedate ulcerative colitis. Gastroenterology 164, 619–629 (2023).
Grännö, O. et al. Preclinical protein signatures of Crohn’s disease and ulcerative colitis: a nested case-control study within large population-based cohorts. Gastroenterology 168, 741–753 (2025).
Raygoza Garay, J. A. et al. Gut microbiome composition is associated with future onset of Crohn’s disease in healthy first-degree relatives. Gastroenterology 165, 670–681 (2023).
Galipeau, H. J. et al. Novel fecal biomarkers that precede clinical diagnosis of ulcerative colitis. Gastroenterology 160, 1532–1545 (2021).
Lee, S. H. et al. Development and validation of an integrative risk score for future risk of Crohn’s disease in healthy first-degree relatives: a multicenter prospective cohort study. Gastroenterology 168, 150–153.e4 (2025).
Zhang, L., Agrawal, M., Ng, S. C. & Jess, T. Early-life exposures and the microbiome: implications for IBD prevention. Gut 73, 541–549 (2024).
D’Haens, G. & Simsek, M. Early Crohn’s disease: can we change the disease course? Nat. Rev. Gastroenterol. Hepatol. 22, 367–368 (2025).
Herold, K. C. et al. An anti-CD3 antibody, teplizumab, in relatives at risk for type 1 diabetes. N. Engl. J. Med. 381, 603–613 (2019).
Cope, A. P. et al. Abatacept in individuals at high risk of rheumatoid arthritis (APIPPRA): a randomised, double-blind, multicentre, parallel, placebo-controlled, phase 2b clinical trial. Lancet 403, 838–849 (2024).
Sanderson, J. et al. A phase 1b clinical trial to determine the safety, tolerability and immunogenicity of simian adenovirus and poxvirus vectored vaccines against a Mycobacterium avium complex subspecies in patients with active Crohn’s disease. eBioMedicine 113, 105570 (2025).
Krienke, C. et al. A noninflammatory mRNA vaccine for treatment of experimental autoimmune encephalomyelitis. Science 371, 145–153 (2021).
Mohammadzamani, M. et al. Insights into the interplay between Epstein-Barr virus (EBV) and multiple sclerosis (MS): a state-of-the-art review and implications for vaccine development. Health Sci. Rep. 7, e1898 (2024).
Bjornevik, K. et al. Longitudinal analysis reveals high prevalence of Epstein-Barr virus associated with multiple sclerosis. Science 375, 296–301 (2022).
Levine, A. et al. Dietary guidance from the International Organization for the Study of Inflammatory Bowel Diseases. Clin. Gastroenterol. Hepatol. 18, 1381–1392 (2020).
Solitano, V. et al. Shaping the future of inflammatory bowel disease: a global research agenda for better management and public health response. Nat. Rev. Gastroenterol. Hepatol. 22, 438–452 (2025).
Hazlewood, G. S. et al. Patient preferences for maintenance therapy in Crohn’s disease: a discrete-choice experiment. PLoS ONE 15, e0227635 (2020).
Vutcovici, M. et al. Patient perspectives of IBD care and services: an integral part of a pan-Canadian quality improvement initiative. J. Can. Assoc. Gastroenterol. 4, 229–233 (2021).
Mathias, H. et al. The 2023 impact of inflammatory bowel disease in Canada: access to and models of care. J. Can. Assoc. Gastroenterol. 6, S111–S121 (2023).
Zhen, J., Marshall, J. K., Nguyen, G. C., Atreja, A. & Narula, N. Impact of digital health monitoring in the management of inflammatory bowel disease. J. Med. Syst. 45, 23 (2021).
Habashi, P., Bouchard, S. & Nguyen, G. C. Transforming access to specialist care for inflammatory bowel disease: the PACE telemedicine program. J. Can. Assoc. Gastroenterol. 2, 186–194 (2019).
Novak, K. et al. Clinic-based point of care transabdominal ultrasound for monitoring Crohn’s disease: impact on clinical decision making. J. Crohns Colitis 9, 795–801 (2015).
Shaffer, S. R. et al. The 2023 impact of inflammatory bowel disease in Canada: special populations — IBD in seniors. J. Can. Assoc. Gastroenterol. 6, S45–S54 (2023).
Crosby, M., Tadrous, M. & Gomes, T. Potential cost implications of mandatory non-medical switching policies for biologics for rheumatic conditions and inflammatory bowel disease in Canada. Clin. Pharmacol. Ther. 109, 739–745 (2021).
Murthy, S. K. et al. The 2023 impact of inflammatory bowel disease in Canada: treatment landscape. J. Can. Assoc. Gastroenterol. 6, S97–S110 (2023).
Silverman, A. L., Shung, D., Stidham, R. W., Kochhar, G. S. & Iacucci, M. How artificial intelligence will transform clinical care, research, and trials for inflammatory bowel disease. Clin. Gastroenterol. Hepatol. 23, 428–439 (2025).
Acknowledgements
The author acknowledges S. Coward, M. Cummings, L. Hracs and J. Windsor for their expertise, contextual insights and editorial contributions to the article. In particular, the author thanks J. Gorospe for her assistance in drafting the figures and J. Windsor for developing the visual representation and guitar-string analogy in Fig. 1. The author’s research is funded by operating grants from the Canadian Institutes of Health Research and the Leona M. and Harry B. Helmsley Charitable Trust.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
G.G.K. has received honoraria for speaking or consultancy from AbbVie, Amgen, Janssen, Pfizer and Takeda; and has received grants for research from Ferring and for educational activities from AbbVie, Bristol Myers Squibb, Ferring, Fresenius-Kabi, Janssen, Pfizer and Takeda. He shares ownership of a patent: Use of mirtazapine in the treatment of inflammatory disorders, autoimmune disease and PBC (patent WO/2019/046959A1, PCT/CA2018/051098).
Peer review
Peer review information
Nature Reviews Gastroenterology & Hepatology thanks Manasi Agrawal, Stefanos Bonovas and Zhihua Ran for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kaplan, G.G. The global burden of inflammatory bowel disease: from 2025 to 2045. Nat Rev Gastroenterol Hepatol 22, 708–720 (2025). https://doi.org/10.1038/s41575-025-01097-1
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41575-025-01097-1