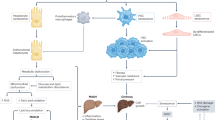
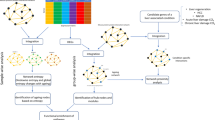
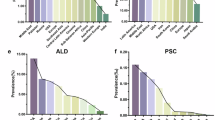
Abstract
As the global population ages, research on the biology of ageing and its role in chronic disease is expanding, alongside a growing clinical focus on the unique needs of older adults. In the past, the liver was not thought to undergo substantial age-related changes, nor was there thought to be any liver disease characteristic of older adults. Current studies challenge this perspective, revealing that ageing substantially influences liver pathophysiology at the organ level and within each of the liver cell types. These observations have implications for understanding the pathogenesis of liver diseases common in older adults, including hepatocellular carcinoma, hypoxic hepatitis and metabolic dysfunction-associated steatotic liver disease. Previously, managing older patients with liver disease mostly addressed age-related changes in drug metabolism and liver function tests. However, current clinical practice increasingly emphasizes age-specific issues such as frailty, sarcopenia, multimorbidity and polypharmacy. Given the liver’s pivotal role in systemic metabolism, immunity and detoxification, ageing of the liver can contribute to systemic diseases. In the future, interventions that target ageing biology might offer new treatment options for liver diseases. Here, we review those age-related changes in the liver that have substantial biological and clinical consequences for older adults.
Key points
-
The liver undergoes ageing processes that are comparable to those of other organs and tissues, contributing to impaired liver function and increased susceptibility to liver diseases in older adults.
-
Age-related changes in hepatocytes and non-parenchymal liver cells drive fibrosis, inflammation, steatosis, metabolic dysfunction, reduced detoxification and impaired regenerative capacity.
-
Elevated serum liver enzyme levels are often a result of comorbidities, whereas low serum alanine transaminase levels are associated with older age, frailty and increased mortality.
-
Liver diseases prevalent in older adults, including hepatocellular carcinoma and metabolic dysfunction-associated steatotic liver disease, share pathogenic mechanisms with the biological processes of ageing.
-
Liver ageing is associated with cardiometabolic syndromes, including dyslipidaemia, insulin resistance and vascular disease, and with neurodegenerative conditions such as dementia.
-
The liver is a key target for longevity-extending interventions, including pharmacological and nutritional strategies to modulate ageing processes that might mitigate liver diseases.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Popper, H. Coming of age. Hepatology 5, 1224–1226 (1985).
Schmucker, D. L. Age-related changes in liver structure and function: implications for disease? Exp. Gerontol. 40, 650–659 (2005).
Tajiri, K. & Shimizu, Y. Liver physiology and liver diseases in the elderly. World J. Gastroenterol. 19, 8459–8467 (2013).
Kim, I. H., Kisseleva, T. & Brenner, D. A. Aging and liver disease. Curr. Opin. Gastroenterol. 31, 184–191 (2015).
Macias, R. I. R. et al. Impact of aging on primary liver cancer: epidemiology, pathogenesis and therapeutics. Aging 13, 23416–23434 (2021).
Sanfeliu-Redondo, D., Gibert-Ramos, A. & Gracia-Sancho, J. Cell senescence in liver diseases: pathological mechanism and theranostic opportunity. Nat. Rev. Gastroenterol. Hepatol. 21, 477–492 (2024).
Wan, Y. et al. Endothelial dysfunction in pathological processes of chronic liver disease during aging. FASEB J. 36, e22125 (2022).
Baiocchi, L. et al. Impact of aging on liver cells and liver disease: focus on the biliary and vascular compartments. Hepatol. Commun. 5, 1125–1137 (2021).
Le Couteur, D. G., Fraser, R., Cogger, V. C. & McLean, A. J. Hepatic pseudocapillarisation and atherosclerosis in ageing. Lancet 359, 1612–1615 (2002).
Morsiani, C. et al. The peculiar aging of human liver: a geroscience perspective within transplant context. Ageing Res. Rev. 51, 24–34 (2019).
United Nations. Global issues: ageing. United Nations www.un.org/en/global-issues/ageing (2024).
de Cabo, R. & Le Couteur, D. in Harrison’s Principles of Internal Medicine Vol. 2 Ch. 476 (eds Loscalzo, J. et al.) 3733–3739 (McGraw Hill, 2021).
Lemoine, M. Defining aging. Biol. Philos. 35, 46 (2020).
Le Couteur, D. G. & Thillainadesan, J. What is an aging-related disease? An epidemiological perspective. J. Gerontol. A Biol. Sci. Med. Sci. 77, 2168–2174 (2022).
Thillainadesan, J., Scott, I. A. & Le Couteur, D. G. Frailty, a multisystem ageing syndrome. Age Ageing 49, 758–763 (2020).
Sierra, F. The emergence of geroscience as an interdisciplinary approach to the enhancement of health span and life span. Cold Spring Harb. Perspect. Med. 6, a025163 (2016).
Rolland, Y. et al. Challenges in developing geroscience trials. Nat. Commun. 14, 5038 (2023).
Le Couteur, D. G., Anderson, R. M. & de Cabo, R. Can we make drug discovery targeting fundamental mechanisms of aging a reality? Expert Opin. Drug Discov. 17, 97–100 (2022).
López-Otín, C., Blasco, M. A., Partridge, L., Serrano, M. & Kroemer, G. J. C. Hallmarks of aging: an expanding universe. Cell 186, 243–278 (2023).
Hunt, N. J., Kang, S. W., Lockwood, G. P., Le Couteur, D. G. & Cogger, V. C. Hallmarks of aging in the liver. Comp. Struct. Biotech. J. 17, 1151–1161 (2019).
Gan, L., Chitturi, S. & Farrell, G. C. Mechanisms and implications of age-related changes in the liver: nonalcoholic fatty liver disease in the elderly. Curr. Gerontol. Geriatr. Res. 2011, 831536 (2011).
Boyer, J. L. Hepatology highlights: the liver does age! Hepatology 33, 487 (2001).
Horvath, S. DNA methylation age of human tissues and cell types. Genome Biol. 14, R115 (2013).
Haghani, A. et al. DNA methylation networks underlying mammalian traits. Science 381, eabq5693 (2023).
Serviddio, G. et al. Bioenergetics in aging: mitochondrial proton leak in aging rat liver, kidney and heart. Redox Rep. 12, 91–95 (2007).
Pandya, J. D. et al. Age- and organ-specific differences in mitochondrial bioenergetics in brown Norway rats. J. Aging Res. 2020, 7232614 (2020).
Baek, J. H., Son, H., Jeong, Y. H., Park, S. W. & Kim, H. J. Chronological aging standard curves of telomere length and mitochondrial DNA copy number in twelve tissues of C57BL/6 male mouse. Cells 8, 247 (2019).
Khawaja, R. R. et al. Sex-specific and cell-type-specific changes in chaperone-mediated autophagy across tissues during aging. Nat. Aging 5, 691–708 (2025).
Tuma, R. F., Irion, G. L., Vasthare, U. S. & Heinel, L. A. Age-related changes in regional blood flow in the rat. Am. J. Physiol. 249, H485–H491 (1985).
Petr, M. A. et al. A cross-sectional study of functional and metabolic changes during aging through the lifespan in male mice. eLife 10, e62952 (2021).
Tabula Muris, C. A single-cell transcriptomic atlas characterizes ageing tissues in the mouse. Nature 583, 590–595 (2020).
Schaum, N. et al. Ageing hallmarks exhibit organ-specific temporal signatures. Nature 583, 596–602 (2020).
Oh, H. S. et al. Organ aging signatures in the plasma proteome track health and disease. Nature 624, 164–172 (2023).
Jiang, M. et al. A biomarker framework for liver aging: the Aging Biomarker Consortium consensus statement. Life Med. 3, lnae004 (2024).
Kwekel, J. C., Desai, V. G., Moland, C. L., Branham, W. S. & Fuscoe, J. C. Age and sex dependent changes in liver gene expression during the life cycle of the rat. BMC Genom. 11, 675 (2010).
Le Couteur, D. G. et al. Nutritional reprogramming of mouse liver proteome is dampened by metformin, resveratrol, and rapamycin. Cell Metab. 33, 2367–2379.e4 (2021).
Schmucker, D. L. Aging and the liver: an update. J. Gerontol. A Biol. Sci. Med. Sci. 53, B315–B320 (1998).
Schmucker, D. L. Liver function and phase I drug metabolism in the elderly: a paradox. Drugs Aging 18, 837–851 (2001).
Terman, A. & Brunk, U. T. Lipofuscin. Int. J. Biochem. Cell Biol. 36, 1400–1404 (2004).
Wakabayashi, H., Nishiyama, Y., Ushiyama, T., Maeba, T. & Maeta, H. Evaluation of the effect of age on functioning hepatocyte mass and liver blood flow using liver scintigraphy in preoperative estimations for surgical patients: comparison with CT volumetry. J. Surg. Res. 106, 246–253 (2002).
Vats, R. et al. Intravital imaging reveals inflammation as a dominant pathophysiology of age-related hepatovascular changes. Am. J. Physiol. Cell Physiol. 322, C508–C520 (2022).
Wiley, C. D. & Campisi, J. The metabolic roots of senescence: mechanisms and opportunities for intervention. Nat. Metab. 3, 1290–1301 (2021).
Ershler, W. B. Interleukin-6: a cytokine for gerontologists. J. Am. Geriatr. Soc. 41, 176–181 (1993).
Heinke, P. et al. Diploid hepatocytes drive physiological liver renewal in adult humans. Cell Syst. 13, 499–507 e412 (2022).
Le Couteur, D. G. et al. Old age and the hepatic sinusoid. Anat. Rec. 291, 672–683 (2008).
Le Couteur, D. G. & Lakatta, E. G. A vascular theory of aging. J. Gerontol. A Biol. Sci. Med. Sci. 65, 1025–1027 (2010).
Gracia-Sancho, J., Caparros, E., Fernandez-Iglesias, A. & Frances, R. Role of liver sinusoidal endothelial cells in liver diseases. Nat. Rev. Gastroenterol. Hepatol. 18, 411–431 (2021).
Le Couteur, D. G. et al. Pseudocapillarization and associated energy limitation in the aged rat liver. Hepatology 33, 537–543 (2001).
McLean, A. J. et al. Age-related pseudocapillarization of the human liver. J. Pathol. 200, 112–117 (2003).
Warren, A. et al. Hepatic pseudocapillarization in aged mice. Exp. Gerontol. 40, 807–812 (2005).
Cogger, V. C. et al. Hepatic sinusoidal pseudocapillarization with aging in the non-human primate. Exp. Gerontol. 38, 1101–1107 (2003).
Cogger, V. C., Hunt, N. J. & Le Couteur, D. G. in The Liver: Biology and Pathobiology Ch. 35 (eds Arias, I. M. et al.) 435–443 (Wiley, 2020).
Cogger, V. C., Roessner, U., Warren, A., Fraser, R. & Le Couteur, D. G. A sieve-raft hypothesis for the regulation of endothelial fenestrations. Comput. Struct. Biotechnol. J. 8, e201308003 (2013).
Svistounov, D. et al. The relationship between fenestrations, sieve plates and rafts in liver sinusoidal endothelial cells. PLoS ONE 7, e46134 (2012).
Mak, K. M., Chu, E., Lau, K. H. & Kwong, A. J. Liver fibrosis in elderly cadavers: localization of collagen types I, III, and IV, α-smooth muscle actin, and elastic fibers. Anat. Rec. 295, 1159–1167 (2012).
Hilmer, S. N. et al. Age-related changes in the hepatic sinusoidal endothelium impede lipoprotein transfer in the rat. Hepatology 42, 1349–1354 (2005).
Mohamad, M. et al. Ultrastructure of the liver microcirculation influences hepatic and systemic insulin activity and provides a mechanism for age-related insulin resistance. Aging Cell 15, 706–715 (2016).
Grosse, L. et al. Defined p16high senescent cell types are indispensable for mouse healthspan. Cell Metab. 32, 87–99.e6 (2020).
Grosse, L. & Bulavin, D. V. LSEC model of aging. Aging 12, 11152–11160 (2020).
Maeso-Diaz, R. et al. Effects of aging on liver microcirculatory function and sinusoidal phenotype. Aging Cell 17, e12829 (2018).
Warren, A. et al. The effects of old age on hepatic stellate cells. Curr. Gerontol. Geriatr. Res. 2011, 439835 (2011).
Marcos, R. & Correia-Gomes, C. Long live the liver: immunohistochemical and stereological study of hepatocytes, liver sinusoidal endothelial cells, Kupffer cells and hepatic stellate cells of male and female rats throughout ageing. Cell Tissue Res. 366, 639–649 (2016).
Cogger, V. C. et al. Preliminary analysis of the sinusoidal endothelium and space of Disse in ageing Papio hamadrayas. Comp. Hepatol. 3, S26 (2004).
Franceschi, C., Garagnani, P., Parini, P., Giuliani, C. & Santoro, A. Inflammaging: a new immune-metabolic viewpoint for age-related diseases. Nat. Rev. Endocrinol. 14, 576–590 (2018).
Hilmer, S. N., Cogger, V. C. & Le Couteur, D. G. Basal activity of Kupffer cells increases with old age. J. Gerontol. A Biol. Sci. Med. Sci. 62, 973–978 (2007).
Knook, D. L. & Brouwer, A. Kupffer cells and the acute phase response: the effect of aging. Immunol. Invest. 18, 339–350 (1989).
Zou, J., Li, J., Wang, X., Tang, D. & Chen, R. Neuroimmune modulation in liver pathophysiology. J. Neuroinflamm. 21, 188 (2024).
Chatterjee, N., Sharma, R., Kale, P. R., Trehanpati, N. & Ramakrishna, G. Is the liver resilient to the process of ageing? Ann. Hepatol. 30, 101580 (2024).
Stell, D. & Wall, W. J. The impact of aging on the liver. Geriatr. Aging 6, 36–37 (2003).
Frith, J., Jones, D. & Newton, J. L. Chronic liver disease in an ageing population. Age Ageing 38, 11–18 (2009).
Le Couteur, D. G. et al. The association of alanine transaminase with aging, frailty, and mortality. J. Gerontol. A Biol. Sci. Med. Sci. 65, 712–717 (2010).
Liu, Z., Que, S., Xu, J. & Peng, T. Alanine aminotransferase — old biomarker and new concept: a review. Int. J. Med. Sci. 11, 925–935 (2014).
McPherson, S. et al. Age as a confounding factor for the accurate non-invasive diagnosis of advanced NAFLD fibrosis. Am. J. Gastroenterol. 112, 740–751 (2017).
Liu, Z. et al. Complex association between alanine aminotransferase activity and mortality in general population: a systematic review and meta-analysis of prospective studies. PLoS ONE 9, e91410 (2014).
Moshkovits, Y., Chetrit, A. & Dankner, R. The association between frailty biomarkers and 20-year all-cause and cardiovascular mortality among community-dwelling older adults. Postgrad. Med. 136, 641–650 (2024).
Fleming, K. M., West, J., Aithal, G. P. & Fletcher, A. E. Abnormal liver tests in people aged 75 and above: prevalence and association with mortality. Aliment. Pharmacol. Ther. 34, 324–334 (2011).
Katzke, V. et al. Circulating liver enzymes and risks of chronic diseases and mortality in the prospective EPIC-Heidelberg case-cohort study. BMJ Open 10, e033532 (2020).
Kim, J. W. et al. Liver function and Alzheimer’s brain pathologies: a longitudinal study: liver and Alzheimer’s pathologies. J. Prev. Alzheimers Dis. 12, 100012 (2025).
Li, C. et al. Serum liver enzymes and risk of stroke: systematic review with meta-analyses and Mendelian randomization studies. Eur. J. Neurol. 31, e16506 (2024).
Cieslak, K. P., Baur, O., Verheij, J., Bennink, R. J. & van Gulik, T. M. Liver function declines with increased age. HPB 18, 691–696 (2016).
Schembri, G. et al. Mebrofenin functional indices in a normal population. J. Nucl. Med. 64, P501 (2023).
McLean, A. J. & Le Couteur, D. G. Aging biology and geriatric clinical pharmacology. Pharmacol. Rev. 56, 163–184 (2004).
Butler, J. M. & Begg, E. J. Free drug metabolic clearance in elderly people. Clin. Pharmacokinet. 47, 297–321 (2008).
Sotaniemi, E. A., Arranto, A. J., Pelkonen, O. & Pasanen, M. Age and cytochrome p450-linked drug metabolism in humans: an analysis of 226 subjects with equal histopathologic conditions. Clin. Pharmacol. Ther. 61, 331–339 (1997).
Lin, L. et al. The burden and trends of primary liver cancer caused by specific etiologies from 1990 to 2017 at the global, regional, national, age, and sex level results from the Global Burden of Disease Study 2017. Liver Cancer 9, 563–582 (2020).
Institute for Health Metrics and Evaluation. Global Burden of Disease Study 2021 (GBD 2021) data resources. IHME https://ghdx.healthdata.org/gbd-2021 (2025).
Villanueva, A. Hepatocellular carcinoma. N. Engl. J. Med. 380, 1450–1462 (2019).
Yi, S. W., Choi, J. S., Yi, J. J., Lee, Y. H. & Han, K. J. Risk factors for hepatocellular carcinoma by age, sex, and liver disorder status: a prospective cohort study in Korea. Cancer 124, 2748–2757 (2018).
He, Y. et al. Emerging role of aging in the progression of NAFLD to HCC. Ageing Res. Rev. 84, 101833 (2023).
Hanahan, D. Hallmarks of cancer: new dimensions. Cancer Discov. 12, 31–46 (2022).
Le Couteur, D. G. & Simpson, S. J. Adaptive senectitude: the prolongevity effects of aging. J. Gerontol. A Biol. Sci. Med. Sci. 66, 179–182 (2011).
Sheedfar, F., Di Biase, S., Koonen, D. & Vinciguerra, M. Liver diseases and aging: friends or foes? Aging Cell 12, 950–954 (2013).
Campisi, J. Aging, cellular senescence, and cancer. Annu. Rev. Physiol. 75, 685–705 (2013).
Abul-Husn, N. S. et al. A protein-truncating HSD17b13 variant and protection from chronic liver disease. N. Engl. J. Med. 378, 1096–1106 (2018).
Hung, A. K. & Guy, J. Hepatocellular carcinoma in the elderly: meta-analysis and systematic literature review. World J. Gastroenterol. 21, 12197–12210 (2015).
Brunot, A., Le Sourd, S., Pracht, M. & Edeline, J. Hepatocellular carcinoma in elderly patients: challenges and solutions. J. Hepatocell. Carcinoma 3, 9–18 (2016).
Stefan, N., Yki-Jarvinen, H. & Neuschwander-Tetri, B. A. Metabolic dysfunction-associated steatotic liver disease: heterogeneous pathomechanisms and effectiveness of metabolism-based treatment. Lancet Diabetes Endocrinol. 13, 134–148 (2025).
Huang, D. Q. et al. Metabolic dysfunction-associated steatotic liver disease in adults. Nat. Rev. Dis. Primers 11, 14 (2025).
Alqahtani, S. A. & Schattenberg, J. M. NAFLD in the elderly. Clin. Interv. Aging 16, 1633–1649 (2021).
Le Couteur, D. G., Raubenheimer, D., Solon-Biet, S., de Cabo, R. & Simpson, S. J. Does diet influence aging? Evidence from animal studies. J. Intern. Med. 295, 400–415 (2024).
Diaz-Ruiz, A., Price, N. L., Ferrucci, L. & de Cabo, R. Obesity and lifespan, a complex tango. Sci. Transl Med. 15, eadh1175 (2023).
Kagansky, N. et al. Non-alcoholic fatty liver disease — a common and benign finding in octogenarian patients. Liver Int. 24, 588–594 (2004).
Malenfant, J. H. & Batsis, J. A. Obesity in the geriatric population — a global health perspective. J. Glob. Health Rep. 3, e2019045 (2019).
Oliveros, E. et al. Hypertension in older adults: assessment, management, and challenges. Clin. Cardiol. 43, 99–107 (2020).
Hashemi, R. et al. High prevalence of comorbidities in older adult patients with type 2 diabetes: a cross-sectional survey. BMC Geriatr. 24, 873 (2024).
Rosada, A. et al. Hyperlipidemias in elderly patients: results from the Berlin Aging Study II (BASEII), a cross-sectional study. Lipids Health Dis. 19, 92 (2020).
Lichtinghagen, R. et al. The enhanced liver fibrosis (ELF) score: normal values, influence factors and proposed cut-off values. J. Hepatol. 59, 236–242 (2013).
Vali, Y. et al. Precision in liver diagnosis: varied accuracy across subgroups and the need for variable thresholds in diagnosis of MASLD. Liver Int. 45, e16240 (2025).
Stine, J. G. & Rinella, M. E. Editorial: age and non-invasive markers of fibrosis in patients with nonalcoholic fatty liver disease: time to adjust the clock? Am. J. Gastroenterol. 112, 752–754 (2017).
Chen, T. P., Lai, M., Lin, W. Y., Huang, K. C. & Yang, K. C. Metabolic profiles and fibrosis of nonalcoholic fatty liver disease in the elderly: a community-based study. J. Gastroenterol. Hepatol. 35, 1636–1643 (2020).
Li, Y., Adeniji, N. T., Fan, W., Kunimoto, K. & Torok, N. J. Non-alcoholic fatty liver disease and liver fibrosis during aging. Aging Dis. 13, 1239–1251 (2022).
Ogrodnik, M. et al. Cellular senescence drives age-dependent hepatic steatosis. Nat. Commun. 8, 15691 (2017).
Ogrodnik, M. & Jurk, D. Senescence explains age- and obesity-related liver steatosis. Cell Stress 1, 70–72 (2017).
Eriksson, S., Fraser, J. R., Laurent, T. C., Pertoft, H. & Smedsrod, B. Endothelial cells are a site of uptake and degradation of hyaluronic acid in the liver. Exp. Cell Res. 144, 223–228 (1983).
Miyao, M. et al. Pivotal role of liver sinusoidal endothelial cells in NAFLD/NASH progression. Lab. Invest. 95, 1130–1144 (2015).
Kus, E. et al. LSEC fenestrae are preserved despite pro-inflammatory phenotype of liver sinusoidal endothelial cells in mice on high fat diet. Front. Physiol. 10, 6 (2019).
McCuskey, R. S. et al. Hepatic microvascular dysfunction during evolution of dietary steatohepatitis in mice. Hepatology 40, 386–393 (2004).
DeLeve, L. D. Liver sinusoidal endothelial cells in hepatic fibrosis. Hepatology 61, 1740–1746 (2015).
Hammoutene, A. et al. A defect in endothelial autophagy occurs in patients with non-alcoholic steatohepatitis and promotes inflammation and fibrosis. J. Hepatol. 72, 528–538 (2020).
Dai, Q. et al. Aging-associated liver sinusoidal endothelial cells dysfunction aggravates the progression of metabolic dysfunction-associated steatotic liver disease. Aging Cell 24, e14502 (2025).
Kim, I. H. et al. Aging increases the susceptibility of hepatic inflammation, liver fibrosis and aging in response to high-fat diet in mice. Age 38, 291–302 (2016).
Maeso-Diaz, R. et al. Aging influences hepatic microvascular biology and liver fibrosis in advanced chronic liver disease. Aging Dis. 10, 684–698 (2019).
Van den Broecke, A. et al. Epidemiology, causes, evolution and outcome in a single-center cohort of 1116 critically ill patients with hypoxic hepatitis. Ann. Intensive Care 8, 15 (2018).
Tapper, E. B., Sengupta, N. & Bonder, A. The incidence and outcomes of ischemic hepatitis: a systematic review with meta-analysis. Am. J. Med. 128, 1314–1321 (2015).
Roedl, K. et al. Occurrence, characteristics, and outcome of hypoxic liver injury among patients aged ≥90 years admitted to the intensive care unit: a retrospective cohort study. Gerontology 69, 728–736 (2023).
Aboelsoud, M. M., Javaid, A. I., Al-Qadi, M. O. & Lewis, J. H. Hypoxic hepatitis — its biochemical profile, causes and risk factors of mortality in critically-ill patients: a cohort study of 565 patients. J. Crit. Care 41, 9–15 (2017).
Rashed, K. A., McNabb, W. R. & Lewis, R. R. Ischaemic hepatitis in the elderly. Gerontology 48, 245–249 (2002).
Martinez, I. et al. The influence of oxygen tension on the structure and function of isolated liver sinusoidal endothelial cells. Comp. Hepatol. 7, 4 (2008).
Le Couteur, D. G. & McLean, A. J. The aging liver. Drug clearance and an oxygen diffusion barrier hypothesis. Clin. Pharmacokinet. 34, 359–373 (1998).
Bjornsson, E. S., Bergmann, O. M., Bjornsson, H. K., Kvaran, R. B. & Olafsson, S. Incidence, presentation, and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology 144, 1419–1425 (2013).
Hoofnagle, J. H. & Navarro, V. J. Drug-induced liver injury: Icelandic lessons. Gastroenterology 144, 1335–1336 (2013).
Hoofnagle, J. H. & Bjornsson, E. S. Drug-induced liver injury — types and phenotypes. N. Engl. J. Med. 381, 264–273 (2019).
Scott, I. A. et al. Reducing inappropriate polypharmacy: the process of deprescribing. JAMA Intern. Med. 175, 827–834 (2015).
Dayoub, J. C., Cortese, F., Anzic, A., Grum, T. & de Magalhaes, J. P. The effects of donor age on organ transplants: a review and implications for aging research. Exp. Gerontol. 110, 230–240 (2018).
Sakai, Y., Zhong, R., Garcia, B., Zhu, L. & Wall, W. J. Assessment of the longevity of the liver using a rat transplant model. Hepatology 25, 421–425 (1997).
Wu, B. J. et al. The virtual 4Ms: a novel curriculum for first year health professional students during COVID-19. J. Am. Geriatr. Soc. 69, E13–E16 (2021).
Fried, L. P. et al. The physical frailty syndrome as a transition from homeostatic symphony to cacophony. Nat. Aging 1, 36–46 (2021).
Rodriguez-Manas, L. & Fried, L. P. Frailty in the clinical scenario. Lancet 385, e7–e9 (2015).
Kim, D. H. & Rockwood, K. Frailty in older adults. N. Engl. J. Med. 391, 538–548 (2024).
Lai, J. C. et al. Development of a novel frailty index to predict mortality in patients with end-stage liver disease. Hepatology 66, 564–574 (2017).
Jutras, G. & Lai, J. C. The liver frailty index: a model for establishing organ-specific frailty metrics across all solid organ transplantation. Curr. Opin. Organ Transplant. 29, 266–270 (2024).
Collard, R. M., Boter, H., Schoevers, R. A. & Oude Voshaar, R. C. Prevalence of frailty in community-dwelling older persons: a systematic review. J. Am. Geriatr. Soc. 60, 1487–1492 (2012).
Gnjidic, D. et al. High-risk prescribing and incidence of frailty among older community-dwelling men. Clin. Pharmacol. Ther. 91, 521–528 (2012).
Kirk, B. et al. The conceptual definition of sarcopenia: Delphi consensus from the Global Leadership Initiative in Sarcopenia (GLIS). Age Ageing 53, e14502 (2024).
Le Couteur, D. G. et al. Sarcopenic obesity and amino acids: Cconcord Health and Ageing in Men Project. J. Gerontol. A Biol. Sci. Med. Sci. 76, 1000–1004 (2021).
Stankevicius, C. et al. Sarcopenia as a risk factor for mortality in NAFLD: how should we diagnose it? J. Dig. Dis. 25, 645–654 (2024).
Zambon Azevedo, V. et al. Impact of sarcopenia on the severity of the liver damage in patients with non-alcoholic fatty liver disease. Front. Nutr. 8, 774030 (2021).
Iwaki, M. et al. Impact of sarcopenia on non-alcoholic fatty liver disease. Nutrients 15, 891 (2023).
Markakis, G. E. et al. Sarcopenia as a predictor of survival and complications of patients with cirrhosis after liver transplantation: a systematic review and meta-analysis. Clin. Transplant. 39, e70088 (2025).
Liao, Y. Sarcopenia with muscle wasting in hepatic cancer predicts therapeutic outcome after hepatic artery intervention. Int. J. Clin. Pharmacol. Ther. 63, 70–76 (2025).
Mikolasevic, I. et al. Nonalcoholic fatty liver disease and sarcopenia: where do we stand? Can. J. Gastroenterol. Hepatol. 2020, 8859719 (2020).
Skou, S. T. et al. Multimorbidity. Nat. Rev. Dis. Primers 8, 48 (2022).
Moreno-Juste, A. et al. Multimorbidity in patients with chronic liver disease: a population-based study in the Epichron Cohort, Spain. J. Clin. Med. 13, 7198 (2024).
Gnjidic, D. et al. Polypharmacy cutoff and outcomes: five or more medicines were used to identify community-dwelling older men at risk of different adverse outcomes. J. Clin. Epidemiol. 65, 989–995 (2012).
Winardi, K. et al. Chronic polypharmacy, monotherapy, and deprescribing: understanding complex effects on the hepatic proteome of aging mice. Aging Cell 24, e14357 (2025).
Wu, H. et al. Comparing effects of polypharmacy on inflammatory profiles in older adults and mice: implications for translational aging research. J. Gerontol. A Biol. Sci. Med. Sci. 77, 1295–1303 (2022).
Hayward, K. L. et al. Changing prevalence of medication use in people with cirrhosis: a retrospective cohort study using Pharmaceutical Benefits Scheme data. Drugs Real World Outcomes 10, 605–618 (2023).
Lee, B. T., Odin, J. A. & Grewal, P. An approach to drug-induced liver injury from the geriatric perspective. Curr. Gastroenterol. Rep. 23, 6 (2021).
Hayward, K. L. & Weersink, R. A. Improving medication-related outcomes in chronic liver disease. Hepatol. Commun. 4, 1562–1577 (2020).
Longbotham, D. et al. The impact of age on post-operative liver function following right hepatectomy: a retrospective, single centre experience. HPB 22, 151–160 (2020).
Ruzzenente, A. et al. Impact of age on short-term outcomes of liver surgery: lessons learned in 10-years’ experience in a tertiary referral hepato-pancreato-biliary center. Medicine 96, e6955 (2017).
Joliat, G. R. et al. Guidelines for perioperative care for liver surgery: Enhanced Recovery After Surgery (ERAS) Society recommendations 2022. World J. Surg. 47, 11–34 (2023).
Thillainadesan, J., Yumol, M. F., Hilmer, S., Aitken, S. J. & Naganathan, V. Interventions to improve clinical outcomes in older adults admitted to a surgical service: a systematic review and meta-analysis. J. Am. Med. Dir. Assoc. 21, 1833–1843.e20 (2020).
Jung, I. et al. Association of metabolic dysfunction-associated fatty liver disease with white matter hyperintensity and cognitive decline: a longitudinal cohort study. Diabetes Obes. Metab. 27, 2271–2279 (2025).
Wang, L., Sang, B. & Zheng, Z. Risk of dementia or cognitive impairment in non-alcoholic fatty liver disease: a systematic review and meta-analysis. Front. Aging Neurosci. 14, 985109 (2022).
Hunt, N. J. et al. Targeting the liver in dementia and cognitive impairment: dietary macronutrients and diabetic therapeutics. Adv. Drug Deliv. Rev. 190, 114537 (2022).
Pinheiro, F. I. et al. Hepatopancreatic metabolic disorders and their implications in the development of Alzheimer’s disease and vascular dementia. Ageing Res. Rev. 96, 102250 (2024).
Kciuk, M. et al. Alzheimer’s disease as type 3 diabetes: understanding the link and implications. Int. J. Mol. Sci. 25, 11955 (2024).
Pujol, A. et al. Metabolic-associated fatty liver disease and cognitive performance in type 2 diabetes: basal data from the Phytate, Neurodegeneration and diabetes (PHYND) study. Biomedicines 12, 1993 (2024).
Tsoy, A., Umbayev, B., Kassenova, A., Kaupbayeva, B. & Askarova, S. Pathology of amyloid-β (aβ) peptide peripheral clearance in Alzheimer’s disease. Int. J. Mol. Sci. 25, 10964 (2024).
Lam, V. et al. Synthesis of human amyloid restricted to liver results in an Alzheimer disease-like neurodegenerative phenotype. PLoS Biol. 19, e3001358 (2021).
Bassendine, M. F., Taylor-Robinson, S. D., Fertleman, M., Khan, M. & Neely, D. Is Alzheimer’s disease a liver disease of the brain? J. Alzheimers Dis. 75, 1–14 (2020).
Cha, W. J. et al. Association between brain amyloid deposition and longitudinal changes of white matter hyperintensities. Alzheimers Res. Ther. 16, 50 (2024).
Kaur, A., Rohit & Aran, K. R. Unraveling the dual role of bilirubin in neurological diseases: a comprehensive exploration of its neuroprotective and neurotoxic effects. Brain Res. 1851, 149472 (2025).
Silvey, S. et al. A possible reversible cause of cognitive impairment: undiagnosed cirrhosis and potential hepatic encephalopathy in patients with dementia. Am. J. Med. 137, 1082–1087.e1 (2024).
Cogger, V. C. et al. Dietary macronutrients and the aging liver sinusoidal endothelial cell. Am. J. Physiol. Heart Circ. Physiol. 310, H1064–H1070 (2016).
O’Reilly, J. N., Cogger, V. C., Fraser, R. & Le Couteur, D. G. The effect of feeding and fasting on fenestrations in the liver sinusoidal endothelial cell. Pathology 42, 255–258 (2010).
Jamieson, H. A. et al. Caloric restriction reduces age-related pseudocapillarization of the hepatic sinusoid. Exp. Gerontol. 42, 374–378 (2007).
Bartke, A. & Darcy, J. GH and ageing: pitfalls and new insights. Best Pract. Res. Clin. Endocrinol. Metab. 31, 113–125 (2017).
Salminen, A., Kauppinen, A. & Kaarniranta, K. FGF21 activates AMPK signaling: impact on metabolic regulation and the aging process. J. Mol. Med. 95, 123–131 (2017).
Gesing, A. et al. A long-lived mouse lacking both growth hormone and growth hormone receptor: a new animal model for aging studies. J. Gerontol. A Biol. Sci. Med. Sci. 72, 1054–1061 (2017).
Sinclair, D. A. A bile acid could explain how calorie restriction slows ageing. Nature 643, 38–40 (2025).
Qu, Q. et al. Lithocholic acid phenocopies anti-ageing effects of calorie restriction. Nature 643, 192–200 (2025).
Qu, Q. et al. Lithocholic acid binds TULP3 to activate sirtuins and AMPK to slow down ageing. Nature 643, 201–209 (2025).
Baur, J. A. et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature 444, 337–342 (2006).
Pearson, K. J. et al. Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span. Cell Metab. 8, 157–168 (2008).
Labbe, A. et al. Resveratrol improves insulin resistance hyperglycemia and hepatosteatosis but not hypertriglyceridemia, inflammation, and life span in a mouse model for werner syndrome. J. Gerontol. A Biol. Sci. Med. Sci. 66, 264–278 (2011).
Pfluger, P. T., Herranz, D., Velasco-Miguel, S., Serrano, M. & Tschop, M. H. Sirt1 protects against high-fat diet-induced metabolic damage. Proc. Natl Acad. Sci. USA 105, 9793–9798 (2008).
Luo, X. et al. Sirtuin 1 ameliorates defenestration in hepatic sinusoidal endothelial cells during liver fibrosis via inhibiting stress-induced premature senescence. Cell Prolif. 54, e12991 (2021).
Le Couteur, D. G. & Barzilai, N. New horizons in life extension, healthspan extension and exceptional longevity. Age Ageing 51, afac156 (2022).
Perazza, F. et al. Metformin and the liver: unlocking the full therapeutic potential. Metabolites 14, 186 (2024).
Martin-Montalvo, A. et al. Metformin improves healthspan and lifespan in mice. Nat. Commun. 4, 2192 (2013).
Alfaras, I. et al. Health benefits of late-onset metformin treatment every other week in mice. npj Aging Mech. Dis. 3, 16 (2017).
Keys, M. T. et al. Reassessing the evidence of a survival advantage in type 2 diabetes treated with metformin compared with controls without diabetes: a retrospective cohort study. Int. J. Epidemiol. 51, 1886–1898 (2022).
Barzilai, N., Crandall, J. P., Kritchevsky, S. B. & Espeland, M. A. Metformin as a tool to target aging. Cell Metab. 23, 1060–1065 (2016).
Chen, H. P. et al. Metformin decreases hepatocellular carcinoma risk in a dose-dependent manner: population-based and in vitro studies. Gut 62, 606–615 (2013).
McCay, C., Crowell, M. & Maynard, L. The effect of retarded growth upon the length of life span and upon the ultimate body size. J. Nutr. 10, 63–79 (1935).
Ingram, D. K. & de Cabo, R. Calorie restriction in rodents: caveats to consider. Ageing Res. Rev. 39, 15–28 (2017).
Mattison, J. A. et al. Caloric restriction improves health and survival of rhesus monkeys. Nat. Commun. 8, 14063 (2017).
Derous, D. et al. The effects of graded levels of calorie restriction: XI. Evaluation of the main hypotheses underpinning the life extension effects of cr using the hepatic transcriptome. Aging 9, 1770–1824 (2017).
de Sousa, D. J. M. et al. Dietary restriction and hepatic cancer: systematic review and meta-analysis of animal studies. Crit. Rev. Oncol. Hematol. 196, 104264 (2024).
Serra, M., Marongiu, F., Pisu, M. G., Serra, M. & Laconi, E. Time-restricted feeding delays the emergence of the age-associated, neoplastic-prone tissue landscape. Aging 11, 3851–3863 (2019).
Haigh, L. et al. The effectiveness and acceptability of Mediterranean diet and calorie restriction in non-alcoholic fatty liver disease (NAFLD): a systematic review and meta-analysis. Clin. Nutr. 41, 1913–1931 (2022).
Simpson, S. J. & Raubenheimer, D. The Nature of Nutrition: A Unifying Framework from Animal Adaptation to Human Obesity (Princeton Univ. Press, 2012).
Solon-Biet, S. M. et al. The ratio of macronutrients, not caloric intake, dictates cardiometabolic health, aging, and longevity in ad libitum-fed mice. Cell Metab. 19, 418–430 (2014).
Gokarn, R. et al. The relationship between dietary macronutrients and hepatic telomere length in aging mice. J. Gerontol. A Biol. Sci. Med. Sci. 73, 446–449 (2018).
Levine, M. E. et al. Low protein intake is associated with a major reduction in IGF-1, cancer, and overall mortality in the 65 and younger but not older population. Cell Metab. 19, 407–417 (2014).
Liao, Y. et al. Amino acid is a major carbon source for hepatic lipogenesis. Cell Metab. 36, 2437–2448.e8 (2024).
Zeng, X. F. et al. The role of dietary modification in the prevention and management of metabolic dysfunction-associated fatty liver disease: an international multidisciplinary expert consensus. Metabolism 161, 156028 (2024).
Honfo, S. H. et al. Evidence for protein leverage on total energy intake, but not body mass index, in a large cohort of older adults. Int. J. Obes. 48, 654–661 (2024).
Raubenheimer, D. & Simpson, S. J. Protein leverage: theoretical foundations and ten points of clarification. Obesity 27, 1225–1238 (2019).
Pibiri, M. Liver regeneration in aged mice: new insights. Aging 10, 1801–1824 (2018).
Maldonado-Rengel, R., Socola-Barsallo, Z. & Vasquez, B. Alterations of liver morphology in senescent rats. Int. J. Mol. Sci. 25, 9846 (2024).
Wang, M. J., Chen, F., Lau, J. T. Y. & Hu, Y. P. Hepatocyte polyploidization and its association with pathophysiological processes. Cell Death Dis. 8, e2805 (2017).
Lysek-Gladysinska, M. et al. Aging-related changes in the ultrastructure of hepatocytes and cardiomyocytes of elderly mice are enhanced in ApoE-deficient animals. Cells 10, 502 (2021).
Zhang, C. & Cuervo, A. M. Restoration of chaperone-mediated autophagy in aging liver improves cellular maintenance and hepatic function. Nat. Med. 14, 959–965 (2008).
Sun, W. B. et al. Effect of aging on cytoskeleton system of Kupffer cell and its phagocytic capacity. World J. Gastroenterol. 4, 77–79 (1998).
de Leeuw, A. M., Brouwer, A., Barelds, R. J. & Knook, D. L. Maintenance cultures of Kupffer cells isolated from rats of various ages: ultrastructure, enzyme cytochemistry, and endocytosis. Hepatology 3, 497–506 (1983).
Saito, Y., Morine, Y. & Shimada, M. Mechanism of impairment on liver regeneration in elderly patients: role of hepatic stellate cell function. Hepatol. Res. 47, 505–513 (2017).
Pinto, C., Ninfole, E., Benedetti, A., Maroni, L. & Marzioni, M. Aging-related molecular pathways in chronic cholestatic conditions. Front. Med. 6, 332 (2019).
Karaman, H. et al. Investigation of genome instability in patients with non-alcoholic steatohepatitis. World J. Gastroenterol. 19, 5295–5301 (2013).
Akazawa, Y. et al. Detection of DNA damage response in nonalcoholic fatty liver disease via p53-binding protein 1 nuclear expression. Mod. Pathol. 32, 997–1007 (2019).
Ningarhari, M. et al. Telomere length is key to hepatocellular carcinoma diversity and telomerase addiction is an actionable therapeutic target. J. Hepatol. 74, 1155–1166 (2021).
In der Stroth, L., Tharehalli, U., Gunes, C. & Lechel, A. Telomeres and telomerase in the development of liver cancer. Cancers 12, 2048 (2020).
Tang, L. et al. The association between telomere length and non-alcoholic fatty liver disease: a prospective study. BMC Med. 21, 427 (2023).
Bacalini, M. G. et al. Molecular aging of human liver: an epigenetic/transcriptomic signature. J. Gerontol. A Biol. Sci. Med. Sci. 74, 1–8 (2019).
Fu, S., Debes, J. D. & Boonstra, A. DNA methylation markers in the detection of hepatocellular carcinoma. Eur. J. Cancer 191, 112960 (2023).
Vachher, M., Bansal, S., Kumar, B., Yadav, S. & Burman, A. Deciphering the role of aberrant DNA methylation in NAFLD and NASH. Heliyon 8, e11119 (2022).
Loomba, R. et al. DNA methylation signatures reflect aging in patients with nonalcoholic steatohepatitis. JCI Insight 3, e96685 (2018).
Negroni, L. et al. Integrative quantitative proteomics unveils proteostasis imbalance in human hepatocellular carcinoma developed on nonfibrotic livers. Mol. Cell Proteom. 13, 3473–3483 (2014).
He, Q. J. et al. Recent advances in age-related metabolic dysfunction-associated steatotic liver disease. World J. Gastroenterol. 30, 652–662 (2024).
Schneider, J. L. & Cuervo, A. M. Liver autophagy: much more than just taking out the trash. Nat. Rev. Gastroenterol. Hepatol. 11, 187–200 (2014).
Yang, S. et al. New insights into autophagy in hepatocellular carcinoma: mechanisms and therapeutic strategies. Am. J. Cancer Res. 9, 1329–1353 (2019).
Han, X. et al. Nicotinamide riboside exerts protective effect against aging-induced NAFLD-like hepatic dysfunction in mice. PeerJ 7, e7568 (2019).
Su, W. W. et al. Association of circulating insulin-like growth factor 1 with hepatocellular carcinoma: one cross-sectional correlation study. J. Clin. Lab. Anal. 24, 195–200 (2010).
Ge, S. et al. PSME4 activates mTOR signaling and promotes the malignant progression of hepatocellular carcinoma. Int. J. Gen. Med. 15, 885–895 (2022).
Chen, G., Li, M. Y., Yang, J. Y. & Zhou, Z. H. Will AMPK be a potential therapeutic target for hepatocellular carcinoma? Am. J. Cancer Res. 14, 3241–3258 (2024).
Shrestha, R. et al. Complete inhibition of liver acetyl-CoA carboxylase activity is required to exacerbate liver tumorigenesis in mice treated with diethylnitrosamine. Cancer Metab. 12, 34 (2024).
Zhao, P. et al. An AMPK-caspase-6 axis controls liver damage in nonalcoholic steatohepatitis. Science 367, 652–660 (2020).
Purushotham, A. et al. Hepatocyte-specific deletion of SIRT1 alters fatty acid metabolism and results in hepatic steatosis and inflammation. Cell Metab. 9, 327–338 (2009).
Marcondes-de-Castro, I. A., Reis-Barbosa, P. H., Marinho, T. S., Aguila, M. B. & Mandarim-de-Lacerda, C. A. AMPK/mTOR pathway significance in healthy liver and non-alcoholic fatty liver disease and its progression. J. Gastroenterol. Hepatol. 38, 1868–1876 (2023).
Muller-Hocker, J. et al. Defects of the respiratory chain in the normal human liver and in cirrhosis during aging. Hepatology 26, 709–719 (1997).
Zeng, L., Zhu, L., Fu, S., Li, Y. & Hu, K. Mitochondrial dysfunction-molecular mechanisms and potential treatment approaches of hepatocellular carcinoma. Mol. Cell Biochem. 480, 2131–2142 (2025).
Perez-Carreras, M. et al. Defective hepatic mitochondrial respiratory chain in patients with nonalcoholic steatohepatitis. Hepatology 38, 999–1007 (2003).
Allaire, M. & Gilgenkrantz, H. The aged liver: beyond cellular senescence. Clin. Res. Hepatol. Gastroenterol. 44, 6–11 (2020).
Schulte, L. A., Lopez-Gil, J. C., Sainz, B. Jr. & Hermann, P. C. The cancer stem cell in hepatocellular carcinoma. Cancers 12, 127–140 (2020).
Gu, L. et al. FBP1 controls liver cancer evolution from senescent MASH hepatocytes. Nature 637, 461–469 (2025).
Hora, S. & Wuestefeld, T. Liver injury and regeneration: current understanding, new approaches, and future perspectives. Cells 12, 2129 (2023).
Lee, T. K., Guan, X. Y. & Ma, S. Cancer stem cells in hepatocellular carcinoma — from origin to clinical implications. Nat. Rev. Gastroenterol. Hepatol. 19, 26–44 (2022).
Nobili, V. et al. Hepatic progenitor cells activation, fibrosis, and adipokines production in pediatric nonalcoholic fatty liver disease. Hepatology 56, 2142–2153 (2012).
Li, Q. et al. Sirt1 promotes the restoration of hepatic progenitor cell (HPC)-mediated liver fatty injury in NAFLD through activating the Wnt/β-catenin signal pathway. Front. Nutr. 8, 791861 (2021).
Chiang, C. H. et al. Decreased circulating endothelial progenitor cell levels and function in patients with nonalcoholic fatty liver disease. PLoS ONE 7, e31799 (2012).
Wang, W. T., Jin, W. L. & Li, X. Intercellular communication in the tumour microecosystem: mediators and therapeutic approaches for hepatocellular carcinoma. Biochim. Biophys. Acta Mol. Basis Dis. 1868, 166528 (2022).
Marrone, G., Shah, V. H. & Gracia-Sancho, J. Sinusoidal communication in liver fibrosis and regeneration. J. Hepatol. 65, 608–617 (2016).
Wang, S. et al. Metabolic disorders, inter-organ crosstalk, and inflammation in the progression of metabolic dysfunction-associated steatotic liver disease. Life Sci. 359, 123211 (2024).
Karin, M. New insights into the pathogenesis and treatment of non-viral hepatocellular carcinoma: a balancing act between immunosuppression and immunosurveillance. Precis. Clin. Med. 1, 21–28 (2018).
Barsch, M. et al. T-cell exhaustion and residency dynamics inform clinical outcomes in hepatocellular carcinoma. J. Hepatol. 77, 397–409 (2022).
Ruf, B., Heinrich, B. & Greten, T. F. Immunobiology and immunotherapy of HCC: spotlight on innate and innate-like immune cells. Cell Mol. Immunol. 18, 112–127 (2021).
Sawada, K., Chung, H., Softic, S., Moreno-Fernandez, M. E. & Divanovic, S. The bidirectional immune crosstalk in metabolic dysfunction-associated steatotic liver disease. Cell Metab. 35, 1852–1871 (2023).
Trivedi, Y. et al. The role of gut microbiome in hepatocellular carcinoma: a systematic review. Cureus 15, e43862 (2023).
Forlano, R. et al. Disruption of gut barrier integrity and host–microbiome interactions underlie MASLD severity in patients with type-2 diabetes mellitus. Gut Microbes 16, 2304157 (2024).
Acknowledgements
The authors acknowledge funding by the Australian National and Health Medical Research Council (Investigator Grant no. 2025511 (to D.G.L.C.) and Programme Grant no. 1149976 (to S.J.S. and D.G.L.C.)) and the MRFF Targeted Translation Research Accelerator Program (to N.J.H., V.C.C. and D.G.L.C.).
Author information
Authors and Affiliations
Contributions
All authors researched data for the article. All authors contributed substantially to discussion of the content. D.G.L.C. and V.C.C. wrote the article. All authors reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
D.G.L.C. has received consultancy fees via the Reimbursement Advisory Expert Panel (REAP) for independent pre-submission advice to pharmaceutical companies on regulatory and funding applications to government. D.G.L.C., N.J.H. and V.C.C. are founder members and N.J.H. is the CEO of a start-up company (Endo Axiom) with Sydney University, Sydney Local Health District and Proto Axiom, which hold patents on nanomedicines for the treatment and prevention of diabetes mellitus. The other authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Gastroenterology & Hepatology thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Le Couteur, D.G., Ngu, M.C., Hunt, N.J. et al. Liver, ageing and disease. Nat Rev Gastroenterol Hepatol 22, 680–695 (2025). https://doi.org/10.1038/s41575-025-01099-z
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41575-025-01099-z