Abstract
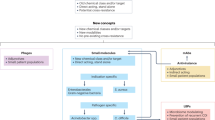
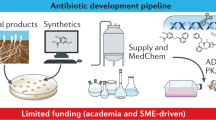
Antibacterial resistance is a global challenge that requires a coordinated international response. The current clinical pipeline largely consists of derivatives of established antibiotic classes, whereas the discovery and preclinical pipeline is diverse and innovative including new direct-acting agents with no cross-resistance with existing antibiotics. These novel compounds target pathways such as lipoprotein synthesis, lipopolysaccharide biosynthesis and transport, outer membrane assembly, peptidoglycan biosynthesis, fatty acid biosynthesis and isoprenoid biosynthesis. If these agents can be developed into safe, effective and affordable drugs, they could address a broad range of infections worldwide, benefiting large patient populations without geographical limitations. However, strategies such as indirect-acting or pathogen-specific treatments are likely to benefit small patient groups, primarily in high-income countries that have advanced health-care systems and diagnostic infrastructure. Although encouraging, the discovery and preclinical pipeline remains insufficiently robust to offset the high attrition rates typical of early-stage drug innovation and to meet global health needs.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Coque, T. M., Cantón, R., Pérez-Cobas, A. E., Fernández-de-Bobadilla, M. D. & Baquero, F. Antimicrobial resistance in the Global Health Network: known unknowns and challenges for efficient responses in the 21st century. Microorganisms 11, 1050 (2023).
Hernando-Amado, S., Coque, T. M., Baquero, F. & Martínez, J. L. Antibiotic resistance: moving from individual health norms to social norms in One Health and Global Health. Front. Microbiol. 11, 1914 (2020).
Okeke, I. N. et al. The scope of the antimicrobial resistance challenge. Lancet 403, 2426–2438 (2024).
Murray, C. J. L. et al. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet 399, 629–655 (2022).
Zhou, N. et al. Global antimicrobial resistance: a system-wide comprehensive investigation using the Global One Health Index. Infect. Dis. Poverty 11, 92 (2022).
Naghavi, M. et al. Global burden of bacterial antimicrobial resistance 1990–2021: a systematic analysis with forecasts to 2050. Lancet 404, 1199–1226 (2024).
Brown, D. R., Henderson, H. I., Ruegsegger, L., Moody, J. & van Duin, D. Socioeconomic disparities in the prevalence of multidrug resistance in Enterobacterales. Infect. Control Hosp. Epidemiol. 44, 2068–2070 (2023).
Alividza, V. et al. Investigating the impact of poverty on colonization and infection with drug-resistant organisms in humans: a systematic review. Infect. Dis. Poverty 7, 76 (2018).
Pallett, S. J. C. et al. The contribution of human conflict to the development of antimicrobial resistance. Commun. Med. 3, 153 (2023).
Li, W. et al. Association between antibiotic resistance and increasing ambient temperature in China: an ecological study with nationwide panel data. Lancet Reg. Health West. Pac. 30, 100628 (2023).
Muloi, D. M. et al. Exploiting genomics for antimicrobial resistance surveillance at One Health interfaces. Lancet Microbe 4, e1056–e1062 (2023).
Burnham, J. P. Climate change and antibiotic resistance: a deadly combination. Ther. Adv. Infect. Dis. 8, 204993612199137 (2021).
Laxminarayan, R. et al. Expanding antibiotic, vaccine, and diagnostics development and access to tackle antimicrobial resistance. Lancet 403, 2534–2550 (2024).
Payne, D. J., Gwynn, M. N., Holmes, D. J. & Pompliano, D. L. Drugs for bad bugs: confronting the challenges of antibacterial discovery. Nat. Rev. Drug Discov. 6, 29–40 (2007).
Tommasi, R., Brown, D. G., Walkup, G. K., Manchester, J. I. & Miller, A. A. ESKAPEing the labyrinth of antibacterial discovery. Nat. Rev. Drug Discov. 14, 529–542 (2015).
Tommasi, R., Iyer, R. & Miller, A. A. Antibacterial drug discovery: some assembly required. ACS Infect. Dis. 4, 686–695 (2018).
Yao, J. & Rock, C. O. Bacterial fatty acid metabolism in modern antibiotic discovery. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 1862, 1300–1309 (2017).
Baquero, F. et al. Allogenous selection of mutational collateral resistance: old drugs select for new resistance within antibiotic families. Front. Microbiol. 12, 757833 (2021).
Alsayed, S. S. R. & Gunosewoyo, H. Tuberculosis: pathogenesis, current treatment regimens and new drug targets. Int. J. Mol. Sci. 24, 5202 (2023).
WHO. WHO antibacterial preclinical pipeline review. https://cdn.who.int/media/docs/default-source/global-observatory-on-health-r-d/who_preclinical_antibacterial_products_data_2023.xlsx?sfvrsn=4868221f_1 (WHO, 2024).
Magiorakos, A.-P. et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 18, 268–281 (2012).
Karakonstantis, S., Rousaki, M., Vassilopoulou, L. & Kritsotakis, E. I. Global prevalence of cefiderocol non-susceptibility in Enterobacterales, Pseudomonas aeruginosa, Acinetobacter baumannii, and Stenotrophomonas maltophilia: a systematic review and meta-analysis. Clin. Microbiol. Infect. 30, 178–188 (2024).
Theuretzbacher, U. Evaluating the innovative potential of the global antibacterial pipeline. Clin. Microbiol. Infect., https://doi.org/10.1016/j.cmi.2023.09.024 (2023).
Iqbal, Z. et al. Recent developments to cope the antibacterial resistance via β-lactamase inhibition. Molecules 27, 3832 (2022).
Alfei, S. & Schito, A. M. β-lactam antibiotics and β-lactamase enzymes inhibitors, Part 2: our limited resources. Pharmaceuticals 15, 476 (2022).
Coleman, K. Diazabicyclooctanes (DBOs): a potent new class of non-β-lactam β-lactamase inhibitors. Curr. Opin. Microbiol. 14, 550–555 (2011).
Vázquez-Ucha, J. C., Arca-Suárez, J., Bou, G. & Beceiro, A. New carbapenemase inhibitors: clearing the way for the β-lactams. Int. J. Mol. Sci. 21, 9308 (2020).
Ranjitkar, S. et al. Identification of mutations in the mrdA gene encoding PBP2 that reduce carbapenem and diazabicyclooctane susceptibility of Escherichia coli clinical isolates with mutations in ftsI (PBP3) and which carry bla NDM-1. mSphere 4, 00074-19 (2019).
Durand-Reville, T. F. et al. Rational design of a new antibiotic class for drug-resistant infections. Nature 597, 698–702 (2021).
Nägeli, M., Rodriguez, S., Manson, A. L., Earl, A. M. & Brennan-Krohn, T. Rapid emergence of resistance to broad-spectrum direct antimicrobial activity of avibactam. Preprint at bioRxiv https://doi.org/10.1101/2024.09.25.615047 (2024).
Mojica, M. F., Rossi, M.-A., Vila, A. J. & Bonomo, R. A. The urgent need for metallo-β-lactamase inhibitors: an unattended global threat. Lancet Infect. Dis. 22, e28–e34 (2022).
Bush, K. Game changers: new β-lactamase inhibitor combinations targeting antibiotic resistance in Gram-negative bacteria. ACS Infect. Dis. 4, 84–87 (2018).
Ooi, N. et al. Restoring carbapenem efficacy: a novel carbapenem companion targeting metallo-β-lactamases in carbapenem-resistant Enterobacterales. Antimicrob. Chemother. 76, 460–466 (2021).
Bertonha, A. F., Silva, C. C. L., Shirakawa, K. T., Trindade, D. M. & Dessen, A. Penicillin-binding protein (PBP) inhibitor development: a 10-year chemical perspective. Exp. Biol. Med. 248, 1657–1670 (2023).
Jacobs, L. M. C., Consol, P. & Chen, Y. Drug discovery in the field of β-lactams: an academic perspective. Antibiotics 13, 59 (2024).
Uehara, T. et al. A new class of penicillin-binding protein inhibitors to address drug-resistant Neisseria gonorrhoeae. Gordon Research Conference 2024: Bacterial Cell Surfaces https://venatorx.com/wp-content/uploads/2024/08/GRC_2024_poster_NgPBPi_Uehara-final.pdf (2024).
Theuretzbacher, U., Blasco, B., Duffey, M. & Piddock, L. J. V. Unrealized targets in the discovery of antibiotics for Gram-negative bacterial infections. Nat. Rev. Drug Discov. 22, 957–975 (2023).
Konovalova, A., Kahne, D. E. & Silhavy, T. J. Outer membrane biogenesis. Annu. Rev. Microbiol. 71, 539–556 (2017).
Tang, X. et al. Structural basis for bacterial lipoprotein relocation by the transporter LolCDE. Nat. Struct. Mol. Biol. 28, 347–355 (2021).
Bei, W. et al. Cryo-EM structures of LolCDE reveal the molecular mechanism of bacterial lipoprotein sorting in Escherichia coli. PLoS Biol. 20, e3001823 (2022).
Breidenstein, E. B. M. et al. SMT-738: a novel small-molecule inhibitor of bacterial lipoprotein transport targeting Enterobacteriaceae. Antimicrob. Agents Chemother. 68, e0069523 (2023).
Liu, G. et al. Deep learning-guided discovery of an antibiotic targeting Acinetobacter baumannii. Nat. Chem. Biol. 19, 1342–1350 (2023).
Muñoz, K. A. et al. A Gram-negative-selective antibiotic that spares the gut microbiome. Nature 630, 429–436 (2024).
Sharma, S. et al. Mechanism of LolCDE as a molecular extruder of bacterial triacylated lipoproteins. Nat. Commun. 12, 4687 (2021).
Tao, K., Narita, S., Okada, U., Murakami, S. & Tokuda, H. Dissection of an ABC transporter LolCDE function analyzed by photo-crosslinking. J. Biochem. 175, 427–437 (2024).
McLeod, S. M. et al. Small-molecule inhibitors of Gram-negative lipoprotein trafficking discovered by phenotypic screening. J. Bacteriol. 197, 1075–1082 (2015).
Nickerson, N. N. et al. A novel inhibitor of the LolCDE ABC transporter essential for lipoprotein trafficking in Gram-negative bacteria. Antimicrob. Agents Chemother. 62, e02151-17 (2018).
Schuster, M. et al. Peptidomimetic antibiotics disrupt the lipopolysaccharide transport bridge of drug-resistant Enterobacteriaceae. Sci. Adv. 9, eadg3683 (2023).
Pahil, K. S. et al. A new antibiotic traps lipopolysaccharide in its intermembrane transporter. Nature 625, E27 (2024).
Zampaloni, C. et al. A novel antibiotic class targeting the lipopolysaccharide transporter. Nature 623, 566–571 (2024).
Seyfert, C. E. et al. New genetically engineered derivatives of antibacterial darobactins underpin their potential for antibiotic development. J. Med. Chem. 66, 16330–16341 (2023).
Kaur, H. et al. The antibiotic darobactin mimics a β-strand to inhibit outer membrane insertase. Nature 593, 125–129 (2021).
Groß, S. et al. Improved broad-spectrum antibiotics against Gram-negative pathogens via darobactin biosynthetic pathway engineering. Chem. Sci. 12, 11882–11893 (2021).
Niu, Z. et al. Small molecule LpxC inhibitors against Gram-negative bacteria: advances and future perspectives. Eur. J. Med. Chem. 253, 115326 (2023).
Fujita, K. et al. TP0586532, a non-hydroxamate LpxC inhibitor, has in vitro and in vivo antibacterial activities against Enterobacteriaceae. J. Antibiot. 75, 98–107 (2022).
Di Leo, R., Cuffaro, D., Rossello, A. & Nuti, E. Bacterial zinc metalloenzyme inhibitors: recent advances and future perspectives. Molecules 28, 4378 (2023).
Romano, K. P. & Hung, D. T. Targeting LPS biosynthesis and transport in Gram-negative bacteria in the era of multi-drug resistance. Biochim. Biophys. Acta Mol. Cell Res. 1870, 119407 (2023).
Zhao, J. et al. Preclinical safety and efficacy characterization of an LpxC inhibitor against Gram-negative pathogens. Sci. Transl. Med. 15, eadf5668 (2023).
Mungula, J. et al. Advanced preclinical in vitro and in vivo characterization of a novel, non-hydroxamate-based LpxC inhibitor for the intravenous and oral treatment of multidrug-resistant Enterobacterales. Gordon Research Conference New Antibacterial Discovery and Development (2024).
Crunkhorn, S. LpxC inhibitor eliminates bacterial infections. Nat. Rev. Drug Discov. 22, 787 (2023).
Krause, K. M. et al. Potent LpxC Inhibitors with in vitro activity against multidrug-resistant Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 63, e00977-19 (2019).
Karthikeyan, D., Kumar, S. & Jayaprakash, N. S. A comprehensive review of recent developments in the Gram-negative bacterial UDP-2,3-diacylglucosamine hydrolase (LpxH) enzyme. Int. J. Biol. Macromol. 267, 131327 (2024).
Wang, H. et al. Cerastecins inhibit membrane lipooligosaccharide transport in drug-resistant Acinetobacter baumannii. Nat. Microbiol. 9, 1244–1255 (2024).
Huseby, D. L. et al. Antibiotic class with potent in vivo activity targeting lipopolysaccharide synthesis in Gram-negative bacteria. Proc. Natl Acad. Sci. USA 121, e2317274121 (2024).
Zhou, P. & Hong, J. Structure- and ligand-dynamics-based design of novel antibiotics targeting lipid A enzymes LpxC and LpxH in Gram-negative bacteria. Acc. Chem. Res. 54, 1623–1634 (2021).
Alexander, M. K. et al. Disrupting Gram-negative bacterial outer membrane biosynthesis through inhibition of the lipopolysaccharide transporter MsbA. Antimicrob. Agents Chemother. 62, e01142-18 (2018).
Ho, H. et al. Structural basis for dual-mode inhibition of the ABC transporter MsbA. Nature 557, 196–201 (2018).
Kadeřábková, N., Mahmood, A. J. S., Furniss, R. C. D. & Mavridou, D. A. I. Making a chink in their armor: current and next-generation antimicrobial strategies against the bacterial cell envelope. Adv. Microb. Physiol. 83, 221–307 (2023).
Pei, S. et al. Discovery of novel tetrahydrobenzothiophene derivatives as MSBA inhibitors for antimicrobial agents. Bioorg Chem. 142, 106932 (2024).
Verma, V. A. et al. Discovery of inhibitors of the lipopolysaccharide transporter MsbA: from a screening hit to potent wild-type Gram-negative activity. J. Med. Chem. 65, 4085–4120 (2022).
Skudlarek, J. W. et al. Cerastecin inhibition of the lipooligosaccharide transporter MsbA to combat Acinetobacter baumannii: from screening impurity to in vivo efficacy. J. Med. Chem. 67, 15620–15675 (2024).
Smith, P. A. et al. Optimized arylomycins are a new class of Gram-negative antibiotics. Nature 561, 189–194 (2018).
De Rosa, M. et al. Design, synthesis and in vitro biological evaluation of oligopeptides targeting E. coli type I signal peptidase (LepB). Bioorg Med. Chem. 25, 897–911 (2017).
Huang, K.-J. et al. Deletion of a previously uncharacterized lipoprotein lirL confers resistance to an inhibitor of type II signal peptidase in Acinetobacter baumannii. Proc. Natl Acad. Sci. USA 119, e2123117119 (2022).
Pantua, H. et al. Unstable mechanisms of resistance to inhibitors of Escherichia coli lipoprotein signal peptidase. mBio 11, e02018-20 (2020).
Garland, K. et al. Optimization of globomycin analogs as novel Gram-negative antibiotics. Bioorg Med. Chem. Lett. 30, 127419 (2020).
Shukla, R. et al. Teixobactin kills bacteria by a two-pronged attack on the cell envelope. Nature 608, 390–396 (2022).
Liu, Y., Liu, Y., Chan-Park, M. B. & Mu, Y. Binding modes of teixobactin to lipid II: molecular dynamics study. Sci. Rep. 7, 17197 (2017).
Parmar, A. et al. Development of teixobactin analogues containing hydrophobic, non-proteogenic amino acids that are highly potent against multidrug-resistant bacteria and biofilms. Eur. J. Med. Chem. 261, 115853 (2023).
Shukla, R. et al. An antibiotic from an uncultured bacterium binds to an immutable target. Cell 186, 4059–4073.e27 (2023).
Lin, J., Zhou, D., Steitz, T. A., Polikanov, Y. S. & Gagnon, M. G. Ribosome-targeting antibiotics: modes of action, mechanisms of resistance, and implications for drug design. Annu. Rev. Biochem. 87, 451–478 (2018).
Paternoga, H. et al. Structural conservation of antibiotic interaction with ribosomes. Nat. Struct. Mol. Biol. 30, 1380–1392 (2023).
Espejo, R. T. & Plaza, N. Multiple ribosomal RNA operons in bacteria; their concerted evolution and potential consequences on the rate of evolution of their 16S rRNA. Front. Microbiol. 9, 1232 (2018).
Pantel, L. et al. Odilorhabdins, antibacterial agents that cause miscoding by binding at a new ribosomal site. Mol. Cell 70, e7 (2018).
Lanois-Nouri, A. et al. The odilorhabdin antibiotic biosynthetic cluster and acetyltransferase self-resistance locus are niche and species specific. mBio 13, e0282621 (2022).
Pantel, L. et al. Missense mutations in the CrrB protein mediate odilorhabdin derivative resistance in Klebsiella pneumoniae. Antimicrob. Agents Chemother. 65, e00139-21 (2021).
Serrano, C. M. et al. Unifying the aminohexopyranose‐ and peptidyl‐nucleoside antibiotics: implications for antibiotic design. Angew. Chem. Int. Ed. 59, 11330–11333 (2020).
Vickers, A., Mushtaq, S., Woodford, N., Doumith, M. & Livermore, D. M. Activity of RX-04 pyrrolocytosine protein synthesis inhibitors against multidrug-resistant Gram-negative bacteria. Antimicrob. Agents Chemother. 62, e00689–18 (2018).
Aron, Z. D. et al. trans-Translation inhibitors bind to a novel site on the ribosome and clear Neisseria gonorrhoeae in vivo. Nat. Commun. 12, 1799 (2021).
Mitcheltree, M. J. et al. A synthetic antibiotic class overcoming bacterial multidrug resistance. Nature 599, 507–512 (2021).
Wu, K. J. Y. et al. An antibiotic preorganized for ribosomal binding overcomes antimicrobial resistance. Science 383, 721–726 (2024).
Lomeli, B. K. et al. Multiple-ascending-dose phase 1 clinical study of the safety, tolerability, and pharmacokinetics of CRS3123, a narrow-spectrum agent with minimal disruption of normal gut microbiota. Antimicrob. Agents Chemother. 64, e01395-19 (2019).
Kim, S.-H., Bae, S. & Song, M. Recent development of aminoacyl-tRNA synthetase inhibitors for human diseases: a future perspective. Biomolecules 10, 1625 (2020).
O’Dwyer, K. et al. Bacterial resistance to leucyl-tRNA synthetase inhibitor GSK2251052 develops during treatment of complicated urinary tract infections. Antimicrob. Agents Chemother. 59, 289–298 (2015).
Diacon, A. H. et al. A first-in-class leucyl-tRNA synthetase inhibitor, ganfeborole, for rifampicin-susceptible tuberculosis: a phase 2a open-label, randomized trial. Nat. Med. 30, 896–904 (2024).
Finn, P., Charlton, M., Edmund G, Jirgensons, A. & Loza, E. 2-Amino-N-(arylsulfinyl)-acetamide compounds as iInhibitors of bacterial aminoacyl-tRNA synthetase and their preparation. Patent WO2121123237A1 (2018).
Knak, T. et al. Over 40 years of fosmidomycin drug research: a comprehensive review and future opportunities. Pharmaceuticals 15, 1553 (2022).
Kesharwani, S. & Sundriyal, S. Non-hydroxamate inhibitors of 1-deoxy-d-xylulose 5-phosphate reductoisomerase (DXR): a critical review and future perspective. Eur. J. Med. Chem. 213, 113055 (2021).
Andaloussi, M. et al. Design, synthesis, and X-ray crystallographic studies of α-aryl substituted fosmidomycin analogues as inhibitors of Mycobacterium tuberculosis 1-deoxy-d-xylulose 5-phosphate reductoisomerase. J. Med. Chem. 54, 4964–4976 (2011).
Bibens, L., Becker, J.-P., Dassonville-Klimpt, A., & Sonnet, P. Review of fatty acid biosynthesis enzyme inhibitors as promising antimicrobial drugs. Pharmaceuticals 16, 425 (2023).
Wittke, F. et al. Afabicin, a first-in-class antistaphylococcal antibiotic, in the treatment of acute bacterial skin and skin structure infections: clinical noninferiority to vancomycin/linezolid. Antimicrob. Agents Chemother. 64, e00250-20 (2020).
Parker, E. N. et al. An iterative approach guides discovery of the FabI inhibitor fabimycin, a late-stage antibiotic candidate with in vivo efficacy against drug-resistant Gram-negative infections. ACS Cent. Sci. 8, 1145–1158 (2022).
Zorman, M., Hrast Rambaher, M., Kokot, M., Minovski, N. & Anderluh, M. The overview of development of novel bacterial topoisomerase inhibitors effective against multidrug-resistant bacteria in an academic environment: from early hits to in vivo active antibacterials. Eur. J. Pharm. Sci. 192, 106632 (2024).
Collin, F., Karkare, S. & Maxwell, A. Exploiting bacterial DNA gyrase as a drug target: current state and perspectives. Appl. Microbiol. Biotechnol. 92, 479–497 (2011).
Perry, C. R. et al. Efficacy and safety of gepotidacin as treatment of uncomplicated urogenital gonorrhea (EAGLE-1): design of a randomized, comparator-controlled, phase 3 study. Infect. Dis. Ther. 12, 2307–2320 (2023).
Hameed, P. S. et al. BWC0977, a broad-spectrum antibacterial clinical candidate to treat multidrug resistant infections. Nat. Commun. 15, 8202 (2024).
Whelan, A. O. et al. In vitro activity of novel topoisomerase inhibitors against Francisella tularensis and Burkholderia pseudomallei. Antibiotics 12, 983 (2023).
Zumbrunn, C. A short history of topoisomerases at Actelion Pharmaceuticals. Chimia 76, 647 (2022).
Cumming, J. G. et al. Novel indane-containing NBTIs with potent anti-Gram-negative activity and minimal hERG inhibition. ACS Med. Chem. Lett. 14, 1791–1799 (2023).
Kolarič, A. & Minovski, N. Novel bacterial topoisomerase inhibitors: challenges and perspectives in reducing hERG toxicity. Future Med. Chem. 10, 2241–2244 (2018).
Bisacchi, G. S. & Manchester, J. I. A new-class antibacterial—almost. Lessons in drug discovery and development: a critical analysis of more than 50 years of effort toward ATPase inhibitors of DNA gyrase and topoisomerase IV. ACS Infect. Dis. 1, 4–41 (2015).
Callaway, E. ‘Groundbreaking’: first treatment targeting ‘super-gonorrhoea’ passes trial. Nature 623, 236 (2023).
Tari, L. W. et al. Tricyclic GyrB/ParE (TriBE) inhibitors: a new class of broad-spectrum dual-targeting antibacterial agents. PLoS ONE 8, e84409 (2013).
Theuretzbacher, U. & Piddock, L. J. V. Non-traditional antibacterial therapeutic options and challenges. Cell Host Microbe 26, 61–72 (2019).
Kang, S.-J., Nam, S. H. & Lee, B.-J. Engineering approaches for the development of antimicrobial peptide-based antibiotics. Antibiotics 11, 1338 (2022).
Wan, F., Torres, M. D. T., Peng, J. & de la Fuente-Nunez, C. Deep-learning-enabled antibiotic discovery through molecular de-extinction. Nat. Biomed. Eng. 8, 854–871 (2024).
Santos-Júnior, C. D. et al. Discovery of antimicrobial peptides in the global microbiome with machine learning. Cell 187, e16 (2024).
Mourtada, R. et al. Design of stapled antimicrobial peptides that are stable, nontoxic and kill antibiotic-resistant bacteria in mice. Nat. Biotechnol. 37, 1186–1197 (2019).
O’Neil, D. A. Innovation in infectious disease therapies from immunology. Drug Discov. Today 26, 2090–2094 (2021).
Murray, E., Draper, L. A., Ross, R. P. & Hill, C. The advantages and challenges of using endolysins in a clinical setting. Viruses 13, 680 (2021).
Carratalá, J. V., Arís, A., Garcia-Fruitós, E. & Ferrer-Miralles, N. Design strategies for positively charged endolysins: insights into artilysin development. Biotechnol. Adv. 69, 108250 (2023).
Jun, S. Y. et al. Pharmacokinetics and tolerance of the phage endolysin-based candidate drug SAL200 after a single intravenous administration among healthy volunteers. Antimicrob. Agents Chemother. 61, e02629-16 (2017).
Pallesen, E. M. H. et al. Endolysin inhibits skin colonization by patient-derived Staphylococcus aureus and malignant T-cell activation in cutaneous T-cell lymphoma. J. Investig. Dermatology 143, 1757–1768 (2023).
Heselpoth, R. D., Euler, C. W., Schuch, R. & Fischetti, V. A. Lysocins: bioengineered antimicrobials that deliver lysins across the outer membrane of Gram-negative bacteria. Antimicrob. Agents Chemother. 63, e00342-19 (2019).
Defraine, V. et al. Efficacy of artilysin Art-175 against resistant and persistent Acinetobacter baumannii. Antimicrob. Agents Chemother. 60, 3480–3488 (2016).
Wesseling, C. M. J. & Martin, N. I. Synergy by perturbing the Gram-negative outer membrane: opening the door for Gram-positive specific antibiotics. ACS Infect. Dis. 8, 1731–1757 (2022).
Kim, S. J., Jo, J., Kim, J., Ko, K. S. & Lee, W. Polymyxin B nonapeptide potentiates the eradication of Gram-negative bacterial persisters. Microbiol. Spectr. 12, e0368723 (2024).
Vaara, M. Polymyxin derivatives that sensitize Gram-negative bacteria to other antibiotics. Molecules 24, 249 (2019).
Eckburg, P. B. et al. Safety, tolerability, pharmacokinetics, and drug interaction potential of SPR741, an intravenous potentiator, after single and multiple ascending doses and when combined with β-lactam antibiotics in healthy subjects. Antimicrob. Agents Chemother. 63, e00892-19 (2019).
Holden, E. R. et al. Mechanisms of action and synergies of a novel lipid IVA biosynthesis inhibitor. Preprint at bioRxiv https://doi.org/10.1101/2023.09.15.557861 (2023).
Compagne, N. et al. Update on the discovery of efflux pump inhibitors against critical priority Gram-negative bacteria. Antibiotics 12, 180 (2023).
Zhang, Y. et al. Evaluation of a conformationally constrained indole carboxamide as a potential efflux pump inhibitor in Pseudomonas aeruginosa. Antibiotics 11, 716 (2022).
Aron, Z. & Opperman, T. J. The hydrophobic trap—the Achilles heel of RND efflux pumps. Res. Microbiol. 169, 393–400 (2018).
Li, X.-Z., Plésiat, P. & Nikaido, H. The challenge of efflux-mediated antibiotic resistance in Gram-negative bacteria. Clin. Microbiol. Rev. 28, 337–418 (2015).
Lomovskaya, O. & Bostian, K. A. Practical applications and feasibility of efflux pump inhibitors in the clinic—a vision for applied use. Biochem. Pharmacol. 71, 910–918 (2006).
Lomovskaya, O., Zgurskaya, H. I., Totrov, M. & Watkins, W. J. Waltzing transporters and ‘the dance macabre’ between humans and bacteria. Nat. Rev. Drug Discov. 6, 56–65 (2007).
Duffey, M. et al. Extending the potency and lifespan of antibiotics: inhibitors of Gram-negative bacterial efflux pumps. ACS Infect. Dis. 10, 1458–1482 (2024).
Gaurav, A., Bakht, P., Saini, M., Pandey, S. & Pathania, R. Role of bacterial efflux pumps in antibiotic resistance, virulence, and strategies to discover novel efflux pump inhibitors. Microbiology 169, 001333 (2023).
Konstantinović, J. et al. Inhibitors of the elastase LasB for the treatment of Pseudomonas aeruginosa lung infections. ACS Cent. Sci. 9, 2205–2215 (2023).
Brodie, G. & Conway, S. J. Disarming Gram-negative bacteria in the fight against antimicrobial resistance. ACS Cent. Sci. 9, 2179–2182 (2023).
Everett, M. J. & Davies, D. T. Pseudomonas aeruginosa elastase (LasB) as a therapeutic target. Drug Discov. Today 26, 2108–2123 (2021).
Ahmad-Mansour, N. et al. Staphylococcus aureus toxins: an update on their pathogenic properties and potential treatments. Toxins 13, 677 (2021).
Kim, M.-K. Staphylococcus aureus toxins: from their pathogenic roles to anti-virulence therapy using natural products. Biotechnol. Bioprocess. Eng. 24, 424–435 (2019).
Jiang, J.-H., Cameron, D. R., Nethercott, C., Aires-de-Sousa, M. & Peleg, A. Y. Virulence attributes of successful methicillin-resistant Staphylococcus aureus lineages. Clin. Microbiol. Rev. 36, e0014822 (2023).
CARB-X Press release. CARB-X is funding a team of top German researchers to develop a drug to treat Staphylococcus aureus infections and prevent exacerbation of life-threatening pneumonia. https://carb-x.org/carb-x-news/carb-x-is-funding-a-team-of-top-german-researchers-to-develop-a-drug-to-treat-staphylococcus-aureus-infections-and-prevent-exacerbation-of-life-threatening-pneumonia/ (2020).
Baković, J. et al. Redox regulation of the quorum-sensing transcription factor AgrA by coenzyme A. Antioxidants 10, 841 (2021).
Trebosc, V. et al. In vitro activity of BV200 anti-virulent small molecules against a large and geographically diverse panel of S. aureus isolates from skin and lung infections. Presented at 32nd European Congress of Clinical Microbiology & Infectious Diseases (2022).
Horna, G. & Ruiz, J. Type 3 secretion system of Pseudomonas aeruginosa. Microbiol. Res. 246, 126719 (2021).
Moir, D. T. et al. A structure-function-inhibition analysis of the Pseudomonas aeruginosa type III secretion needle protein PscF. J. Bacteriol. 202, e00055-20 (2020).
Simonis, A. et al. Discovery of highly neutralizing human antibodies targeting Pseudomonas aeruginosa. Cell 186, 5098–5113.e19 (2023).
Elmassry, M. M., Colmer-Hamood, J. A., Kopel, J., San Francisco, M. J. & Hamood, A. N. Anti-Pseudomonas aeruginosa vaccines and therapies: an assessment of clinical trials. Microorganisms 11, 916 (2023).
Williams, J. D. et al. Synthesis and structure–activity relationships of novel phenoxyacetamide inhibitors of the Pseudomonas aeruginosa type III secretion system (T3SS). Bioorg Med. Chem. 23, 1027–1043 (2015).
Berube, B. J. et al. Impact of type III secretion effectors and of phenoxyacetamide inhibitors of type III secretion on abscess formation in a mouse model of Pseudomonas aeruginosa infection. Antimicrob. Agents. Chemother. 61, e01202-17 (2017).
Strathdee, S. A., Hatfull, G. F., Mutalik, V. K. & Schooley, R. T. Phage therapy: from biological mechanisms to future directions. Cell 186, 17–31 (2023).
Hitchcock, N. M. et al. Current clinical landscape and global potential of bacteriophage therapy. Viruses 15, 1020 (2023).
Green, S. I. et al. A retrospective, observational study of 12 cases of expanded-access customized phage therapy: production, characteristics, and clinical outcomes. Clin. Infect. Dis. 77, 1079–1091 (2023).
Szijártó, V. et al. Bactericidal monoclonal antibodies specific to the lipopolysaccharide O antigen from multidrug-resistant Escherichia coli clone ST131-O25b:H4 elicit protection in mice. Antimicrob. Agents Chemother. 59, 3109–3116 (2015).
Bruno, J. G. Applications in which aptamers are needed or wanted in diagnostics and therapeutics. Pharmaceuticals 15, 693 (2022).
Kristian, S. A. et al. Retargeting pre-existing human antibodies to a bacterial pathogen with an alpha-Gal conjugated aptamer. J. Mol. Med. 93, 619–631 (2015).
Chadborn, T. et al. An approach for embedding behavioural science in antimicrobial resistance One Health research. J. Infect. Public Health 16, 134–140 (2023).
Lewnard, J. A. et al. Burden of bacterial antimicrobial resistance in low-income and middle-income countries avertible by existing interventions: an evidence review and modelling analysis. Lancet 403, 2439–2454 (2024).
Mendelson, M. et al. Antimicrobial resistance and the great divide: inequity in priorities and agendas between the Global North and the Global South threatens global mitigation of antimicrobial resistance. Lancet Glob. Health 12, e516–e521 (2024).
Theuretzbacher, U. et al. Critical analysis of antibacterial agents in clinical development. Nat. Rev. Microbiol. 18, 286–298 (2020).
Paterson, D. L. Antibacterial agents active against Gram negative bacilli in phase I, II, or III clinical trials. Expert Opin. Investig. Drugs 33, 371–387 (2024).
McDowell, L. L., Quinn, C. L., Leeds, J. A., Silverman, J. A. & Silver, L. L. Perspective on antibacterial lead identification challenges and the role of hypothesis-driven strategies. SLAS Discov. 24, 440–456 (2019).
Miethke, M. et al. Towards the sustainable discovery and development of new antibiotics. Nat. Rev. Chem. 5, 726–749 (2021).
Piddock, L. J. V., Malpani, R. & Hennessy, A. Challenges and opportunities with antibiotic discovery and exploratory research. ACS Infect. Dis. 10, 2445–2447 (2024).
Silver, L. L. Challenges of antibacterial discovery. Clin. Microbiol. Rev. 24, 71–109 (2011).
Theuretzbacher, U., Baraldi, E., Ciabuschi, F. & Callegari, S. Challenges and shortcomings of antibacterial discovery projects. Clin. Microbiol. Inf. 29, 610–615 (2023).
Theuretzbacher, U. Antibiotic innovation for future public health needs. Clin. Microbiol. Inf. 23, 713–717 (2017).
Butler, M. S., Henderson, I. R., Capon, R. J. & Blaskovich, M. A. T. Antibiotics in the clinical pipeline as of December 2022. J. Antibiot. 76, 431–473 (2023).
Butler, M. S. et al. Analysis of the clinical pipeline of treatments for drug-resistant bacterial infections: despite progress, more action is needed. Antimicrob. Agents Chemother. 66, e0199121 (2022).
Theuretzbacher, U., Outterson, K., Engel, A. & Karlén, A. The global preclinical antibacterial pipeline. Nat. Rev. Microbiol. 18, 275–285 (2020).
Bergkessel, M., Forte, B. & Gilbert, I. H. Small-molecule antibiotic drug development: need and challenges. ACS Infect. Dis. 9, 2062–2071 (2023).
Cohn, J. et al. Improving equitable access for effective antibacterial: an ecosystem approach. Clin. Microbiol. Infect. 31, 339–344 (2025).
Cohn, J. et al. Accelerating antibiotic access and stewardship: a new model to safeguard public health. Lancet Infect. Dis. 24, e584–e590 (2024).
Tacconelli, E. et al. Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect. Dis. 18, 318–327 (2018).
Martínez, J. L., Baquero, F. & Andersson, D. I. Beyond serial passages: new methods for predicting the emergence of resistance to novel antibiotics. Curr. Opin. Pharmacol. 11, 439–445 (2011).
Kaplan, N. et al. Mode of action, in vitro activity, and in vivo efficacy of AFN-1252, a selective antistaphylococcal FabI inhibitor. Antimicrob. Agents Chemother. 56, 5865–5874 (2012).
Hill, A. M., Barber, M. J. & Gotham, D. Estimated costs of production and potential prices for the WHO Essential Medicines List. BMJ Glob. Health 3, e000571 (2018).
Acknowledgements
The authors thank M. Duffey, GARDP, for generating the draft of Fig. 4.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Microbiology thanks Mark Blaskovich and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
BEAM Alliance: https://beam-alliance.eu
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Theuretzbacher, U., Jumde, R.P., Hennessy, A. et al. Global health perspectives on antibacterial drug discovery and the preclinical pipeline. Nat Rev Microbiol 23, 474–490 (2025). https://doi.org/10.1038/s41579-025-01167-w
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41579-025-01167-w
This article is cited by
-
Biomaterials mediated 3R (remove-remodel-repair) strategy: holistic management of Helicobacter pylori infection
Journal of Nanobiotechnology (2025)
-
A unique inhibitor conformation selectively targets the DNA polymerase PolC of Gram-positive priority pathogens
Nature Communications (2025)
-
Bacterial cell envelope-targeting antibiotics
Nature Reviews Microbiology (2025)