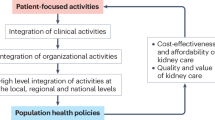
Abstract
Scientific reductionism has been the basis of disease classification and understanding for more than a century. However, the reductionist approach of characterizing diseases from a limited set of clinical observations and laboratory evaluations has proven insufficient in the face of an exponential growth in data generated from transcriptomics, proteomics, metabolomics and deep phenotyping. A new systematic method is necessary to organize these datasets and build new definitions of what constitutes a disease that incorporates both biological and environmental factors to more precisely describe the ever-growing complexity of phenotypes and their underlying molecular determinants. Network medicine provides such a conceptual framework to bridge these vast quantities of data while providing an individualized understanding of disease. The modern application of network medicine principles is yielding new insights into the pathobiology of chronic kidney diseases and renovascular disorders by expanding the understanding of pathogenic mediators, novel biomarkers and new options for renal therapeutics. These efforts affirm network medicine as a robust paradigm for elucidating new advances in the diagnosis and treatment of kidney disorders.
Key points
-
Network medicine applies the principles of network theory to disease diagnostics and therapeutics to provide a novel understanding of disease.
-
Biological networks can be constructed from several different biomolecules, representing simple to complex inter-relationships between these entities.
-
The network basis of disease is the disease module or subnetwork.
-
Kidney diseases are well-positioned for exploitation by network medicine approaches to achieve a more personalized and precise approach to treatment.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Kauffman, S. & Clayton, P. On emergence, agency, and organization. Biol. Philos. 21, 501–521 (2006).
Kesic, S. Systems biology, emergence and antireductionism. Saudi J. Biol. Sci. 23, 584–591 (2016).
Novikoff, A. B. The concept of integrative levels and biology. Science 101, 209–215 (1945).
Gevers, T. J. & Drenth, J. P. Diagnosis and management of polycystic liver disease. Nat. Rev. Gastroenterol. Hepatol. 10, 101–108 (2013).
Perrone, R. D., Malek, A. M. & Watnick, T. Vascular complications in autosomal dominant polycystic kidney disease. Nat. Rev. Nephrol. 11, 589–598 (2015).
Lanktree, M. B. & Chapman, A. B. New treatment paradigms for ADPKD: moving towards precision medicine. Nat. Rev. Nephrol. 13, 750–768 (2017).
Barabasi, A. L. & Oltvai, Z. N. Network biology: understanding the cell’s functional organization. Nat. Rev. Genet. 5, 101–113 (2004).
Menche, J. et al. Disease networks. Uncovering disease–disease relationships through the incomplete interactome. Science 347, 1257601 (2015).
Yildirim, M. A., Goh, K. I., Cusick, M. E., Barabasi, A. L. & Vidal, M. Drug–target network. Nat. Biotechnol. 25, 1119–1126 (2007).
Ravasz, E., Somera, A. L., Mongru, D. A., Oltvai, Z. N. & Barabasi, A. L. Hierarchical organization of modularity in metabolic networks. Science 297, 1551–1555 (2002).
Peel, L., Peixoto, T. P. & De Domenico, M. Statistical inference links data and theory in network science. Nat. Commun. 13, 6794 (2022).
Barabási, A. L. & Albert, R. Emergence of scaling in random networks. Science 286, 509–512 (1999).
Jeong, H., Tombor, B., Albert, R., Oltvai, Z. N. & Barabási, A. L. The large-scale organization of metabolic networks. Nature 407, 651–654 (2000).
Cohen, R. & Havlin, S. Scale-free networks are ultrasmall. Phys. Rev. Lett. 90, 058701 (2003).
Broido, A. D. & Clauset, A. Scale-free networks are rare. Nat. Commun. 10, 1017 (2019).
Zhou, B., Meng, X. & Stanley, H. E. Power-law distribution of degree–degree distance: a better representation of the scale-free property of complex networks. Proc. Natl Acad. Sci. USA 117, 14812–14818 (2020).
Serafino, M. et al. True scale-free networks hidden by finite size effects. Proc. Natl Acad. Sci. USA https://doi.org/10.1073/pnas.2013825118 (2021).
Voitalov, I., van der Hoorn, P., van der Hofstad, R. & Krioukov, D. Scale-free networks well done. Phys. Rev. Res. 1, 033034 (2019).
Albert, R., Jeong, H. & Barabási, A. L. Error and attack tolerance of complex networks. Nature 406, 378–382 (2000).
Jeong, H., Mason, S. P., Barabási, A. L. & Oltvai, Z. N. Lethality and centrality in protein networks. Nature 411, 41–42 (2001).
Liang, Y. & Kelemen, A. Dynamic modeling and network approaches for omics time course data: overview of computational approaches and applications. Brief. Bioinform. 19, 1051–1068 (2018).
Emmert-Streib, F., Dehmer, M. & Haibe-Kains, B. Gene regulatory networks and their applications: understanding biological and medical problems in terms of networks. Front. Cell Dev. Biol. 2, 38 (2014).
Levine, M. & Davidson, E. H. Gene regulatory networks for development. Proc. Natl Acad. Sci. USA 102, 4936–4942 (2005).
Karlebach, G. & Shamir, R. Modelling and analysis of gene regulatory networks. Nat. Rev. Mol. Cell Biol. 9, 770–780 (2008).
Davidson, E. H. et al. A genomic regulatory network for development. Science 295, 1669–1678 (2002).
Laub, M. T., McAdams, H. H., Feldblyum, T., Fraser, C. M. & Shapiro, L. Global analysis of the genetic network controlling a bacterial cell cycle. Science 290, 2144–2148 (2000).
Cai, L., Friedman, N. & Xie, X. S. Stochastic protein expression in individual cells at the single molecule level. Nature 440, 358–362 (2006).
Segal, E., Raveh-Sadka, T., Schroeder, M., Unnerstall, U. & Gaul, U. Predicting expression patterns from regulatory sequence in Drosophila segmentation. Nature 451, 535–540 (2008).
Chen, K. C. et al. Integrative analysis of cell cycle control in budding yeast. Mol. Biol. Cell 15, 3841–3862 (2004).
Friedman, N., Linial, M., Nachman, I. & Pe’er, D. Using Bayesian networks to analyze expression data. J. Comput. Biol. 7, 601–620 (2000).
Huynh-Thu, V. A., Irrthum, A., Wehenkel, L. & Geurts, P. Inferring regulatory networks from expression data using tree-based methods. PLoS ONE https://doi.org/10.1371/journal.pone.0012776 (2010).
Gat-Viks, I., Tanay, A., Raijman, D. & Shamir, R. A probabilistic methodology for integrating knowledge and experiments on biological networks. J. Comput. Biol. 13, 165–181 (2006).
Huynh-Thu, V. A. & Geurts, P. dynGENIE3: dynamical GENIE3 for the inference of gene networks from time series expression data. Sci. Rep. 8, 3384 (2018).
Simao, E., Remy, E., Thieffry, D. & Chaouiya, C. Qualitative modelling of regulated metabolic pathways: application to the tryptophan biosynthesis in E. coli. Bioinformatics 21, ii190–ii196 (2005).
Covert, M. W. & Palsson, B. O. Transcriptional regulation in constraints-based metabolic models of Escherichia coli. J. Biol. Chem. 277, 28058–28064 (2002).
Covert, M. W., Knight, E. M., Reed, J. L., Herrgard, M. J. & Palsson, B. O. Integrating high-throughput and computational data elucidates bacterial networks. Nature 429, 92–96 (2004).
Ryu, J. Y., Kim, H. U. & Lee, S. Y. Framework and resource for more than 11,000 gene-transcript–protein-reaction associations in human metabolism. Proc. Natl Acad. Sci. USA 114, E9740–E9749 (2017).
Forster, J., Famili, I., Fu, P., Palsson, B. O. & Nielsen, J. Genome-scale reconstruction of the Saccharomyces cerevisiae metabolic network. Genome Res. 13, 244–253 (2003).
Pols, T. et al. A synthetic metabolic network for physicochemical homeostasis. Nat. Commun. 10, 4239 (2019).
Gu, C., Kim, G. B., Kim, W. J., Kim, H. U. & Lee, S. Y. Current status and applications of genome-scale metabolic models. Genome Biol. 20, 121 (2019).
Boccard, J. et al. Gaining insights into metabolic networks using chemometrics and bioinformatics: chronic kidney disease as a clinical model. Front. Mol. Biosci. 8, 682559 (2021).
Thiele, I. et al. Personalized whole-body models integrate metabolism, physiology, and the gut microbiome. Mol. Syst. Biol. 16, e8982 (2020).
Brunk, E. et al. Recon3D enables a three-dimensional view of gene variation in human metabolism. Nat. Biotechnol. 36, 272–281 (2018).
Duarte, N. C. et al. Global reconstruction of the human metabolic network based on genomic and bibliomic data. Proc. Natl Acad. Sci. USA 104, 1777–1782 (2007).
Ma, H. et al. The Edinburgh human metabolic network reconstruction and its functional analysis. Mol. Syst. Biol. 3, 135 (2007).
Bhalla, U. S. & Iyengar, R. Emergent properties of networks of biological signaling pathways. Science 283, 381–387 (1999).
Jordan, J. D., Landau, E. M. & Iyengar, R. Signaling networks: the origins of cellular multitasking. Cell 103, 193–200 (2000).
Handly, L. N., Yao, J. & Wollman, R. Signal transduction at the single-cell level: approaches to study the dynamic nature of signaling networks. J. Mol. Biol. 428, 3669–3682 (2016).
Csabai, L. et al. SignaLink3: a multi-layered resource to uncover tissue-specific signaling networks. Nucleic Acids Res. 50, D701–D709 (2022).
Licata, L. et al. SIGNOR 2.0, the signaling network open resource 2.0: 2019 update. Nucleic Acids Res. 48, D504–D510 (2020).
Weidmann, C. A., Mustoe, A. M., Jariwala, P. B., Calabrese, J. M. & Weeks, K. M. Analysis of RNA-protein networks with RNP-MaP defines functional hubs on RNA. Nat. Biotechnol. 39, 347–356 (2021).
Lin, Y. et al. RNAInter in 2020: RNA interactome repository with increased coverage and annotation. Nucleic Acids Res. 48, D189–D197 (2020).
Piazza, I. et al. A map of protein-metabolite interactions reveals principles of chemical communication. Cell 172, 358–372.e23 (2018).
Luzarowski, M. et al. Global mapping of protein–metabolite interactions in Saccharomyces cerevisiae reveals that Ser-Leu dipeptide regulates phosphoglycerate kinase activity. Commun. Biol. 4, 181 (2021).
Lee, L. Y., Pandey, A. K., Maron, B. A. & Loscalzo, J. Network medicine in cardiovascular research. Cardiovasc. Res. 117, 2186–2202 (2021).
Celine, S. & Jörg, M. in Networks of Networks in Biology: Concepts, Tools and Applications (eds Gomez-Cabrero, D., Bianconi, G. & Kiani, N. A.) 147–171 (Cambridge Univ. Press, 2021).
Kanehisa, M. et al. From genomics to chemical genomics: new developments in KEGG. Nucleic Acids Res. 34, D354–D357 (2006).
Fabregat, A. et al. The reactome pathway knowledgebase. Nucleic Acids Res. 44, D481–D487 (2016).
Cerami, E. G. et al. Pathway commons, a web resource for biological pathway data. Nucleic Acids Res. 39, D685–D690 (2011).
Kelder, T. et al. WikiPathways: building research communities on biological pathways. Nucleic Acids Res. 40, D1301–D1307 (2012).
Kramer, A., Green, J., Pollard, J. Jr. & Tugendreich, S. Causal analysis approaches in ingenuity pathway analysis. Bioinformatics 30, 523–530 (2014).
Cusick, M. E. et al. Literature-curated protein interaction datasets. Nat. Methods 6, 39–46 (2009).
Hakes, L., Pinney, J. W., Robertson, D. L. & Lovell, S. C. Protein–protein interaction networks and biology – what’s the connection? Nat. Biotechnol. 26, 69–72 (2008).
Skinnider, M. A., Stacey, R. G. & Foster, L. J. Genomic data integration systematically biases interactome mapping. PLoS Comput. Biol. 14, e1006474 (2018).
Schaefer, M. H., Serrano, L. & Andrade-Navarro, M. A. Correcting for the study bias associated with protein–protein interaction measurements reveals differences between protein degree distributions from different cancer types. Front. Genet. 6, 260 (2015).
Cheng, F. et al. Comprehensive characterization of protein–protein interactions perturbed by disease mutations. Nat. Genet. 53, 342–353 (2021).
Ruepp, A. et al. CORUM: the comprehensive resource of mammalian protein complexes. Nucleic Acids Res. 36, D646–D650 (2008).
Oughtred, R. et al. The BioGRID database: a comprehensive biomedical resource of curated protein, genetic, and chemical interactions. Protein Sci. 30, 187–200 (2021).
Szklarczyk, D. et al. The STRING database in 2021: customizable protein–protein networks, and functional characterization of user-uploaded gene/measurement sets. Nucleic Acids Res. 49, D605–D612 (2021).
Keshava Prasad, T. S. et al. Human protein reference database – 2009 update. Nucleic Acids Res. 37, D767–D772 (2009).
Gandhi, T. K. et al. Analysis of the human protein interactome and comparison with yeast, worm and fly interaction datasets. Nat. Genet. 38, 285–293 (2006).
Huttlin, E. L. et al. Architecture of the human interactome defines protein communities and disease networks. Nature 545, 505–509 (2017).
Makhnevych, T. et al. Global map of SUMO function revealed by protein–protein interaction and genetic networks. Mol. Cell 33, 124–135 (2009).
Hein, M. Y. et al. A human interactome in three quantitative dimensions organized by stoichiometries and abundances. Cell 163, 712–723 (2015).
Petschnigg, J. et al. The mammalian-membrane two-hybrid assay (MaMTH) for probing membrane–protein interactions in human cells. Nat. Methods 11, 585–592 (2014).
Rolland, T. et al. A proteome-scale map of the human interactome network. Cell 159, 1212–1226 (2014).
Stelzl, U. et al. A human protein–protein interaction network: a resource for annotating the proteome. Cell 122, 957–968 (2005).
Luo, Y., Batalao, A., Zhou, H. & Zhu, L. Mammalian two-hybrid system: a complementary approach to the yeast two-hybrid system. Biotechniques 22, 350–352 (1997).
Kovacs, I. A. et al. Network-based prediction of protein interactions. Nat. Commun. 10, 1240 (2019).
Keskin, O., Tuncbag, N. & Gursoy, A. Predicting protein–protein interactions from the molecular to the proteome level. Chem. Rev. 116, 4884–4909 (2016).
Zhang, B., Tian, Y. & Zhang, Z. Network biology in medicine and beyond. Circ. Cardiovasc. Genet. 7, 536–547 (2014).
Schaefer, M. H. et al. Adding protein context to the human protein–protein interaction network to reveal meaningful interactions. PLoS Comput. Biol. 9, e1002860 (2013).
Lopes, T. J. et al. Tissue-specific subnetworks and characteristics of publicly available human protein interaction databases. Bioinformatics 27, 2414–2421 (2011).
Kotlyar, M., Pastrello, C., Malik, Z. & Jurisica, I. IID 2018 update: context-specific physical protein–protein interactions in human, model organisms and domesticated species. Nucleic Acids Res. 47, D581–D589 (2019).
Skinnider, M. A. et al. An atlas of protein–protein interactions across mouse tissues. Cell 184, 4073–4089.e17 (2021).
Bader, G. D. & Hogue, C. W. Analyzing yeast protein–protein interaction data obtained from different sources. Nat. Biotechnol. 20, 991–997 (2002).
Gavin, A. C. et al. Proteome survey reveals modularity of the yeast cell machinery. Nature 440, 631–636 (2006).
Stumpf, M. P. et al. Estimating the size of the human interactome. Proc. Natl Acad. Sci. USA 105, 6959–6964 (2008).
Luck, K. et al. A reference map of the human binary protein interactome. Nature 580, 402–408 (2020).
Vinayagam, A. et al. A directed protein interaction network for investigating intracellular signal transduction. Sci. Signal. 4, rs8 (2011).
Cao, M. et al. New directions for diffusion-based network prediction of protein function: incorporating pathways with confidence. Bioinformatics 30, i219–i227 (2014).
Silverbush, D. & Sharan, R. A systematic approach to orient the human protein–protein interaction network. Nat. Commun. 10, 3015 (2019).
Goh, K. I. et al. The human disease network. Proc. Natl Acad. Sci. USA 104, 8685–8690 (2007).
Fraser, H. B., Hirsh, A. E., Steinmetz, L. M., Scharfe, C. & Feldman, M. W. Evolutionary rate in the protein interaction network. Science 296, 750–752 (2002).
Yu, H. et al. High-quality binary protein interaction map of the yeast interactome network. Science 322, 104–110 (2008).
Feldman, I., Rzhetsky, A. & Vitkup, D. Network properties of genes harboring inherited disease mutations. Proc. Natl Acad. Sci. USA 105, 4323–4328 (2008).
Milo, R. et al. Network motifs: simple building blocks of complex networks. Science 298, 824–827 (2002).
Xu, J. & Li, Y. Discovering disease-genes by topological features in human protein–protein interaction network. Bioinformatics 22, 2800–2805 (2006).
Kitsak, M. et al. Tissue specificity of human disease module. Sci. Rep. 6, 35241 (2016).
Ghiassian, S. D. et al. Endophenotype network models: common core of complex diseases. Sci. Rep. 6, 27414 (2016).
Larsen, S. J., Schmidt, H. H. H. W. & Baumbach, J. De novo and supervised endophenotyping using network-guided ensemble learning. Syst. Med. 3, 8–21 (2020).
Iossifov, I., Zheng, T., Baron, M., Gilliam, T. C. & Rzhetsky, A. Genetic-linkage mapping of complex hereditary disorders to a whole-genome molecular-interaction network. Genome Res. 18, 1150–1162 (2008).
Krauthammer, M., Kaufmann, C. A., Gilliam, T. C. & Rzhetsky, A. Molecular triangulation: bridging linkage and molecular-network information for identifying candidate genes in Alzheimer’s disease. Proc. Natl Acad. Sci. USA 101, 15148–15153 (2004).
Li, Z. et al. Integrating mouse and human genetic data to move beyond GWAS and identify causal genes in cholesterol metabolism. Cell Metab. 31, 741–754.e5 (2020).
Sharma, A. et al. Controllability in an islet specific regulatory network identifies the transcriptional factor NFATC4, which regulates type 2 diabetes associated genes. NPJ Syst. Biol. Appl. 4, 25 (2018).
Rhodes, C. J. et al. Whole-blood RNA profiles associated with pulmonary arterial hypertension and clinical outcome. Am. J. Respir. Crit. Care Med. 202, 586–594 (2020).
Wang, T., Peng, Q., Liu, B., Liu, Y. & Wang, Y. Disease module identification based on representation learning of complex networks integrated from GWAS, eQTL summaries, and human interactome. Front. Bioeng. Biotechnol. 8, 418 (2020).
Oti, M., Snel, B., Huynen, M. A. & Brunner, H. G. Predicting disease genes using protein–protein interactions. J. Med. Genet. 43, 691–698 (2006).
Ghiassian, S. D., Menche, J. & Barabási, A. L. A disease module detection (DIAMOnD) algorithm derived from a systematic analysis of connectivity patterns of disease proteins in the human interactome. PLoS Comput. Biol. 11, e1004120 (2015).
Silberberg, Y., Kupiec, M. & Sharan, R. GLADIATOR: a global approach for elucidating disease modules. Genome Med. 9, 48 (2017).
Huang, S. S. & Fraenkel, E. Integrating proteomic, transcriptional, and interactome data reveals hidden components of signaling and regulatory networks. Sci. Signal. 2, ra40 (2009).
Wang, R. S. & Loscalzo, J. Network-based disease module discovery by a novel seed connector algorithm with pathobiological implications. J. Mol. Biol. 430, 2939–2950 (2018).
Kohler, S., Bauer, S., Horn, D. & Robinson, P. N. Walking the interactome for prioritization of candidate disease genes. Am. J. Hum. Genet. 82, 949–958 (2008).
Li, L., Wang, Y., An, L., Kong, X. & Huang, T. A network-based method using a random walk with restart algorithm and screening tests to identify novel genes associated with Menière’s disease. PLoS ONE 12, e0182592 (2017).
Wei, P. J. et al. Prioritizing cancer genes based on an improved random walk method. Front. Genet. 11, 377 (2020).
Liu, W. et al. Topologically inferring risk-active pathways toward precise cancer classification by directed random walk. Bioinformatics 29, 2169–2177 (2013).
Lazareva, O., Baumbach, J., List, M. & Blumenthal, D. B. On the limits of active module identification. Brief. Bioinform. https://doi.org/10.1093/bib/bbab066 (2021).
Levi, H., Elkon, R. & Shamir, R. DOMINO: a network-based active module identification algorithm with reduced rate of false calls. Mol. Syst. Biol. 17, e9593 (2021).
Page, I. H. Pathogenesis of arterial hypertension. J. Am. Med. Assoc. 140, 451–458 (1949).
Page, I. H. The mosaic theory of arterial hypertension – its interpretation. Perspect. Biol. Med. 10, 325–333 (1967).
Guyton, A. C. & Coleman, T. G. Quantitative analysis of the pathophysiology of hypertension. Circ. Res. 24, 1–19 (1969).
Guyton, A. C. et al. Systems analysis of arterial pressure regulation and hypertension. Ann. Biomed. Eng. 1, 254–281 (1972).
Guyton, A. C., Coleman, T. G. & Granger, H. J. Circulation: overall regulation. Annu. Rev. Physiol. 34, 13–46 (1972).
Montani, J. P. & Van Vliet, B. N. Understanding the contribution of Guyton’s large circulatory model to long-term control of arterial pressure. Exp. Physiol. 94, 382–388 (2009).
He, L. et al. The glomerular transcriptome and a predicted protein–protein interaction network. J. Am. Soc. Nephrol. 19, 260–268 (2008).
Gadegbeku, C. A. et al. Design of the nephrotic syndrome study network (NEPTUNE) to evaluate primary glomerular nephropathy by a multidisciplinary approach. Kidney Int. 83, 749–756 (2013).
Hansen, J. et al. A reference tissue atlas for the human kidney. Sci. Adv. 8, eabn4965 (2022).
Bhavnani, S. K. et al. Network analysis of genes regulated in renal diseases: implications for a molecular-based classification. BMC Bioinformatics 10, S3 (2009).
Bhavnani, S. K. et al. Discovering hidden relationships between renal diseases and regulated genes through 3D network visualizations. BMC Res. Notes 3, 296 (2010).
Song, X. et al. Systems biology of autosomal dominant polycystic kidney disease (ADPKD): computational identification of gene expression pathways and integrated regulatory networks. Hum. Mol. Genet. 18, 2328–2343 (2009).
Pandey, P., Qin, S., Ho, J., Zhou, J. & Kreidberg, J. A. Systems biology approach to identify transcriptome reprogramming and candidate microRNA targets during the progression of polycystic kidney disease. BMC Syst. Biol. 5, 56 (2011).
Hajarnis, S. et al. microRNA-17 family promotes polycystic kidney disease progression through modulation of mitochondrial metabolism. Nat. Commun. 8, 14395 (2017).
Yheskel, M., Lakhia, R., Cobo-Stark, P., Flaten, A. & Patel, V. Anti-microRNA screen uncovers miR-17 family within miR-17~92 cluster as the primary driver of kidney cyst growth. Sci. Rep. 9, 1920 (2019).
Podrini, C., Cassina, L. & Boletta, A. Metabolic reprogramming and the role of mitochondria in polycystic kidney disease. Cell Signal. 67, 109495 (2020).
Ju, W. et al. Tissue transcriptome-driven identification of epidermal growth factor as a chronic kidney disease biomarker. Sci. Transl. Med. 7, 316ra193 (2015).
Segarra-Medrano, A. et al. Value of urinary levels of interleukin-6, epidermal growth factor, monocyte chemoattractant protein type1 and transforming growth factor β1 in predicting the extent of fibrosis lesions in kidney biopsies of patients with IgA nephropathy. Nefrologia 37, 531–538 (2017).
Azukaitis, K. et al. Low levels of urinary epidermal growth factor predict chronic kidney disease progression in children. Kidney Int. 96, 214–221 (2019).
Norvik, J. V. et al. Urinary excretion of epidermal growth factor and rapid loss of kidney function. Nephrol. Dial. Transpl. 36, 1882–1892 (2021).
Sas, K. M. et al. Tissue-specific metabolic reprogramming drives nutrient flux in diabetic complications. JCI Insight 1, e86976 (2016).
Ahmed, M. M. et al. Identification of pathogenic genes associated with CKD: an integrated bioinformatics approach. Front. Genet. 13, 891055 (2022).
Berthier, C. C. et al. Enhanced expression of Janus kinase-signal transducer and activator of transcription pathway members in human diabetic nephropathy. Diabetes 58, 469–477 (2009).
Tao, J. et al. JAK-STAT signaling is activated in the kidney and peripheral blood cells of patients with focal segmental glomerulosclerosis. Kidney Int. 94, 795–808 (2018).
Sellmayr, M. et al. Only hyperuricemia with crystalluria, but not asymptomatic hyperuricemia, drives progression of chronic kidney disease. J. Am. Soc. Nephrol. 31, 2773–2792 (2020).
Brosius, F. C., Tuttle, K. R. & Kretzler, M. JAK inhibition in the treatment of diabetic kidney disease. Diabetologia 59, 1624–1627 (2016).
Botzer, A., Grossman, E., Moult, J. & Unger, R. A system view and analysis of essential hypertension. J. Hypertens. 36, 1094–1103 (2018).
Zhao, Y. et al. Integrative genomics analysis unravels tissue-specific pathways, networks, and key regulators of blood pressure regulation. Front. Cardiovasc. Med. 6, 21 (2019).
Huan, T. et al. Integrative network analysis reveals molecular mechanisms of blood pressure regulation. Mol. Syst. Biol. 11, 799 (2015).
Ehret, G. B. et al. Genetic variants in novel pathways influence blood pressure and cardiovascular disease risk. Nature 478, 103–109 (2011).
Saleh, M. A. et al. Lymphocyte adaptor protein LNK deficiency exacerbates hypertension and end-organ inflammation. J. Clin. Invest. 125, 1189–1202 (2015).
Dale, B. L. & Madhur, M. S. Linking inflammation and hypertension via LNK/SH2B3. Curr. Opin. Nephrol. Hypertens. 25, 87–93 (2016).
Keefe, J. A. et al. Evidence for a causal role of the SH2B3-β2M axis in blood pressure regulation. Hypertension 73, 497–503 (2019).
Alexander, M. R. et al. A single nucleotide polymorphism in SH2B3/LNK promotes hypertension development and renal damage. Circ. Res. 131, 731–747 (2022).
Rudemiller, N. P. et al. Mutation of SH2B3 (LNK), a genome-wide association study candidate for hypertension, attenuates Dahl salt-sensitive hypertension via inflammatory modulation. Hypertension 65, 1111–1117 (2015).
Batchu, N. et al. Role of Axl in T-lymphocyte survival in salt-dependent hypertension. Arterioscler. Thromb. Vasc. Biol. 36, 1638–1646 (2016).
Van Beusecum, J. P. et al. Growth arrest specific-6 and Axl coordinate inflammation and hypertension. Circ. Res. 129, 975–991 (2021).
You, S. et al. Comprehensive bioinformatics analysis identifies POLR2I as a key gene in the pathogenesis of hypertensive nephropathy. Front. Genet. 12, 698570 (2021).
Rinschen, M. M. et al. Metabolic rewiring of the hypertensive kidney. Sci. Signal. https://doi.org/10.1126/scisignal.aax9760 (2019).
Berthier, C. C. et al. Cross-species transcriptional network analysis defines shared inflammatory responses in murine and human lupus nephritis. J. Immunol. 189, 988–1001 (2012).
Arazi, A. et al. The immune cell landscape in kidneys of patients with lupus nephritis. Nat. Immunol. 20, 902–914 (2019).
Eddy, S. et al. Inflammatory and JAK-STAT pathways as shared molecular targets for ANCA-associated vasculitis and nephrotic syndrome. Preprint at bioRxiv https://doi.org/10.1101/427898 (2018).
Martini, S. et al. Integrative biology identifies shared transcriptional networks in CKD. J. Am. Soc. Nephrol. 25, 2559–2572 (2014).
Hopkins, A. L. Network pharmacology: the next paradigm in drug discovery. Nat. Chem. Biol. 4, 682–690 (2008).
Keiser, M. J. et al. Predicting new molecular targets for known drugs. Nature 462, 175–181 (2009).
Iida, M., Iwata, M. & Yamanishi, Y. Network-based characterization of disease–disease relationships in terms of drugs and therapeutic targets. Bioinformatics 36, i516–i524 (2020).
Cheng, F. et al. Network-based approach to prediction and population-based validation of in silico drug repurposing. Nat. Commun. 9, 2691 (2018).
Guney, E., Menche, J., Vidal, M. & Barabási, A. L. Network-based in silico drug efficacy screening. Nat. Commun. 7, 10331 (2016).
Paci, P. et al. Comprehensive network medicine-based drug repositioning via integration of therapeutic efficacy and side effects. NPJ Syst. Biol. Appl. 8, 12 (2022).
Varghese, R. & Majumdar, A. A new prospect for the treatment of nephrotic syndrome based on network pharmacology analysis. Curr. Res. Physiol. 5, 36–47 (2022).
Hickey, S. L., McKim, A., Mancuso, C. A. & Krishnan, A. A network-based approach for isolating the chronic inflammation gene signatures underlying complex diseases towards finding new treatment opportunities. Front. Pharmacol. 13, 995459 (2022).
Lamb, J. et al. The Connectivity Map: using gene-expression signatures to connect small molecules, genes, and disease. Science 313, 1929–1935 (2006).
Williams, V. R. et al. Connectivity mapping of a chronic kidney disease progression signature identified lysine deacetylases as novel therapeutic targets. Kidney Int. 98, 116–132 (2020).
Wilmes, A. et al. Application of integrated transcriptomic, proteomic and metabolomic profiling for the delineation of mechanisms of drug induced cell stress. J. Proteom. 79, 180–194 (2013).
Azeloglu, E. U. et al. Interconnected network motifs control podocyte morphology and kidney function. Sci. Signal. 7, ra12 (2014).
Sonawane, A. R. et al. Understanding tissue-specific gene regulation. Cell Rep. 21, 1077–1088 (2017).
Glass, K., Huttenhower, C., Quackenbush, J. & Yuan, G. C. Passing messages between biological networks to refine predicted interactions. PLoS One 8, e64832 (2013).
Thul, P. J. et al. A subcellular map of the human proteome. Science https://doi.org/10.1126/science.aal3321 (2017).
Mele, M. et al. Human genomics. The human transcriptome across tissues and individuals. Science 348, 660–665 (2015).
Karlsson, M. et al. A single-cell type transcriptomics map of human tissues. Sci. Adv. https://doi.org/10.1126/sciadv.abh2169 (2021).
Sadegh, S. et al. Network medicine for disease module identification and drug repurposing with the NeDRex platform. Nat. Commun. 12, 6848 (2021).
Kuijjer, M. L., Tung, M. G., Yuan, G., Quackenbush, J. & Glass, K. Estimating sample-specific regulatory networks. iScience 14, 226–240 (2019).
Maron, B. A. et al. Individualized interactomes for network-based precision medicine in hypertrophic cardiomyopathy with implications for other clinical pathophenotypes. Nat. Commun. 12, 873 (2021).
Acknowledgements
The authors thank S. C. Tribuna for expert administrative assistance. This work was supported in part by NIH grants HL119145, HL155107, HL155096 and HG007690, and by American Heart Association grants D700382 and CV-19 to J.L.
Author information
Authors and Affiliations
Contributions
Both authors wrote the article, researched the data for the article, contributed substantially to discussion of the content and reviewed and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
J.L. is the co-founder and a member of the Scientific Advisory Board (2013 to present) of Scipher Medicine, which is a for-profit company. He is also a member of the Scientific Advisory Board of Applied BioMath and a consultant for Naring Health. A.K.P. declares no competing interests.
Peer review
Peer review information
Nature Reviews Nephrology thanks David Blumenthal, Marcus Rinschen and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Glossary
- Bipartite network
-
A network consisting of nodes divided into two independent sets in which edges connect nodes between the two sets.
- Monte Carlo simulation
-
A computational algorithm that relies on repeated randomly generated inputs followed by deterministic computations on those inputs.
- Ordinary differential equations
-
Equations in which the independent variable is a derivative of a single-variable function.
- Pareto’s rule
-
For many events, approximately 80% of all effects arise from 20% of all causes.
- Random walker algorithm
-
An algorithm based on iterative paths to randomly selected neighbouring nodes to assign correlations between nodes and uncover information on network topology.
- Stochastic partial differential equations
-
Generalized form of partial differential equations in which coefficients are random numbers or functions.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pandey, A.K., Loscalzo, J. Network medicine: an approach to complex kidney disease phenotypes. Nat Rev Nephrol 19, 463–475 (2023). https://doi.org/10.1038/s41581-023-00705-0
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41581-023-00705-0