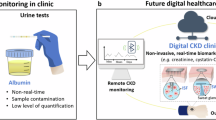
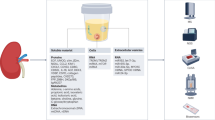
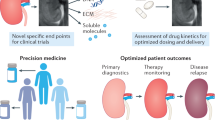
Abstract
Established clinical practices for monitoring kidney health and disease — including biopsy and serum biomarker analysis — suffer from practical limitations in monitoring frequency and lack adequate sensitivity for early disease detection. Engineering advances in biosensors have led to the development of wearable and implantable systems for monitoring of kidney health. Non-invasive microfluidic systems have demonstrated utility in the detection of kidney-relevant biomarkers, such as creatinine, urea and electrolytes in peripheral body fluids such as sweat, interstitial fluid, tears and saliva. Implantable systems may aid the identification of early transplant rejection through analysis of organ temperature and perfusion, and enable real-time assessment of inflammation through the use of thermal sensors. These technologies enable continuous, real-time monitoring of kidney health, offering complementary information to standard clinical procedures to alert physicians of changes in kidney health for early intervention. In this Review, we explore devices for monitoring renal biomarkers in peripheral biofluids and discuss developments in implantable sensors for the direct measurement of the local, biophysical properties of kidney tissue. We also describe potential clinical applications, including monitoring of chronic kidney disease, acute kidney injury and allograft health.
Key points
-
Advances in biosensors have led to the development of wearable and implantable systems for detecting indices of kidney health.
-
Wearable biosensors are non-invasive alternatives to tests for biomarkers in blood, and include non-invasive microfluidic and microneedle-based systems with optical or electrochemical mechanisms to measure concentrations of kidney-relevant biomarkers in biofluids such as sweat, interstitial fluid, tears and saliva.
-
Implantable devices enable direct measurements of the physical properties of the kidney, including tissue oxygenation, perfusion and temperature.
-
Compared with blood tests and radiological procedures, these electronic devices enable the real-time capture of physiological data and may enable continuous monitoring over periods of time.
-
Successful commercial translations of wearable biosensing devices are expected to benefit patients by reducing costs and providing invaluable real-time biochemical information for clinical decision making.
-
Translational studies using large animal models with sufficiently large populations are needed to assess the predictive value of implantable biophysical sensors.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Francis, A. et al. Chronic kidney disease and the global public health agenda: an international consensus. Nat. Rev. Nephrol. 20, 473–485 (2024).
Webster, A. C., Nagler, E. V., Morton, R. L. & Masson, P. Chronic kidney disease. Lancet 389, 1238–1252 (2017).
Kellum, J. A. et al. Acute kidney injury. Nat. Rev. Dis. Primers 7, 52 (2021).
Tucker, E. L. et al. Life and expectations post-kidney transplant: a qualitative analysis of patient responses. BMC Nephrol. 20, 175 (2019).
Giwa, S. et al. The promise of organ and tissue preservation to transform medicine. Nat. Biotechnol. 35, 530–542 (2017).
Hariharan, S., Israni, A. K. & Danovitch, G. Long-term survival after kidney transplantation. N. Engl. J. Med. 385, 729–743 (2021).
Delanaye, P., Cavalier, E. & Pottel, H. Serum creatinine: not so simple! Nephron 136, 302–308 (2017).
Ostermann, M. et al. Biomarkers in acute kidney injury. Ann. Intensive Care 14, 145 (2024).
Seki, M. et al. Blood urea nitrogen is independently associated with renal outcomes in Japanese patients with stage 3–5 chronic kidney disease: a prospective observational study. BMC Nephrol. 20, 1–10 (2019).
Sharma, S. & Smyth, B. From proteinuria to fibrosis: an update on pathophysiology and treatment options. Kidney Blood Press. Res. 46, 411–420 (2021).
Carrero, J. J. et al. Albuminuria changes are associated with subsequent risk of end-stage renal disease and mortality. Kidney Int. 91, 244–251 (2017).
Menon, M. C., Murphy, B. & Heeger, P. S. Moving biomarkers toward clinical implementation in kidney transplantation. J. Am. Soc. Nephrol. 28, 735–747 (2017).
Bloom, R. D. & Augustine, J. J. Beyond the biopsy: monitoring immune status in kidney recipients. Clin. J. Am. Soc. Nephrol. 16, 1413–1422 (2021).
El-Bandar, N. et al. Kidney perfusion in contrast-enhanced ultrasound (CEUS) correlates with renal function in living kidney donors. J. Clin. Med. 11, 791 (2022).
Singla, R. K., Kadatz, M., Rohling, R. & Nguan, C. Kidney ultrasound for nephrologists: a review. Kidney Med. 4, 100464 (2022).
Thurman, J. & Gueler, F. Recent advances in renal imaging. F1000Res 7, F1000 (2018).
Francis, S. T., Selby, N. M. & Taal, M. W. Magnetic resonance imaging to evaluate kidney structure, function, and pathology: moving toward clinical application. Am. J. Kidney Dis. 82, 491–504 (2023).
Hull, K. L., Adenwalla, S. F., Topham, P. & Graham-Brown, M. P. Indications and considerations for kidney biopsy: an overview of clinical considerations for the non-specialist. Clin. Med. 22, 34–40 (2022).
Schnuelle, P. Renal biopsy for diagnosis in kidney disease: indication, technique, and safety. J. Clin. Med. 12, 6424 (2023).
Poggio, E. D. et al. Systematic review and meta-analysis of native kidney biopsy complications. Clin. J. Am. Soc. Nephrol. 15, 1595–1602 (2020).
Bufkin, K. B., Karim, Z. A. & Silva, J. Review of the limitations of current biomarkers in acute kidney injury clinical practices. SAGE Open. Med. 12, 20503121241228446 (2024).
Chung, H. U. et al. Binodal, wireless epidermal electronic systems with in-sensor analytics for neonatal intensive care. Science 363, eaau0780 (2019).
Gao, W. et al. Fully integrated wearable sensor arrays for multiplexed in situ perspiration analysis. Nature 529, 509–514 (2016).
Mickle, A. D. et al. A wireless closed-loop system for optogenetic peripheral neuromodulation. Nature 565, 361–365 (2019).
Zhao, C., Park, J., Root, S. E. & Bao, Z. Skin-inspired soft bioelectronic materials, devices and systems. Nat. Rev. Bioeng. 2, 671–690 (2024).
Xu, S., Kim, J., Walter, J. R., Ghaffari, R. & Rogers, J. A. Translational gaps and opportunities for medical wearables in digital health. Sci. Transl. Med. 14, eabn6036 (2022).
Kukkar, D., Zhang, D., Jeon, B. H. & Kim, K.-H. Recent advances in wearable biosensors for non-invasive monitoring of specific metabolites and electrolytes associated with chronic kidney disease: performance evaluation and future challenges. TrAC. Trends Anal. Chem. 150, 116570 (2022).
Tricoli, A. & Neri, G. Miniaturized bio-and chemical-sensors for point-of-care monitoring of chronic kidney diseases. Sensors 18, 942 (2018).
Strauss, C., Booke, H., Forni, L. & Zarbock, A. Biomarkers of acute kidney injury: from discovery to the future of clinical practice. J. Clin. Anesth. 95, 111458 (2024).
Dhondup, T. & Qian, Q. Acid-base and electrolyte disorders in patients with and without chronic kidney disease: an update. Kidney Dis. 3, 136–148 (2017).
Tesch, G. H. Review: serum and urine biomarkers of kidney disease: a pathophysiological perspective. Nephrology 15, 609–616 (2010).
Gowda, S. et al. Markers of renal function tests. N. Am. J. Med. Sci. 2, 170–173 (2010).
Kim, J., Campbell, A. S., de Avila, B. E. & Wang, J. Wearable biosensors for healthcare monitoring. Nat. Biotechnol. 37, 389–406 (2019).
Baker, L. B. Physiology of sweat gland function: the roles of sweating and sweat composition in human health. Temperature 6, 211–259 (2019).
Zhang, Y. et al. Passive sweat collection and colorimetric analysis of biomarkers relevant to kidney disorders using a soft microfluidic system. Lab. Chip 19, 1545–1555 (2019).
Choi, J., Ghaffari, R., Baker, L. B. & Rogers, J. A. Skin-interfaced systems for sweat collection and analytics. Sci. Adv. 4, eaar3921 (2018).
Kim, S. B. et al. Soft, skin-interfaced microfluidic systems with wireless, battery-free electronics for digital, real-time tracking of sweat loss and electrolyte composition. Small 14, e1802876 (2018).
Koh, A. et al. A soft, wearable microfluidic device for the capture, storage, and colorimetric sensing of sweat. Sci. Transl. Med. 8, 366ra165 (2016).
Choi, J., Kang, D., Han, S., Kim, S. B. & Rogers, J. A. Thin, soft, skin-mounted microfluidic networks with capillary bursting valves for chrono-sampling of sweat. Adv. Healthc. Mater. 6, 1601355 (2017).
Sempionatto, J. R. et al. An epidermal patch for the simultaneous monitoring of haemodynamic and metabolic biomarkers. Nat. Biomed. Eng. 5, 737–748 (2021).
al-Tamer, Y. Y., Hadi, E. A. & al-Baldrani, I. I.Sweat urea, uric acid and creatinine concentrations in uraemic patients. Urol. Res. 25, 337–340 (1997).
Yang, Y. R. et al. A laser-engraved wearable sensor for sensitive detection of uric acid and tyrosine in sweat. Nat. Biotechnol. 38, 217 (2020).
Adelaars, S. et al. The correlation of urea and creatinine concentrations in sweat and saliva with plasma during hemodialysis: an observational cohort study. Clin. Chem. Lab. Med. 62, 1118–1125 (2024).
Altamer, Y. Y. & Hadi, E. A. Age-dependent reference intervals of glucose, urea, protein, lactate and electrolytes in thermally-induced sweat. Eur. J. Clin. Chem. Clin 32, 71–77 (1994).
Kwon, K. et al. An on-skin platform for wireless monitoring of flow rate, cumulative loss and temperature of sweat in real time. Nat. Electron. 4, 302–312 (2021).
Kim, H. S. et al. Hand-held Raman spectrometer-based dual detection of creatinine and cortisol in human sweat using silver nanoflakes. Anal. Chem. 93, 14996–15004 (2021).
Kalasin, S., Sangnuang, P. & Surareungchai, W. Satellite-based sensor for environmental heat-stress sweat creatinine monitoring: the remote artificial intelligence-assisted epidermal wearable sensing for health evaluation. ACS Biomater. Sci. Eng. 7, 322–334 (2021).
Kalasin, S. & Sangnuang, P. Multiplex wearable electrochemical sensors fabricated from sodiated polymers and mxene nanosheet to measure sodium and creatinine levels in sweat. ACS Appl. Nano Mater. 6, 18209–18221 (2023).
Rakesh Kumar, R. K., Shaikh, M. O. & Chuang, C. H. A review of recent advances in non-enzymatic electrochemical creatinine biosensing. Anal. Chim. Acta 1183, 338748 (2021).
Hussain, S. & Park, S. Y. Sweat-based noninvasive skin-patchable urea biosensors with photonic interpenetrating polymer network films integrated into PDMS chips. Acs Sens. 5, 3988–3998 (2020).
Promphet, N. et al. Cotton thread-based wearable sensor for non-invasive simultaneous diagnosis of diabetes and kidney failure. Sens. Actuators B: Chem. 321, 128549 (2020).
Singh, S., Sharma, M. & Singh, G. Recent advancements in urea biosensors for biomedical applications. IET Nanobiotechnol. 15, 358–379 (2021).
Ibáñez-Redín, G. et al. Wearable potentiometric biosensor for analysis of urea in sweat. Biosens. Bioelectron. 223, 114994 (2023).
Liu, Y. L. et al. Flexible electrochemical urea sensor based on surface molecularly imprinted nanotubes for detection of human sweat. Anal. Chem. 90, 13081–13087 (2018).
Xu, Z. Y. et al. A conducting polymer PEDOT:PSS hydrogel based wearable sensor for accurate uric acid detection in human sweat. Sens. Actuators B: Chem. 348, 130674 (2021).
Huang, C. T., Chen, M. L., Huang, L. L. & Mao, I. F. Uric acid and urea in human sweat. Chin. J. Physiol. 45, 109–115 (2002).
Pirovano, P. et al. A wearable sensor for the detection of sodium and potassium in human sweat during exercise. Talanta 219, 121145 (2020).
Yuan, Z. et al. A multi-modal sweat sensing patch for cross-verification of sweat rate, total ionic charge, and Na+ concentration. Lab. Chip 19, 3179–3189 (2019).
Nyein, H. Y. Y. et al. A wearable microfluidic sensing patch for dynamic sweat secretion analysis. ACS Sens. 3, 944–952 (2018).
Alizadeh, A. et al. A wearable patch for continuous monitoring of sweat electrolytes during exertion. Lab. Chip 18, 2632–2641 (2018).
Parrilla, M. et al. Wearable potentiometric ion patch for on-body electrolyte monitoring in sweat: toward a validation strategy to ensure physiological relevance. Anal. Chem. 91, 8644–8651 (2019).
Friedel, M. et al. Opportunities and challenges in the diagnostic utility of dermal interstitial fluid. Nat. Biomed. Eng. 7, 1541–1555 (2023).
Xu, N. et al. Microneedle-based technology: toward minimally invasive disease diagnostics. Adv. Mater. Technol-Us 7, 2101595 (2022).
Zheng, H. et al. Reverse iontophoresis with the development of flexible electronics: a review. Biosens. Bioelectron. 223, 115036 (2023).
Metry, G. S., Attman, P. O., Lonnroth, P., Beshara, S. N. & Aurell, M. Urea kinetics during hemodialysis measured by microdialysis-a novel technique. Kidney Int. 44, 622–629 (1993).
Wascotte, V. et al. Non-invasive diagnosis and monitoring of chronic kidney disease by reverse iontophoresis of urea in vivo. Eur. J. Pharm. Biopharm. 69, 1077–1082 (2008).
Ebah, L. M. et al. Reverse iontophoresis of urea in health and chronic kidney disease: a potential diagnostic and monitoring tool? Eur. J. Clin. Invest. 42, 840–847 (2012).
Varadharaj, E. K. & Jampana, N. Non-invasive potentiometric sensor for measurement of blood urea in human subjects using reverse iontophoresis. J. Electrochem. Soc. 163, B340 (2016).
Zheng, L., Zhu, D., Xiao, Y., Zheng, X. & Chen, P. Microneedle coupled epidermal sensor for multiplexed electrochemical detection of kidney disease biomarkers. Biosens. Bioelectron. 237, 115506 (2023).
Dervisevic, M., Jara Fornerod, M. J., Harberts, J., Zangabad, P. S. & Voelcker, N. H. Wearable microneedle patch for transdermal electrochemical monitoring of urea in interstitial fluid. ACS Sens. 9, 932–941 (2024).
Miller, P. R. et al. Microneedle-based transdermal sensor for on-chip potentiometric determination of K(+). Adv. Healthc. Mater. 3, 876–881 (2014).
Parrilla, M. et al. Wearable all-solid-state potentiometric microneedle patch for intradermal potassium detection. Anal. Chem. 91, 1578–1586 (2019).
Shukla, S., Machekposhti, S. A., Joshi, N., Joshi, P. & Narayan, R. J. Microneedle-integrated device for transdermal sampling and analyses of targeted biomarkers. Small Sci. 3, 2200087 (2023).
Huang, X. S. et al. 3D-assembled microneedle ion sensor-based wearable system for the transdermal monitoring of physiological ion fluctuations. Microsyst. Nanoeng. 9, 25 (2023).
Molinero-Fernández, A., Casanova, A., Wang, Q. Y., Cuartero, M. & Crespo, G. A. In vivo transdermal multi-ion monitoring with a potentiometric microneedle-based sensor patch. ACS Sensors 8, 158–166 (2022).
Li, H. et al. Microneedle-based potentiometric sensing system for continuous monitoring of multiple electrolytes in skin interstitial fluids. ACS Sens. 6, 2181–2190 (2021).
Zhu, D. D. et al. Microneedle-coupled epidermal sensors for in-situ-multiplexed ion detection in interstitial fluids. ACS Appl. Mater. Interfaces https://doi.org/10.1021/acsami.3c00573 (2023).
Zheng, Y. B. et al. A wearable microneedle-based extended gate transistor for real-time detection of sodium in interstitial fluids. Adv. Mater. 34, e2108607 (2022).
Li, M. S. et al. Current and future perspectives on microfluidic tear analytic devices. ACS Sens. 7, 1300–1314 (2022).
Giardini, A. & Roberts, J. R. Concentration of glucose and total chloride in tears. Br. J. Ophthalmol. 34, 737–743 (1950).
Kang, J., Fulop, G. & Friedman, A. H. Tear urea nitrogen and creatinine levels in renal patients. Acta Ophthalmol. 66, 407–412 (1988).
Thomas, N., Lähdesmäki, I. & Parviz, B. A. A contact lens with an integrated lactate sensor. Sens. Actuators B: Chem. 162, 128–134 (2012).
Liu, H., Yan, X., Gu, Z., Xiu, G. & Xiao, X. Electrochemical sensing in contact lenses. Electroanalysis 34, 227–236 (2021).
Yang, X. et al. Flexible, wearable microfluidic contact lens with capillary networks for tear diagnostics. J. Mater. Sci. 55, 9551–9561 (2020).
Badugu, R., Szmacinski, H., Reece, E. A., Jeng, B. H. & Lakowicz, J. R. Fluorescent contact lens for continuous non-invasive measurements of sodium and chloride ion concentrations in tears. Anal. Biochem. 608, 113902 (2020).
Moreddu, R. et al. Integration of paper microfluidic sensors into contact lenses for tear fluid analysis. Lab. Chip 20, 3970–3979 (2020).
Moreddu, R. et al. Lab-on-a-contact lens platforms fabricated by multi-axis femtosecond laser ablation. Small 17, e2102008 (2021).
Mukundan, G. & Badhulika, S. Nickel-cobalt metal-organic frameworks based flexible hydrogel as a wearable contact lens for electrochemical sensing of urea in tear samples. Mikrochim. Acta 191, 252 (2024).
Lakowicz, J. R., Badugu, R., Sivashanmugan, K. & Reece, A. Remote measurements of tear electrolyte concentrations on both sides of an inserted contact lens. Chemosensors 11, 463 (2023).
Ku, M. et al. Smart, soft contact lens for wireless immunosensing of cortisol. Sci. Adv. 6, eabb2891 (2020).
Badugu, R., Szmacinski, H., Reece, E. A., Jeng, B. H. & Lakowicz, J. R. Sodium-sensitive contact lens for diagnostics of ocular pathologies. Sens. Actuators B Chem. 331, 129434 (2021).
Park, J. et al. Soft, smart contact lenses with integrations of wireless circuits, glucose sensors, and displays. Sci. Adv. 4, eaap9841 (2018).
Keum, D. H. et al. Wireless smart contact lens for diabetic diagnosis and therapy. Sci. Adv. 6, eaba3252 (2020).
Sempionatto, J. R. et al. Eyeglasses-based tear biosensing system: non-invasive detection of alcohol, vitamins and glucose. Biosens. Bioelectron. 137, 161–170 (2019).
Kalasin, S., Sangnuang, P. & Surareungchai, W. Lab-on-eyeglasses to monitor kidneys and strengthen vulnerable populations in pandemics: machine learning in predicting serum creatinine using tear creatinine. Anal. Chem. 93, 10661–10671 (2021).
Xu, J., Tao, X., Liu, X. & Yang, L. Wearable eye patch biosensor for noninvasive and simultaneous detection of multiple biomarkers in human tears. Anal. Chem. 94, 8659–8667 (2022).
Tiffany, T. O., Jansen, J. M., Burtis, C. A., Overton, J. B. & Scott, C. D. Enzymatic kinetic rate and end-point analyses of substrate, by use of a GeMSAEC fast analyzer. Clin. Chem. 18, 829–840 (1972).
Jaffé, M. Ueber den Niederschlag, welchen Pikrinsäure in normalem Harn erzeugt und über eine neue Reaction des Kreatinins. Biol. Chem. 10, 391–400 (1886).
Jung, D., Biggs, H., Erikson, J. & Ledyard, P. U. New colorimetric reaction for end-point, continuous-flow, and kinetic measurement of urea. Clin. Chem. 21, 1136–1140 (1975).
Yetisen, A. K. et al. Scleral lens sensor for ocular electrolyte analysis. Adv. Mater. 32, e1906762 (2020).
Moonla, C. et al. Lab-in-a-mouth and advanced point-of-care sensing systems: detecting bioinformation from the oral cavity and saliva. ECS Sens. Plus 1, 021603 (2022).
Swetha, P., Balijapalli, U. & Feng, S.-P. Wireless accessing of salivary biomarkers based wearable electrochemical sensors: a mini-review. Electrochem. Commun. 140, 107314 (2022).
Haji Mohammadi, M. et al. Saliva lab-on-a-chip biosensors: recent novel ideas and applications in disease detection. Microchem. J. 168, 106506 (2021).
Lee, Y. et al. Wireless, intraoral hybrid electronics for real-time quantification of sodium intake toward hypertension management. Proc. Natl Acad. Sci. USA 115, 5377–5382 (2018).
Kim, J. et al. Non-invasive mouthguard biosensor for continuous salivary monitoring of metabolites. Analyst 139, 1632–1636 (2014).
Kim, J. et al. Wearable salivary uric acid mouthguard biosensor with integrated wireless electronics. Biosens. Bioelectron. 74, 1061–1068 (2015).
Lim, H. R. et al. Smart bioelectronic pacifier for real-time continuous monitoring of salivary electrolytes. Biosens. Bioelectron. 210, 114329 (2022).
Temilola, D. O. et al. Salivary creatinine as a diagnostic tool for evaluating patients with chronic kidney disease. BMC Nephrol. 20, 387 (2019).
Bilancio, G. et al. Saliva for assessing creatinine, uric acid, and potassium in nephropathic patients. BMC Nephrol. 20, 242 (2019).
Soni, A., Surana, R. K. & Jha, S. K. Smartphone based optical biosensor for the detection of urea in saliva. Sens. Actuators B: Chem. 269, 346–353 (2018).
Labat, C. et al. Differential associations for salivary sodium, potassium, calcium, and phosphate levels with carotid intima media thickness, heart rate, and arterial stiffness. Dis. Markers 2018, 3152146 (2018).
Kallapur, B. et al. Quantitative estimation of sodium, potassium and total protein in saliva of diabetic smokers and nonsmokers: a novel study. J. Nat. Sci. Biol. Med. 4, 341–345 (2013).
Holden, B. A., Sweeney, D. F., Vannas, A., Nilsson, K. T. & Efron, N. Effects of long-term extended contact lens wear on the human cornea. Invest. Ophthalmol. Vis. Sci. 26, 1489–1501 (1985).
Ghaffari, R., Rogers, J. A. & Ray, T. R. Recent progress, challenges, and opportunities for wearable biochemical sensors for sweat analysis. Sens. Actuators B Chem. 332, 129447 (2021).
Cho, S. et al. A skin-interfaced microfluidic platform supports dynamic sweat biochemical analysis during human exercise. Sci. Transl. Med. 16, eado5366 (2024).
Zargartalebi, H. et al. Active-reset protein sensors enable continuous in vivo monitoring of inflammation. Science 386, 1146–1153 (2024).
Thompson, I. A. P. et al. An antibody-based molecular switch for continuous small-molecule biosensing. Sci. Adv. 9, eadh4978 (2023).
Hariri, A. A. et al. Modular aptamer switches for the continuous optical detection of small-molecule analytes in complex media. Adv. Mater. 36, e2304410 (2024).
Poudineh, M. et al. A fluorescence sandwich immunoassay for the real-time continuous detection of glucose and insulin in live animals. Nat. Biomed. Eng. 5, 53–63 (2021).
Pinto, M. & Dobson, S. BK and JC virus: a review. J. Infect. 68, S2–S8 (2014).
Reploeg, M. D., Storch, G. A. & Clifford, D. B. Bk virus: a clinical review. Clin. Infect. Dis. 33, 191–202 (2001).
Lo, D. J., Kaplan, B. & Kirk, A. D. Biomarkers for kidney transplant rejection. Nat. Rev. Nephrol. 10, 215–225 (2014).
Shi, C. et al. Application of a sub-0.1-mm(3) implantable mote for in vivo real-time wireless temperature sensing. Sci. Adv. 7, eabf6312 (2021).
Kang, S. K. et al. Bioresorbable silicon electronic sensors for the brain. Nature 530, 71–76 (2016).
Mariello, M., Kim, K., Wu, K., Lacour, S. P. & Leterrier, Y. Recent advances in encapsulation of flexible bioelectronic implants: materials, technologies, and characterization methods. Adv. Mater. 34, e2201129 (2022).
Sang, M., Kim, K., Shin, J. & Yu, K. J. Ultra-thin flexible encapsulating materials for soft bio-integrated electronics. Adv. Sci. 9, e2202980 (2022).
Doloff, J. C. et al. The surface topography of silicone breast implants mediates the foreign body response in mice, rabbits and humans. Nat. Biomed. Eng. 5, 1115–1130 (2021).
Guo, H. et al. Wireless implantable optical probe for continuous monitoring of oxygen saturation in flaps and organ grafts. Nat. Commun. 13, 3009 (2022).
Zhang, H. et al. Wireless, battery-free optoelectronic systems as subdermal implants for local tissue oximetry. Sci. Adv. 5, eaaw0873 (2019).
Humar, A. & Matas, A. J. Surgical complications after kidney transplantation. Semin. Dial. 18, 505–510 (2005).
Salvadori, M., Rosso, G. & Bertoni, E. Update on ischemia-reperfusion injury in kidney transplantation: pathogenesis and treatment. World J. Transpl. 5, 52–67 (2015).
Park, J., Seok, H. S., Kim, S. S. & Shin, H. Photoplethysmogram analysis and applications: an integrative review. Front. Physiol. 12, 808451 (2021).
Traverso, G. et al. First-in-human trial of an ingestible vitals-monitoring pill. Device 1, 100125 (2023).
Srinivasan, S. S. et al. A vibrating ingestible bioelectronic stimulator modulates gastric stretch receptors for illusory satiety. Sci. Adv. 9, eadj3003 (2023).
Ouyang, W. et al. An implantable device for wireless monitoring of diverse physio-behavioral characteristics in freely behaving small animals and interacting groups. Neuron 112, 1764–1777.e1765 (2024).
Lee, K. et al. Mechano-acoustic sensing of physiological processes and body motions via a soft wireless device placed at the suprasternal notch. Nat. Biomed. Eng. 4, 148–158 (2020).
Boutry, C. M. et al. A stretchable and biodegradable strain and pressure sensor for orthopaedic application. Nat. Electron. 1, 314–321 (2018).
Kim, J. et al. A wireless, implantable bioelectronic system for monitoring urinary bladder function following surgical recovery. Proc. Natl Acad. Sci. USA 121, e2400868121 (2024).
Kang, S. K., Koo, J., Lee, Y. K. & Rogers, J. A. Advanced materials and devices for bioresorbable electronics. Acc. Chem. Res. 51, 988–998 (2018).
Zhang, Y. et al. Advances in bioresorbable materials and electronics. Chem. Rev. 123, 11722–11773 (2023).
Lu, D. et al. Implantable, wireless, self-fixing thermal sensors for continuous measurements of microvascular blood flow in flaps and organ grafts. Biosens. Bioelectron. 206, 114145 (2022).
Madhvapathy, S. R. et al. Implantable bioelectronic systems for early detection of kidney transplant rejection. Science 381, 1105–1112 (2023).
Madhvapathy, S. R. et al. Advanced thermal sensing techniques for characterizing the physical properties of skin. Appl. Phys. Rev. 9, 041307 (2022).
Crawford, K. E. et al. Advanced approaches for quantitative characterization of thermal transport properties in soft materials using thin, conformable resistive sensors. Extreme Mech. Lett. 22, 27–35 (2018).
Madhvapathy, S. R. et al. Miniaturized implantable temperature sensors for the long-term monitoring of chronic intestinal inflammation. Nat. Biomed. Eng. 8, 1040–1052 (2024).
Pizarro, T. T. et al. SAMP1/YitFc mouse strain: a spontaneous model of Crohn’s disease-like ileitis. Inflamm. Bowel Dis. 17, 2566–2584 (2011).
Clayton, P. A., McDonald, S. P., Russ, G. R. & Chadban, S. J. Long-term outcomes after acute rejection in kidney transplant recipients: an ANZDATA analysis. J. Am. Soc. Nephrol. 30, 1697–1707 (2019).
Singh, N., Pirsch, J. & Samaniego, M. Antibody-mediated rejection: treatment alternatives and outcomes. Transpl. Rev. 23, 34–46 (2009).
Levitsky, J. et al. Acute rejection increases risk of graft failure and death in recent liver transplant recipients. Clin. Gastroenterol. Hepatol. 15, 584–593.e582 (2017).
Hopkins, P. M. et al. Prospective analysis of 1,235 transbronchial lung biopsies in lung transplant recipients. J. Heart Lung Transpl. 21, 1062–1067 (2002).
Han, Z. et al. Vitrification and nanowarming enable long-term organ cryopreservation and life-sustaining kidney transplantation in a rat model. Nat. Commun. 14, 3407 (2023).
He, X. & Bischof, J. C. Analysis of thermal stress in cryosurgery of kidneys. J. Biomech. Eng. 127, 656–661 (2005).
Natesan, H. et al. A micro-thermal sensor for focal therapy applications. Sci. Rep. 6, 21395 (2016).
Sharma, A. et al. Vitrification and nanowarming of kidneys. Adv. Sci. 8, e2101691 (2021).
O’Brien, T. J. et al. The development of a thin-filmed noninvasive tissue perfusion sensor to quantify capillary pressure occlusion of explanted organs. IEEE Trans. Biomed. Eng. 64, 1631–1637 (2017).
Liapis, H. et al. Banff histopathological consensus criteria for preimplantation kidney biopsies. Am. J. Transpl. 17, 140–150 (2017).
van Stralen, K. J. et al. Diagnostic methods I: sensitivity, specificity, and other measures of accuracy. Kidney Int. 75, 1257–1263 (2009).
Hall, I. E., Doshi, M. D., Poggio, E. D. & Parikh, C. R. A comparison of alternative serum biomarkers with creatinine for predicting allograft function after kidney transplantation. Transplantation 91, 48–56 (2011).
Barone, D. G. et al. Prevention of the foreign body response to implantable medical devices by inflammasome inhibition. Proc. Natl Acad. Sci. USA 119, e2115857119 (2022).
Veiseh, O. et al. Size- and shape-dependent foreign body immune response to materials implanted in rodents and non-human primates. Nat. Mater. 14, 643–651 (2015).
Kaasch, A. J. et al. Effect of clinically uninfected orthopedic implants and pacemakers/AICDs in low-risk staphylococcus aureus bloodstream infection on crude mortality rate: a post hoc analysis of a large cohort study. Open. Forum Infect. Dis. 6, ofz170 (2019).
Jensen, M. J. et al. Cochlear implant material effects on inflammatory cell function and foreign body response. Hear. Res. 426, 108597 (2022).
Carnicer-Lombarte, A., Chen, S. T., Malliaras, G. G. & Barone, D. G. Foreign body reaction to implanted biomaterials and its impact in nerve neuroprosthetics. Front. Bioeng. Biotechnol. 9, 622524 (2021).
Li, C. et al. Design of biodegradable, implantable devices towards clinical translation. Nat. Rev. Mater. 5, 61–81 (2020).
Ciatti, J. L. et al. An autonomous implantable device for the prevention of death from opioid overdose. Sci. Adv. 10, eadr3567 (2024).
Won, S. M., Cai, L., Gutruf, P. & Rogers, J. A. Wireless and battery-free technologies for neuroengineering. Nat. Biomed. Eng. 7, 405–423 (2023).
Liu, H. C. et al. Wearable bioadhesive ultrasound shear wave elastography. Sci. Adv. 10, eadk8426 (2024).
Mac, Q. D. et al. Non-invasive early detection of acute transplant rejection via nanosensors of granzyme B activity. Nat. Biomed. Eng. 3, 281–291 (2019).
Mason, P. Blood tests used to investigate liver, thyroid or kidney function and disease. Pharm. J. 272, 446–448 (2004).
Kayashima, S. et al. Suction effusion fluid from skin and constituent analysis: new candidate for interstitial fluid. Am. J. Physiol. 263, H1623–H1627 (1992).
Pandya, D., Nagrajappa, A. K. & Ravi, K. S. Assessment and correlation of urea and creatinine levels in saliva and serum of patients with chronic kidney disease, diabetes and hypertension- a research study. J. Clin. Diagn. Res. 10, ZC58–ZC62 (2016).
Ebah, L., Brenchley, P., Coupes, B. & Mitra, S. A modified in vivo flow variation technique of microdialysis for sampling uremic toxins in the subcutaneous interstitial compartment. Blood Purif. 32, 96–103 (2011).
Mendelsohn, M., Abramson, D., Senft, S., Servodidio, C. & Gamache, P. Uric acid in the aqueous humor and tears of retinoblastoma patients. J. AAPOS 2, 369–371 (1998).
Asadi, M., Nadhum Bahjat, M. & Hosseini, M. A review on wearable sensors for sodium detection in human sweat. Anal. Bioanal. Electrochem. 15, 794–814 (2023).
Madden, J., O’Mahony, C., Thompson, M., O’Riordan, A. & Galvin, P. Biosensing in dermal interstitial fluid using microneedle based electrochemical devices. Sens. Biosens. Res. 29, 100348 (2020).
Van Haeringen, N. J. Clinical biochemistry of tears. Surv. Ophthalmol. 26, 84–96 (1981).
Ray, T. R. et al. Soft, skin-interfaced sweat stickers for cystic fibrosis diagnosis and management. Sci. Transl. Med. 13, eabd8109 (2021).
Gonçalves, A. C. et al. Chloride and sodium ion concentrations in saliva and sweat as a method to diagnose cystic fibrosis. J. Pediatr. 95, 443–450 (2019).
Senel, M., Dervisevic, M. & Voelcker, N. H. Gold microneedles fabricated by casting of gold ink used for urea sensing. Mater. Lett. 243, 50–53 (2019).
Chen, Y. J. et al. Microneedle patches integrated with lateral flow cassettes for blood-free chronic kidney disease point-of-care testing during a pandemic. Biosens. Bioelectron. 208, 114234 (2022).
Acknowledgements
The authors thank Tatiana Gandlin for providing the initial version of Fig. 1 and Sarena Wapnick (Northwestern University, IL, USA) for useful discussions about the contents of the manuscript prior to submission.
Author information
Authors and Affiliations
Contributions
S.R.M., S. C., Y.X., L.G., E.F., E.G. and J.A.R. wrote the paper. All authors reviewed and edited the manuscript prior to submission.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Nephrology thanks Jonathan Himmelfarb, Sihong Wang and Wei Gao for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Glossary
- Amperometric
-
An electroanalytical technique that measures current generated by the oxidation and reduction of an electroactive biological analyte.
- Aptamer-based molecular switches
-
Molecular mechanisms by which aptamers bind to the target and undergo structural conformational changes.
- Aptamer-based sensors
-
A biosensor category that uses short, single-stranded DNA or RNA to specifically bind to target analytes.
- Electrochemistry
-
The study of the relationship between electrical and chemical processes, often applied to biosensors for detection of target analytes.
- Impedance
-
An electroanalytical technique that measures changes in the electrical impedance of an electrode surface in the presence of the target molecule.
- Interpenetrating polymer network
-
Polymer chains, comprising two or more networks, that are interlaced at molecular scales.
- Iontophoresis
-
An electrical technique that passes a weak electrical current through the skin to deliver ions or drugs for extraction of sweat or interstitial fluid.
- Polyaniline ink
-
A highly conducting polymeric ink, frequently used for biosensing applications.
- Potentiometric
-
An electroanalytical technique that measures electrical potential as an analytical signal generated by an electrochemical reaction.
- Surface-enhanced Raman scattering
-
A signal amplification technique that enhances Raman scattering by surface roughness for detection of target analytes.
- Ultradian rhythms
-
Biological cycles that occur with periods shorter than 24 h.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Madhvapathy, S.R., Cho, S., Gessaroli, E. et al. Implantable bioelectronics and wearable sensors for kidney health and disease. Nat Rev Nephrol 21, 443–463 (2025). https://doi.org/10.1038/s41581-025-00961-2
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41581-025-00961-2
This article is cited by
-
Mechanics and bio-interface engineering in flexible biosensors for continuous health monitoring
npj Flexible Electronics (2026)
-
Next-generation biosensing for in situ monitoring
Nature Sensors (2026)
-
Urine as a source of biomarkers and biological knowledge in chronic kidney disease
Nature Reviews Nephrology (2026)
-
Advances and perspectives in fiber-based electronic devices for next-generation soft systems
npj Flexible Electronics (2025)