Abstract
Acute kidney injury (AKI) during pregnancy and the postpartum period, known as pregnancy-associated AKI (PrAKI), is an important health concern and driver of health inequity worldwide. Causes of PrAKI include sepsis, autoimmune disorders and pregnancy-specific pathologies such as hypertensive disorders. Common risk factors include maternal comorbidities and use of nephrotoxic medications. PrAKI accounts for a substantial proportion of maternal mortality and morbidity, particularly in low-income and middle-income countries, and may also adversely affect the fetus, resulting in death, premature birth and small for gestational age. In this Consensus Statement, we present recommendations on the causes, diagnosis, management and follow-up of PrAKI from the 32nd Acute Disease Quality Initiative meeting, which involved international experts in obstetrics, midwifery, obstetric medicine, paediatrics, internal medicine, anaesthesiology, nephrology and critical care. We suggest that pregnant and postpartum women at a high risk of PrAKI should be identified to enable prevention, surveillance and timely diagnosis. The multidisciplinary management of these patients should be tailored to treat their specific causes of PrAKI to optimize short-term and long-term neonatal and maternal outcomes. Further observational and interventional studies are needed to address existing gaps in knowledge of PrAKI and improve maternal and fetal outcomes.
Similar content being viewed by others
Introduction
Pregnancy-associated acute kidney injury (PrAKI) is a risk factor for maternal and fetal mortality and morbidity. The reported incidence of PrAKI ranges from 40 to 100 cases per 10,000 pregnancies, with a higher frequency in lower-resource settings1. PrAKI is associated with a 13-fold increase in the odds of maternal mortality, a 9-fold increase in the odds of cardiovascular events and a 30–60% increase in the risk of fetal mortality and morbidity1,2,3,4,5,6,7,8,9. However, robust data to support advocacy and action to reduce the risk and improve the management of PrAKI are lacking.
The 32nd Acute Disease Quality Initiative (ADQI) meeting was convened in January 2024 in Hyderabad, India, to evaluate the global burden of acute kidney injury (AKI) during pregnancy and the postpartum period, focusing on epidemiology, risk factors, aetiology, diagnosis, management and follow-up. Participants were selected to reflect the global multidisciplinary workforce that cares for patients with or at risk of PrAKI, including representatives from obstetrics, midwifery, paediatrics, internal medicine, obstetric medicine, critical care, nephrology and anaesthesiology. The primary aim was to develop evidence-based consensus statements to educate and guide health-care practitioners regarding PrAKI and to improve the care and outcomes of pregnant women and their children. Priority areas for further research were also identified.
Methods
Before the in-person meeting, the working groups met virtually to identify key questions for discussion and consensus and to review the literature up to December 2023. The identified studies were heterogeneous, used differing definitions of AKI, included patients from different health-care settings and data were often limited to cohort and observational reports. During the meeting, a modified Delphi method was applied10. The working groups presented key questions to the entire panel for feedback. During break-out sessions, the groups refined the questions and formulated answers, supported by evidence when available and by expert opinion when no clear evidence was available. Statements were presented to the panel several times and re-revised iteratively in response to feedback until consensus was achieved.
The panel resolved that the term ‘PrAKI’ included AKI due to pregnancy-specific pathologies such as pre-eclampsia, hyperemesis, postpartum haemorrhage or chorioamnionitis as well as AKI that develops during pregnancy with coincidental causes. Although abortion (induced termination of pregnancy) was distinguished from miscarriage (spontaneous pregnancy loss), the panel acknowledge that both septic miscarriage and septic abortion may cause AKI.
The certainty of evidence was rated using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) system11. Where evidence was lacking and consensus could not be reached, the group made recommendations for future research. A priori, agreement by 85% of votes was defined as necessary for consensus, as per ADQI methodology12. The final anonymous voting round took place electronically after the meeting.
In this document, we use the terms ‘woman’ and ‘women’s health’ to refer to cisgender women as most research on PrAKI has been conducted in cisgender cohorts. However, kidney and obstetric services and care delivery must be appropriate, inclusive and sensitive to the needs of individuals whose gender identity does not align with the sex they were assigned at birth.
Spectrum of pregnancy-associated acute kidney injury
What is known about the epidemiology of pregnancy-associated acute kidney injury?
Consensus statement:
-
PrAKI is an important global public health problem that is associated with maternal and perinatal morbidity and mortality, which varies in incidence, severity and outcome according to socio-economic factors, health-care expenditure and access to antenatal care. Pre-eclampsia, sepsis and haemorrhage are common causes of PrAKI.
The reported incidence of PrAKI ranges from 40 to 100 cases per 10,000 pregnancies1,4,5,6,7,8,9. Postpartum PrAKI has been reported to occur in 16–40 per 10,000 pregnancies, accounting for 20–50% of PrAKI1,9,13,14,15. The reported incidence of PrAKI has increased over the past 20 years, but heterogeneity is observed across regions, partly due to differences in geography and income4,16,17,18. An inverse relationship seems to exist between total national health expenditure as a percentage of gross domestic product and PrAKI incidence. High-income countries (HICs) report the lowest incidences of PrAKI17,18, with higher incidences observed in low-income and middle-income countries19. The incidence of severe PrAKI that meets the Kidney Disease Improving Global Outcomes (KDIGO) AKI stage 3 criteria is highest in Africa, with an incidence of 77–233 per 10,000 pregnancies15,20.
Approximately 6–12% of PrAKI is associated with maternal mortality14,15, and 16–37% of women with PrAKI require haemodialysis1,16. Studies that included patients with less severe PrAKI reported lower incidences of need for haemodialysis and maternal mortality than those that included patients with more severe PrAKI. PrAKI is associated with a 3.5-fold (95% CI 1.9–6.5) increase in the odds of adverse pregnancy outcomes, including miscarriage, preterm birth, stillbirth and perinatal death, compared with pregnancies without AKI18,19,20,21,22. Fetal death or miscarriage has been reported in 22–36% of cases of PrAKI14,19,22. Pre-eclampsia or eclampsia is the most common aetiology of PrAKI, followed by haemorrhage and sepsis8,14,16,19,20,22.
What are the risk factors for pregnancy-associated acute kidney injury?
Consensus statement:
-
Risk factors for PrAKI include underlying comorbidities, maternal health during pregnancy, access to prenatal care and other socio-demographic factors.
The discrepancies in incidence and outcomes of PrAKI between higher-resource and lower-resource settings, clearly indicate that factors beyond pregnancy have an impact on the risk. PrAKI risk factors are likely to overlap with risk factors that contribute to maternal mortality. For instance, the incidence of hypertensive disorders of pregnancy has increased in HICs (perhaps owing to older maternal age and obesity) but seems to have decreased in low-income and middle-income countries as maternal care has improved. Even within HICs, PrAKI is more common among minority ethnic groups and vulnerable populations23. Dramatic differences in risk of PrAKI suggest that underlying inequities and social determinants of health related to where a woman grew up and what opportunities she had as a child, teenager and young woman are important contributors via structural, cultural and socio-economic mechanisms. A woman’s birth history or prior pregnancy history, medical comorbidities and access to health care will impact her biomedical health and susceptibility to pre-eclampsia, chronic kidney disease (CKD) and PrAKI24. Outcomes in PrAKI are associated with individual physical and clinical risk factors but are also substantially impacted by the timeliness with which a woman seeks care, and the quality of the care that she receives25.
How can high-risk persons be identified for primary prevention of pregnancy-associated acute kidney injury?
Consensus statement:
-
Risk profile assessments before and during pregnancy can identify women at an increased risk of PrAKI and inform strategies for primary prevention. These strategies include optimizing an individual’s general, pregnancy and kidney health and addressing social determinants of health.
Early identification of high-risk pregnant individuals enables increased surveillance for PrAKI and provides opportunities for early interventions. PrAKI risk profiles encompass social determinants of health, clinical characteristics, medical comorbidities and pregnancy-specific and kidney-specific risk factors (Box 1). Primary prevention of PrAKI should be provided at the population, health-care system and patient levels26,27. Assessment of environmental, socio-economic and/or cultural factors as well as processes of care is of particular importance in resource-limited regions where these factors are major contributors to PrAKI risk. To prevent PrAKI, modifiable risk exposures must be identified and remedied. The provision of regular and quality antenatal and peripartum care and equity of access to this care are key interventions. Antenatal and peripartum care must include measures to reduce the risk of sepsis and haemorrhage, as well as the prevention, early diagnosis and management of pre-eclampsia.
Pathophysiology and risk factors
How does pregnancy physiology influence the pathophysiology of acute kidney injury?
Consensus statement:
-
Physiological changes in pregnancy may alter susceptibility to PrAKI. The presence of the fetal–placental unit (FPU) is unique to pregnancy, resulting in haemodynamic, neurohumoral, immunological, vascular and structural changes that are also influenced by pre-existing comorbidities.
AKI is a multifactorial syndrome28. Pregnancy induces lower peripheral vascular resistance, higher heart rate, lower arterial blood pressure and increased cardiac output. An increase in plasma flow rate due to circulatory vasodilation leads to an increase in glomerular filtration rate (GFR) and a decrease in serum creatinine (SCr). These circulatory alterations may mask volume depletion or exaggerate organ hypoperfusion as a consequence of other exposures, for example, sepsis or hypovolaemia29,30. At the microvascular level, glomerular endothelial cells may become mildly swollen and vacuolated31. Some pregnancy-associated physiological changes can predispose to PrAKI (Fig. 1). For instance, humoral and cellular changes in the maternal immune system enhance tolerance to the FPU but also increase the risk of infection, autoimmune disorders and PrAKI. Mechanical pressure from the gravid uterus and pregnancy-associated hormonal changes can lead to physiological dilatation of the urinary collecting system with reduced peristalsis32. These structural changes increase the risk of ascending urinary tract infection, potentially leading to acute pyelonephritis and PrAKI33,34. Patients with pre-existing structural renal abnormalities, such as autosomal-dominant polycystic kidney disease, nephrolithiasis and previous upper urinary tract infections, are particularly at risk.
Pregnancy-associated acute kidney injury (AKI) has multiple different risk factors and aetiologies. Pregnancy-associated haemodynamic, immunological and structural changes can increase the risk of causes of AKI such as infections, autoimmune disorders and hypertensive disorders of pregnancy. Maternal factors such as comorbidities and pregnancy-specific pathologies, and general AKI risk factors, including the use of nephrotoxic medications, can also increase the risk of pregnancy-associated AKI. The risks vary according to gestational age. HELLP, haemolysis, elevated liver enzyme levels and low platelet count.
A healthy pregnancy is an immune-tolerant state. The FPU is a physical interface between the mother and the fetus, with paternal antigens expressed by the fetus. As paternal antigens are foreign to the mother, a successful pregnancy depends on immune adaptation, involving suppression of the adaptive maternal immune system but only minimal changes in the innate immune system35. Trophoblast cells form a specific placental cell layer that protects the embryo from the maternal immune system, which would otherwise destroy the foreign tissue. The main mechanisms of immune escape at the maternal–fetal interface are a reduction in the expression of major histocompatibility complex (HLA) molecules by trophoblast cells, the presence of complement regulators, activation of regulatory T cells and an increase in immune checkpoints36. The major HLA class II is not expressed and only a few alleles of HLA class I (HLA-E, HLA-F and HLA-G) are expressed by trophoblast cells in the first trimester. Several factors, such as chemokines, growth factors, cytokines, and angiogenic factors, such as placental growth factor (PlGF) and vascular endothelial growth factor (VEGF), are produced by the trophoblast. In addition, complement regulatory proteins (MCP (also known as CD46), DAF (also known as CD55) and MIRL (also known as CD59)) are secreted by the placenta, avoiding complement activation during pregnancy37. The presence of a healthy FPU protects the mother and the fetus but any alteration in the immune balance or the crosstalk between trophoblasts and maternal cells can lead to adverse pregnancy outcomes, including AKI38.
Pre-eclampsia, eclampsia and haemolysis, elevated liver enzyme levels and low platelet count (HELLP) syndrome are pregnancy-specific syndromes that result from placental dysfunction (Fig. 1). Placental ischaemia leads to the production of anti-angiogenic factors (for example, soluble FMS-like tyrosine kinase 1 (sFLT1) and soluble endoglin), inflammation and immune responses that induce a maternal angiogenic imbalance and an inflammatory state, resulting in maternal endothelial and multisystem dysfunction characteristic of pre-eclampsia39. These changes can cause hypertension, proteinuria and sometimes AKI. Maternal predispositions to AKI include pre-existing chronic hypertension, previous hypertensive disorders of pregnancy and/or fetal growth restriction, CKD, diabetes, previous AKI and autoimmune disorders. The incidence of acute cortical necrosis (ACN) is also increased during pregnancy. ACN may occur as a consequence of pre-eclampsia, placental abruption, postpartum haemorrhage, puerperal sepsis or, rarely, thrombotic microangiopathy (TMA)40.
Pregnancy-associated immunological changes can lead to flares in certain autoimmune conditions. Women with systemic lupus erythematosus and antiphospholipid antibodies are at an increased risk of adverse pregnancy outcomes41. Pregnancy can result in a lupus flare or even a primary manifestation of the disease with the attendant risk of lupus nephritis as well as pre-eclampsia and PrAKI42,43,44. In general, primary TMAs are rare but pregnancy is a predisposing factor. Although complement regulatory proteins of the placenta provide protection during the first and second trimesters, AKI due to complement-mediated TMA may occur in the late stages of pregnancy or postpartum.
How does pregnancy affect the acute kidney injury course and its recovery?
Consensus statement:
-
Compared with other AKI syndromes, the presence of the FPU alters recovery of AKI and maternal morbidity and mortality.
During pregnancy, hyperfiltration, increased renal blood flow and a decrease in renal functional reserve are well described45,46. These changes not only increase the risk of PrAKI but may also lead to slower recovery of renal function or more maladaptive repair45,47. ACN is often associated with poor prognosis and progression to kidney failure.
Among hospitalized pregnant women, those who had PrAKI during hospitalization had a higher likelihood of inpatient mortality than those who did not have PrAKI while in hospital (OR 13.50; 95% CI 10.47–17.42)27. In the long term, women with PrAKI are at an increased risk of cardiovascular events (OR 9.74; 95% CI 9.08–10.46), CKD, kidney failure and kidney-related hospitalization27.
What is the effect of pregnancy-associated acute kidney injury on the fetus?
Consensus statement:
-
The underlying aetiology of PrAKI and other predisposing factors may result in preterm birth, small for gestational age (SGA) and intra-uterine growth restriction by direct and indirect mechanisms.
During pregnancy, PrAKI can lead to fetotoxicity owing to increased maternal serum concentrations of uraemic toxins, potassium or acids48,49. Other potential effects of PrAKI on the fetus include placenta hypoperfusion (for example, due to volume depletion, sepsis or haemorrhage) or vascular occlusion (for example, due to TMA or malaria); placental dysfunction due to systemic diseases (for example, sepsis, lupus nephritis or acute fatty liver of pregnancy (AFLP)); or abnormal placentation due to pre-eclampsia. The fetal effects of placental dysfunction include fetal growth restriction, still-birth and preterm delivery. As a result, babies born to mothers with PrAKI often need admission to the intensive care unit50.
Diagnosis of pregnancy-associated acute kidney injury
What is the role of kidney health assessment in pregnancy?
Consensus statements:
-
We recommend that a kidney health assessment (KHA) be performed at the first encounter in all pregnant women. In high-risk women and those with ongoing insults, we recommend ongoing KHA. (Best practice statement)
-
We suggest that, at a minimum, serum creatinine (using a standardized assay) and blood pressure measurement, urinalysis, urine dipstick or quantitative proteinuria assessment where feasible, be used for KHA. (Weak recommendation, grade B)
-
We recommend against using estimated GFR (eGFR) to assess kidney function in pregnancy. (Strong recommendation, grade B)
A systematic KHA in pregnancy is essential. The components of KHA include a comprehensive evaluation of PrAKI risk factors, SCr and blood pressure measurement, urine dipstick or quantitative proteinuria and a review of medications51. SCr is suggested for kidney function monitoring during pregnancy but several challenges exist, including the availability and standardization of assays and potential confounding factors52,53. Published reference ranges for SCr during pregnancy are 0.53–0.87 mg/dl (47–77 µmol/l) but vary by gestational age (reaching their nadir in the middle of the second trimester) and SCr assay methods54. Values above pregnancy-related reference SCr might reflect a substantial decline in renal function and warrant serial monitoring as they may indicate gestational changes, AKI, acute kidney disease (AKD) or pre-existing CKD.
Standard eGFR formulae are not validated in pregnancy. The Modification of Diet in Renal Disease equation underestimates and the Cockcroft–Gault equation overestimates the actual GFR depending on the stage of pregnancy55. Measurement of timed creatinine clearance is cumbersome but might be used for drug dosing56. The current evidence does not support the use of salivary urea nitrogen or cystatin C during pregnancy57,58.
Urinalysis might identify proteinuria and/or haematuria. Proteinuria may represent physiological changes during pregnancy as a result of an increase in GFR (a level of <300 mg daily is considered normal) but may also indicate pre-eclampsia, glomerular or tubular disease, or pre-existing CKD59. Ideally, quantitative proteinuria assessment is recommended, but point-of-care urine dipstick might be the only available tool, especially in limited-resource settings. The presence of microalbuminuria (≥2+) should prompt an assessment for kidney dysfunction60. Approximately 1 in 5 women have dipstick-positive haematuria at some point during their pregnancy61. When examined further, almost 40% have normal urine microscopic results but approximately 15% have true haematuria, indicating glomerular or structural kidney disease. Urogenital malignancy is a potential cause of haematuria but is very rare62.
Pre-existing comorbidities (such as hypertension and diabetes) require optimization of management during pregnancy. High-risk women or those experiencing new health challenges should undergo ongoing KHA at each clinical encounter.
When is pregnancy-associated acute kidney injury suspected?
Consensus statements:
-
We suggest that PrAKI be suspected in high-risk women with associated exposures and/or risk factors, and in those with specific signs and symptoms. (Best practice statement)
-
We recommend documenting each episode and distinguishing specific causes of PrAKI to guide treatment and determine future risks. (Best practice statement)
The initial step towards early recognition of PrAKI begins with identifying high-risk individuals. Health-care providers and pregnant women should be educated on how to recognize signs and symptoms of PrAKI throughout pregnancy. Appreciating clinical scenarios associated with PrAKI, such as hyperemesis, hypertensive disorders of pregnancy, HELLP syndrome, AFLP, various causes of antepartum, intrapartum and postpartum haemorrhage, infections, TMA, medications and obstetric complications, is crucial and informs further investigations.
Multiple episodes of PrAKI can complicate pregnancy. It is important to recognize each episode, differentiate its causes and plan for prevention, treatment and follow-up accordingly.
How is pregnancy-associated acute kidney injury diagnosed?
Consensus statements:
-
We recommend that change in SCr from a previous baseline (preconception or at the first antenatal visit), when available, is used to diagnose PrAKI. (Strong recommendation, grade B)
-
In the absence of a baseline SCr, the published reference ranges in pregnancy may be used as a reference SCr. (Weak recommendation, grade B)
-
We recommend serial monitoring in patients with SCr above the reference concentrations and in those with an increase in SCr above baseline to diagnose PrAKI, AKD or CKD. (Strong recommendation, grade B)
-
We suggest that PrAKI be considered if there is any increase in SCr from baseline concentrations associated with a new insult or prolonged oliguria or anuria in the antenatal or postpartum period. (Weak recommendation, grade B)
-
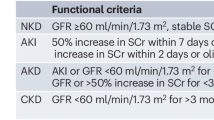
We suggest the KDIGO consensus criteria be used for AKI staging and prognostication. KDIGO stage 1 needs to be interpreted within the clinical context. (Weak recommendation, grade B)
-
We suggest that complete recovery from AKI be defined as a return of SCr to preconception baseline or reference SCr. (Weak recommendation, grade B)
No standardized definition of PrAKI exists. The KDIGO consensus criteria for AKI are based on an increase in SCr and/or a decrease in urine output, but are less studied in pregnancy63. Published reference ranges for SCr during pregnancy vary. Notably, the availability and standardization of urine testing and creatinine assays may be limited in some settings.
Oliguria may be a peripartum physiological response64. However, prolonged oliguria (≥6 h in the antenatal and postpartum period or ≥12 h in the peripartum period) should be considered abnormal. In about 20% of patients with PrAKI stage 1, no apparent cause could be identified, and the episodes were not associated with worse outcomes23. KDIGO AKI stages 2 and 3 during pregnancy are linked to poor maternal and fetal outcomes65,66. Complete recovery should be defined as a return of SCr to its baseline or reference value.
How can pregnancy-associated acute kidney injury be investigated?
Consensus statements:
-
We recommend investigating PrAKI using a combination of blood and/or urine tests, kidney imaging and kidney biopsy based on aetiology and available resources. (Strong recommendation, grade B)
-
We suggest that selected pregnancy-specific biomarkers be incorporated into clinical assessment to differentiate pre-eclampsia from other causes of PrAKI or from underlying CKD. Insufficient data are available to recommend the use of kidney-specific biomarkers to diagnose and stage PrAKI. (Weak recommendation, grade C)
The causes of PrAKI involve functional alterations and/or structural damage. As functional causes are most prevalent during the first trimester, the timing of PrAKI provides valuable insights into potential causes (Fig. 2). The PrAKI work-up can be tailored based on the suspected aetiology and available resources67,68,69 (Supplementary Table 1). Data on alternative biomarkers for PrAKI diagnosis and risk assessment are limited and inconclusive70,71. However, measurement of serum concentrations of anti-angiogenic factors (for example, sFLT1 and PlGF) may aid the diagnosis of pre-eclampsia72.
Risk factors for pregnancy-associated acute kidney injury (PrAKI) include advanced maternal age, Black race, obesity, hypertension and diabetes mellitus. The potential causes of PrAKI differ according to gestational age. COVID-19, coronavirus disease 2019; DIC, disseminated intravascular coagulation, HELLP, haemolysis, elevated liver enzyme levels and low platelet count; HUS, haemolytic uraemic syndrome; OHSS, ovarian hyperstimulation syndrome; PPI, proton pump inhibitor; TTP, thrombotic thrombocytopenic purpura; UTI, urinary tract infection.
The decision to perform a kidney biopsy in pregnancy is complex and should include a multidisciplinary discussion with the patient. Kidney biopsy can be justified when the results will guide treatment, help to prolong the course of the pregnancy and improve fetal outcomes, for example, in patients with unexplained rapidly declining kidney function, de novo nephrotic-range proteinuria in the absence of pre-eclampsia, active urine sediment or suspected lupus flare, or in kidney transplant recipients with PrAKI. The risk of kidney biopsy complications (ranging from small haematomas to major bleeding requiring blood transfusion) is higher during pregnancy (7%) than in the postpartum period (1%)73. The biopsy should ideally be performed before 23 weeks of gestation. Kidney biopsy may inform treatment and help to prolong pregnancy in a carefully selected subgroup of pregnant women3,73. A systematic review demonstrated that 66% of kidney biopsies performed in pregnancy resulted in therapeutic changes73.
Management of pregnancy-associated acute kidney injury
How can risk assessment inform the prevention and management of PrAKI to improve maternal and neonatal outcomes?
Consensus statement:
-
Individualized risk assessment incorporating pre-existing and current risk factors for PrAKI, as well as ongoing surveillance and monitoring, may enable the implementation of care plans to prevent PrAKI or facilitate accurate and timely diagnosis and management for the mother and baby.
PrAKI may be asymptomatic, and kidney function testing prepartum or postpartum is rarely performed or is not possible, particularly in low-resource settings3,25. Approaches to promote early diagnosis and intervention for PrAKI, such as e-alerts and point-of-care testing, have not been studied or validated in pregnancy. Therefore, risk assessment for PrAKI is a cornerstone of primary prevention and management74. Ongoing iterative risk assessments for PrAKI throughout pregnancy and the peripartum period should include assessment of situational risk (for example, access to antenatal and intrapartum care and nutritional health); pre-existing individual risk (for example, diabetes, hypertension and CKD); and identification of trigger events for PrAKI (for example, hyperemesis gravidarum, sepsis, obstetric haemorrhage and nephrotoxins). Timely recognition of PrAKI trigger events and appropriate treatment of the underlying trigger is imperative to prevent AKI progression and improve outcomes (Box 2). If immediate treatment of the cause of PrAKI is not possible, for example, if the decision is made to prolong pregnancy in a woman with pre-eclampsia to optimize fetal condition, close observation and optimization of haemodynamics and fluid status are crucial. Continuity of care and multidisciplinary joint decision making are essential to improve maternal and neonatal outcomes75.
What interventions for pregnancy-associated acute kidney injury can be used to improve maternal and neonatal outcomes?
Consensus statement:
-
Determining the specific cause of AKI, and medication management, informs the treatment of PrAKI. Fluid balance and volume status assessment must also consider the unique physiological and pathophysiological changes in pregnancy.
A fundamental principle in PrAKI management is that treatment must be directed towards the specific cause76 (Box 2). Fluid resuscitation is critical when AKI is related to a decrease in effective blood volume and should be directed to maintain renal and uteroplacental perfusion26,77. However, fluid administration should be performed with caution in women with pre-eclampsia or cardiomyopathy owing to the increased risk of pulmonary oedema. If oliguria persists despite adequate fluid resuscitation, further fluid infusion should be minimized.
A careful medication review must be undertaken, and any drugs with the potential to cause further renal injury (for example, non-steroidal anti-inflammatory drugs (often used as first-line pain relief after caesarean section) or aminoglycoside antibiotics) should be stopped, if possible78. Women with CKD or hypertensive disorders are particularly susceptible to nephrotoxic exposures. Prescription of any medication during PrAKI should balance the risks and benefits to the mother with any potential perceived harm to the fetus76. Renally cleared medications require dose adjustment according to the degree of renal impairment79. Given the increase in the volume of distribution in pregnancy and the risk of AKI, therapeutic drug monitoring, if available, may aid in the monitoring and dosing of nephrotoxic medications (for example, vancomycin and tacrolimus).
Differentiating between conditions in which delivery of the fetus is central to stopping the disease process and diseases that are not affected by delivery can be challenging. In cases of pre-eclampsia, placental abruption, HELLP syndrome and AFLP, prompt delivery of the fetus must be considered to reverse maternal organ dysfunction80. Delivery may also be needed to control bleeding or to remove the source of sepsis (for example, in cases of septic abortion or chorioamnionitis)81,82 (Box 2). Intrinsic pathologies, such as glomerulonephritides, do not necessarily warrant delivery. Thrombotic thrombocytopenic purpura should be treated with plasma exchange, and improved outcomes have been demonstrated with complement inhibitors (for example, eculizumab or ravulizumab) in complement-mediated TMA83,84. Glomerulonephritis flares are often treated with steroids and other pregnancy-compatible immunosuppressants78,85,86. Management of biopsy-proven acute tubular necrosis is supportive, with restoration of intravascular volume, avoidance of nephrotoxins and provision of kidney replacement therapy (KRT). ACN may be diagnosed using kidney biopsy or non-enhanced magnetic resonance imaging and management is supportive87. In cases of post-renal AKI, relieving the obstruction is urgent and may require ureteric stenting or placement of percutaneous nephrostomies, as appropriate, with minimization or avoidance of radiation exposure or delivery of the fetus if the obstruction is due to the gravid uterus.
How can kidney replacement therapy be applied in pregnancy-associated acute kidney injury to improve maternal and neonatal outcomes?
Consensus statement:
-
The maternal indications for KRT do not differ from those of non-pregnant individuals.
KRT may be necessary in cases of progressive AKI and complications of AKI, including fluid overload, refractory hyperkalaemia, uraemic symptoms and severe metabolic acidosis88,89. The obstetrics literature suggests that in pregnant patients with CKD, KRT should be considered when plasma urea is >17–20 mmol/l to prevent fetal uraemic toxicity when the risks of pre-term delivery outweigh those of KRT initiation90. For women receiving haemodialysis during pregnancy, we suggest a dialysis dose prescription aiming for a pre-dialysis urea concentration <12.5 mmol/l90, or blood urea nitrogen concentration <50 mg/dl91,92,93. A dialysis dose that targets these urea concentrations is associated with improved fetal outcomes, probably due to reduced uraemia and a consequent lower risk of polyhydramnios and prematurity, so should be the standard of care in pregnancy92,94. However, a need exists for more precise data on urea thresholds in the PrAKI context.
The choice of KRT modality must be based on patient preference, stage of pregnancy, availability of modalities and the expected efficiency of the modality for that individual95. Intermittent haemodialysis is used for most patients. For critically ill pregnant patients with PrAKI requiring continuous KRT, evidence from non-pregnant populations suggests that the dose prescription should be at least an effluent flow of 20–25 ml/kg/h of pre-pregnancy body weight95.
What is the role of breastfeeding and contraception in pregnancy-associated acute kidney injury?
Consensus statement:
-
PrAKI management includes informed discussion of feeding choices and timely contraceptive counselling.
The World Health Organization recommends exclusive breastfeeding for 6 months, with supplemental breastfeeding for an additional 2 years based on substantial evidence of nutritional, immunological and other broader benefits for the mother and infant96. The benefits of breastfeeding are greater for premature infants. Feeding choices should be implemented in the care of postpartum women, irrespective of the level of care and independent of PrAKI. Medications compatible with breastfeeding should be prescribed where possible, although safety data informing prescribing practices in lactation are sparse.
Facilitating access to contraceptive choices is imperative for health promotion, and to support choice in childbearing and pregnancy spacing, as unintended pregnancy is associated with increased risk to the mother and the fetus97.
Long-term renal and non-renal complications of pregnancy-associated acute kidney injury for mother and fetus
What is the role of a risk profile assessment post-pregnancy-associated acute kidney injury?
Consensus statement:
-
We suggest that all women and children should have a post-PrAKI risk profile assessment documented before discharge from the index PrAKI health-care encounter. Given the risk of renal and non-renal complications after PrAKI, a comprehensive health review facilitates tailored follow-up. (Best practice statement)
Data on long-term outcomes after PrAKI are limited, with some studies describing an association between ongoing proteinuria or albuminuria and the development of CKD13,87,98,99,100,101,102,103,104,105,106,107,108,109 (Supplementary Table 2). However, most studies do not test for the resolution of PrAKI or differentiate between women with pre-eclampsia and those with PrAKI9,110. Indeed, PrAKI may be diagnosed erroneously and represent (previously unknown) CKD, explaining the lack of complete resolution. As non-pregnant patients with AKI are at an increased risk of CKD, cardiovascular morbidity and mortality, these outcomes are also likely to be pertinent to women with PrAKI111. The underlying causes of PrAKI are also likely to influence long-term outcomes. For instance, the long-term outcomes of conditions such as pre-eclampsia differ from those of atypical haemolytic uraemic syndrome112,113,114,115.
In light of these risks, all women should undergo a post-PrAKI risk profile assessment after the index health-care encounter, including those who have experienced pregnancy loss or termination (Fig. 3). The assessment involves consideration of pre-pregnancy risk factors, obstetric-related aspects and renal and non-renal postpartum factors (Supplementary Table 3).
Suggested paradigm for the follow-up care of patients who experience pregnancy-associated acute kidney injury (PrAKI). The degree of nephrology-based follow-up increases with the number of comorbidities, the severity of acute kidney injury (AKI) and the severity of pregnancy complications and adverse pregnancy outcome (miscarriage, preterm birth, stillbirth and perinatal death). The timing and nature of follow-up are suggestions, as limited data are available to inform this process. The items in each bucket follow the ‘OR’ rule; therefore, each patient should follow the most severe bucket if they meet one criterion of that bucket. Follow-up should include assessment of kidney function, advocacy, medications, blood pressure, pregnancy planning and sick-day management (KAMPPS). KRT, kidney replacement therapy; SCr, serum creatinine.
Although a substantial number of patients diagnosed with PrAKI receive their diagnosis within a hospital setting, individuals with milder cases of AKI, those who have experienced an abortion, miscarriage or stillbirth, or those residing in resource-limited environments may be identified in the community. The optimal time for conducting risk stratification is upon discharge from the health-care encounter, regardless of the setting.
PrAKI is also associated with adverse fetal and/or obstetric consequences, including fetal growth restriction and preterm birth6,98,100,110,116,117,118,119,120,121,122. Despite the current absence of long-term follow-up data for children born to mothers with PrAKI, a growing body of evidence suggests a potential association with adverse long-term outcomes such as frequent hospitalization and increased risk of ischaemic heart disease and stroke, independent of pre-term or SGA birth120,121,122, particularly after pre-eclampsia. These neonates must therefore have a comprehensive health check-up, including consideration of maternal, PrAKI-related and paediatric risk factors tailored to resource availability (Fig. 4).
Suggested paradigm for the follow-up care of children whose mothers experienced pregnancy-associated acute kidney injury (PrAKI). Follow-up should be based on risk assessment. The timing and nature of follow-up are suggestions, as limited data are available to inform this process. LBW, low birth weight. *High-risk newborns include sick newborns and those requiring neonatal intensive care. Limited data are available on the long-term kidney health of these newborns and their follow-up may be tailored based on their neonatal course. ‡The timing of the repeat check may depend on the degree of abnormality at discharge and on the feasibility of follow-up, including the available resources and maternal health.
What is the suggested management of women after pregnancy-associated acute kidney injury?
Consensus statement:
Management and follow-up of women after PrAKI is tailored based on their individual risk profile and health-care setting and includes consideration of medical and psychological needs, customized education and subsequent pregnancy planning.
Long-term follow-up of women after PrAKI is informed by their risk profile. This follow-up should include monitoring of renal health and overall well-being using a comprehensive multidisciplinary approach dependent on resource setting. Until data to inform specific follow-up for women with PrAKI are available, a modified approach based on the ADQI quality improvement goals for AKI framework is recommended51 (Fig. 3). Access to testing of SCr and a urine dipstick should be universal for all women at the primary care level. The frequency should be determined by the severity of PrAKI and resolution, as well as comorbidities and other health-care needs. Similar to follow-up after other types of AKI, the key components include assessment of kidney function, advocacy, medications, blood pressure, pregnancy planning and sick-day management (KAMPPS) (Box 3). Persistent kidney abnormalities, including regression of proteinuria and hypertension, may necessitate further investigations, such as renal imaging or biopsy, to identify any structural damage due to AKD or pre-existing CKD, with consideration of conditions that may be more common in pregnancy (for example, ACN or atypical haemolytic uraemic syndrome). Medication stewardship needs to include contraception, breastfeeding status and future pregnancy planning123.
Psychosocial support is an often overlooked but essential component of long-term follow-up. Women who have experienced PrAKI may have undergone a traumatic pregnancy and birth experience requiring emotional and mental health support124.
What is the suggested management of children born to mothers with pregnancy-associated acute kidney injury?
Consensus statement:
-
Prematurity and fetal growth restriction are more common with PrAKI and are associated with long-term complications. Follow-up management tailored to the individual risk profile is essential.
PrAKI can be associated with haemodynamic instability, a pro-inflammatory uraemic milieu, and use of potentially harmful medications, all of which may impact fetal development98,100,108,110,116,117,118,119,120,121,122. No specific data are available on the long-term outcomes of children born to mothers with PrAKI, particularly for those born at term or without any perinatal complications. However, infants who are premature and/or SGA are at risk of long-term sequelae, including cardiovascular, renal and respiratory disease, and adverse neurodevelopmental and obstetric outcomes120,122. Long-term risks including hypertension, CKD and metabolic disorders may not manifest in the first years of life and should be part of extended follow-up planning. Neonates born to mothers with PrAKI must therefore have a comprehensive health check-up, including consideration of maternal, PrAKI-related and paediatric risk factors tailored to resource availability. A post-PrAKI Paediatric Risk Profile Assessment, which includes risk factors in the birthing mother, details of PrAKI and postnatal course, performed at discharge from the birth encounter, can help to individualize follow-up and management (Fig. 4 and Supplementary Table 4). When deciding on kidney follow-up, the balance between the availability of resources and the risk of medicalizing infants who have a relatively normal postnatal course and development needs to be recognized.
Children born premature and/or SGA may benefit from a comprehensive KHA performed at 2 years of corrected age, including assessment of blood pressure, urine for protein, child’s growth and development, kidney function and sonography for baseline kidney size and appearance (where feasible) (Fig. 4) Components of blood pressure, urine for protein, child’s growth and development, kidney function and sonography for baseline kidney size and appearance may be re-assessed every 1–2 years during childhood based on the initial abnormality.
Regular collaboration between obstetricians, nephrologists, paediatricians and primary care providers ensures a comprehensive and holistic approach to long-term follow-up to promote health and quality of life125. Education on the importance of adherence to medical recommendations, regular monitoring, and signs of progressive disease empowers women and their advocates (for example, partners, family, friends and/or community) to actively participate in their ongoing care.
Conclusions
PrAKI is an important public health concern that requires urgent attention. Improved antenatal care and education of health-care practitioners in the recognition and management of PrAKI will undoubtedly improve outcomes for mothers and newborns and alleviate the global burden of PrAKI.
The frequency of PrAKI is increased during pre-eclampsia, sepsis and haemorrhage. Defining and diagnosing PrAKI presents multiple challenges because of pathophysiological changes in pregnancy and socio-economic and cultural differences, potentially leading to late identification of the condition and delay of necessary interventions. Efforts to improve prevention, recognition and management of PrAKI by treating the underlying cause (when known) while optimizing maternal and neonatal outcomes require multi-faceted interventions, including enhanced antenatal care, early identification of high-risk patients, timely diagnosis and swift initiation of treatment, including dialysis in severe cases, and timely delivery of the fetus.
Future research has huge potential to improve PrAKI outcomes (Supplemental Table 5). Long-term studies are also needed to understand the impact of PrAKI on maternal health, particularly the risk of CKD. Research into novel interventions for early detection and management of PrAKI, including exploring the potential for targeted therapies based on molecular and genetic findings, could have the potential to revolutionize care for pregnant women worldwide.
References
Gurrieri, C. et al. Kidney injury during pregnancy: associated comorbid conditions and outcomes. Arch. Gynecol. Obstet. 286, 567–573 (2012).
Taber-Hight, E. & Shah, S. Acute kidney injury in pregnancy. Adv. Chronic Kidney Dis. 27, 455–460 (2020).
Piccoli, G. B. et al. Acute kidney injury in pregnancy: the need for higher awareness. a pragmatic review focused on what could be improved in the prevention and care of pregnancy-related AKI, in the year dedicated to women and kidney diseases. J Clin Med. 7, 318 (2018).
Huang, C. & Chen, S. Acute kidney injury during pregnancy and puerperium: a retrospective study in a single center. BMC Nephrol. 18, 146 (2017).
Meca, D. C., Varlas, V. N., Mehedințu, C. & Cîrstoiu, M. M. Correlations between maternal and fetal outcomes in pregnant women with kidney failure. J. Clin. Med. 12, 832 (2023).
Sachan, R., Shukla, S., Shyam, R., Sachan, P. L. & Patel, M. L. Feto-maternal outcome of pregnancy related acute kidney injury in a North Indian population. J. Fam. Community Med. 29, 204–211 (2022).
Thakur, G. et al. Acute kidney injury and its outcome following maternal near miss event: a prospective observational study from a tertiary care hospital. Obstet. Med. 16, 48–51 (2023).
Coles, H., Steer, P., Hughes, P. & Cauldwell, M. Factors associated with acute kidney injury during delivery or the postpartum period: a case control study. Eur. J. Obstet. Gynecol. Reprod. Biol. 261, 200–204 (2021).
Gama, R. M. et al. Acute kidney injury e-alerts in pregnancy: rates, recognition and recovery. Nephrol. Dial. Transpl. 36, 1023–1030 (2021).
Kellum, J. A., Palevsky, P., Mehta, R., Bellomo, R. & Ronco, C. Acute dialysis quality initiative: methodology. Curr. Opin. Crit. Care 8, 500–501 (2002).
Granholm, A., Alhazzani, W. & Møller, M. H. Use of the GRADE approach in systematic reviews and guidelines. Br. J. Anaesth. 123, 554–559 (2019).
Kellum, J. A., Bellomo, R. & Ronco, C. Acute dialysis quality initiative (ADQI): methodology. Int. J. Artif. Organs 31, 90–93 (2008).
Prakash, J. et al. Acute kidney injury in pregnancy with special reference to pregnancy-specific disorders: a hospital based study (2014–2016). J. Nephrol. 31, 79–85 (2018).
Rage, H. I., Kumar Jha, P., Hashi, H. A. & Abdillahi, N. I. Pregnancy-related AKI: a tertiary care hospital experience in Somaliland. Kidney Int. Rep. 8, 388–391 (2023).
Kharkongor, D. et al. Acute kidney injury in obstetrics: hospital-based cohort study. Trop. Doct. 53, 356–361 (2023).
Cooke, W. R. et al. Incidence, aetiology and outcomes of obstetric-related acute kidney injury in Malawi: a prospective observational study. BMC Nephrol. 19, 25 (2018).
Mehrabadi, A., Dahhou, M., Joseph, K. S. & Kramer, M. S. Investigation of a rise in obstetric acute renal failure in the United States, 1999–2011. Obstet. Gynecol. 127, 899–906 (2016).
Mehrabadi, A. et al. Hypertensive disorders of pregnancy and the recent increase in obstetric acute renal failure in Canada: population based retrospective cohort study. BMJ 349, g4731 (2014).
Iqbal Anvar, M., Talwar, S. & Mallapur, S. A retrospective study on clinical outcomes of pregnancy-related acute kidney injury patients at a south Indian tertiary care hospital. Cureus 15, e49610 (2023).
Gaber, T. Z. et al. Acute kidney injury during pregnancy and puerperium: an Egyptian hospital-based study. J. Nephrol. 34, 1611–1619 (2021).
Liu, Y. M., Bao, H. D., Jiang, Z. Z., Huang, Y. J. & Wang, N. S. Pregnancy-related acute kidney injury and a review of the literature in China. Intern. Med. 54, 1695–1703 (2015).
Liu, D. et al. Epidemiology of acute kidney injury in hospitalized pregnant women in China. BMC Nephrol. 20, 67 (2019).
Gama, R. M. et al. Ethnic disparities in pregnancy-related acute kidney injury in a United Kingdom population. J. Nephrol. 36, 777–787 (2023).
The impact of kidney development on the life course: a consensus document for action. Nephron. 136, 3–49 (2017).
Davidson, B. et al. Pregnancy-associated acute kidney injury in low-resource settings: progress over the last decade. Semin. Nephrol. 42, 151317 (2022).
Vinturache, A., Popoola, J. & Watt-Coote, I. The changing landscape of acute kidney injury in pregnancy from an obstetrics perspective. J. Clin. Med. 8, 1396 (2019).
Shah, S. et al. Pregnancy-related acute kidney injury in the United States: clinical outcomes and health care utilization. Am. J. Nephrol. 51, 216–226 (2020).
Ostermann, M. et al. Acute kidney injury. Lancet 405, 241–256 (2025).
Varga, I., Rigó, J. Jr., Somos, P., Joó, J. G. & Nagy, B. Analysis of maternal circulation and renal function in physiologic pregnancies; parallel examinations of the changes in the cardiac output and the glomerular filtration rate. J. Matern. Fetal Med. 9, 97–104 (2000).
Chapman, A. B. et al. Temporal relationships between hormonal and hemodynamic changes in early human pregnancy. Kidney Int. 54, 2056–2063 (1998).
Strevens, H. et al. Glomerular endotheliosis in normal pregnancy and pre-eclampsia. BJOG 110, 831–836 (2003).
Stanhewicz, A. E., Nuckols, V. R. & Pierce, G. L. Maternal microvascular dysfunction during preeclamptic pregnancy. Clin. Sci. 135, 1083–1101 (2021).
Faúndes, A., Brícola-Filho, M. & Pinto e Silva, J. L. Dilatation of the urinary tract during pregnancy: proposal of a curve of maximal caliceal diameter by gestational age. Am. J. Obstet. Gynecol. 178, 1082–1086 (1998).
Millar, L. K. & Cox, S. M. Urinary tract infections complicating pregnancy. Infect. Dis. Clin. North Am. 11, 13–26 (1997).
Morgan, B. P. & Holmes, C. H. Immunology of reproduction: protecting the placenta. Curr. Biol. 10, R381–R383 (2000).
Boulanger, H. et al. Immunologic aspects of preeclampsia. AJOG Glob. Rep. 4, 100321 (2024).
Alrahmani, L. et al. Quantitative alterations in complement alternative pathway and related genetic analysis in severe phenotype preeclampsia. Kidney360 2, 1463–1472 (2021).
Yagel, S., Cohen, S. M. & Goldman-Wohl, D. An integrated model of preeclampsia: a multifaceted syndrome of the maternal cardiovascular-placental-fetal array. Am. J. Obstet. Gynecol. 226, S963–s972 (2022).
Levine, R. J. et al. Circulating angiogenic factors and the risk of preeclampsia. N. Engl. J. Med. 350, 672–683 (2004).
Sahay, M., Swain, M. & Padua, M. Renal cortical necrosis in tropics. Saudi J. Kidney Dis. Transpl. 24, 725–730 (2013).
Kim, M. Y. et al. Angiogenic factor imbalance early in pregnancy predicts adverse outcomes in patients with lupus and antiphospholipid antibodies: results of the PROMISSE study. Am. J. Obstet. Gynecol. 214, 108.e1–108.e14 (2016).
Wagner, S. J. et al. Maternal and foetal outcomes in pregnant patients with active lupus nephritis. Lupus 18, 342–347 (2009).
Buyon, J. P. et al. Predictors of pregnancy outcomes in patients with lupus: a cohort study. Ann. Intern. Med. 163, 153–163 (2015).
Mok, C. C. & Wong, R. W. Pregnancy in systemic lupus erythematosus. Postgrad. Med. J. 77, 157–165 (2001).
Ronco, C. et al. Renal functional reserve in pregnancy. Nephrol. Dial. Transpl. 3, 157–161 (1988).
Webster, P., Lightstone, L., McKay, D. B. & Josephson, M. A. Pregnancy in chronic kidney disease and kidney transplantation. Kidney Int. 91, 1047–1056 (2017).
Forni, L. G. et al. Renal recovery after acute kidney injury. Intensive Care Med. 43, 855–866 (2017).
Takahashi, Y., Kawabata, I., Shinohara, A. & Tamaya, T. Transient fetal blood flow redistribution induced by maternal diabetic ketoacidosis diagnosed by Doppler ultrasonography. Prenat. Diagn. 20, 524–525 (2000).
Montoro, M. N. et al. Outcome of pregnancy in diabetic ketoacidosis. Am. J. Perinatol. 10, 17–20 (1993).
Reidy, K. J. et al. Advocating for the inclusion of kidney health outcomes in neonatal research: best practice recommendations by the neonatal kidney collaborative. J. Perinatol. 44, 1863–1873 (2024).
Kashani, K. et al. Quality improvement goals for acute kidney injury. Clin. J. Am. Soc. Nephrol. 14, 941–953 (2019).
Kashani, K., Rosner, M. H. & Ostermann, M. Creatinine: from physiology to clinical application. Eur. J. Intern. Med. 72, 9–14 (2020).
Ostermann, M., Kashani, K. & Forni, L. G. The two sides of creatinine: both as bad as each other? J. Thorac. Dis. 8, E628–E630 (2016).
Wiles, K. et al. Serum creatinine in pregnancy: a systematic review. Kidney Int. Rep. 4, 408–419 (2019).
Gao, M., Vilayur, E., Ferreira, D., Nanra, R. & Hawkins, J. Estimating the glomerular filtration rate in pregnancy: the evaluation of the Nanra and CKD-EPI serum creatinine-based equations. Obstet. Med. 14, 31–34 (2021).
Côté, A. M. et al. The 24-hour urine collection: gold standard or historical practice? Am. J. Obstet. Gynecol. 199, 625.e1–6 (2008).
Evans, R. D. R. et al. A salivary urea nitrogen dipstick to detect obstetric-related acute kidney disease in Malawi. Kidney Int. Rep. 3, 178–184 (2018).
Obrenovic, R., Petrovic, D., Majkic-Singh, N., Trbojevic-Stankovic, J. & Stojimirovic, B. Serum cystatin C levels in normal pregnancy. Clin. Nephrol. 76, 174–179 (2011).
Higby, K., Suiter, C. R., Phelps, J. Y., Siler-Khodr, T. & Langer, O. Normal values of urinary albumin and total protein excretion during pregnancy. Am. J. Obstet. Gynecol. 171, 984–989 (1994).
Teeuw, H. M., Amoakoh, H. B., Ellis, C. A., Lindsley, K. & Browne, J. L. Diagnostic accuracy of urine dipstick tests for proteinuria in pregnant women suspected of preeclampsia: a systematic review and meta-analysis. Pregnancy Hypertens. 27, 123–130 (2022).
Brown, M. A. et al. Microscopic hematuria in pregnancy: relevance to pregnancy outcome. Am. J. Kidney Dis. 45, 667–673 (2005).
Peteinaris, A. et al. Bladder cancer during pregnancy: a review of the literature. J. Pers. Med. 13, 1418 (2023).
Thomas, M. E. et al. The definition of acute kidney injury and its use in practice. Kidney Int. 87, 62–73 (2015).
Mackenzie, M. J., Woolnough, M. J., Barrett, N., Johnson, M. R. & Yentis, S. M. Normal urine output after elective caesarean section: an observational study. Int. J. Obstet. Anesth. 19, 379–383 (2010).
Waziri, B. et al. Risk factors and outcomes associated with pregnancy-related acute kidney injury in a high-risk cohort of women in Nigeria. J. Nephrol. 37, 587–596 (2024).
Bentata, Y. et al. Acute kidney injury according to KDIGO stages and maternal mortality in the intensive care unit. Intensive Care Med. 41, 555–556 (2015).
Shah, S. & Verma, P. Pregnancy-related acute kidney injury: do we know what to do? Nephron 147, 35–38 (2023).
Yadav, A. et al. Acute kidney injury during pregnancy in kidney transplant recipients. Clin. Transpl. 36, e14668 (2022).
Ostermann, M. & Joannidis, M. Acute kidney injury 2016: diagnosis and diagnostic workup. Crit. Care 20, 299 (2016).
Naqvi, R. et al. Efficacy of multiple biomarkers: NGAL, KIM1, cystatin C and IL18 in predicting pregnancy related acute kidney injury. Pak. J. Med. Sci. 39, 34–40 (2023).
Ostermann, M. et al. Biomarkers in acute kidney injury. Ann. Intensive Care 14, 145 (2024).
Rolfo, A. et al. Chronic kidney disease may be differentially diagnosed from preeclampsia by serum biomarkers. Kidney Int. 83, 177–181 (2013).
Piccoli, G. B. et al. Kidney biopsy in pregnancy: evidence for counselling? A systematic narrative review. BJOG 120, 412–427 (2013).
Say, L. et al. Global causes of maternal death: a WHO systematic analysis. Lancet Glob. Health 2, e323–e333 (2014).
Guillén, A. O., Shemies, R. S., Ankawi, G. A., Jesudason, S. & Piccoli, G. B. Women should not die of pregnancy-related acute kidney injury (PRAKI): revealing the underwater iceberg of maternal health. J. Nephrol. 37, 543–546 (2024).
Wiles, K. et al. Clinical practice guideline on pregnancy and renal disease. BMC Nephrol. 20, 401 (2019).
Ostermann, M., Liu, K. & Kashani, K. Fluid management in acute kidney injury. Chest 156, 594–603 (2019).
Hall, D. R. & Conti-Ramsden, F. Acute kidney injury in pregnancy including renal disease diagnosed in pregnancy. Best Pract. Res. Clin. Obstet. Gynaecol. 57, 47–59 (2019).
Ostermann, M. et al. Drug management in acute kidney disease — report of the acute disease quality initiative XVI meeting. Br. J. Clin. Pharmacol. 84, 396–403 (2018).
Shields, A. D., Plante, L. A., Pacheco, L. D. & Louis, J. M. Society for maternal-fetal medicine consult series #67: maternal sepsis. Am. J. Obstet. Gynecol. 229, B2–B19 (2023).
World Health Organization. WHO Recommendations for the Prevention and Treatment of Postpartum Haemorrhage (WHO, 2012).
Muñoz, M. et al. Patient blood management in obstetrics: prevention and treatment of postpartum haemorrhage. A NATA consensus statement. Blood Transfus. 17, 112–136 (2019).
Fakhouri, F., Schwotzer, N. & Frémeaux-Bacchi, V. How I diagnose and treat atypical hemolytic uremic syndrome. Blood 141, 984–995 (2023).
Le Clech, A. et al. Atypical and secondary hemolytic uremic syndromes have a distinct presentation and no common genetic risk factors. Kidney Int. 95, 1443–1452 (2019).
Fakhouri, F. et al. Glomerular diseases in pregnancy: pragmatic recommendations for clinical management. Kidney Int. 103, 264–281 (2023).
Piccoli, G. B. et al. Maternal-foetal outcomes in pregnant women with glomerulonephritides. Are all glomerulonephritides alike in pregnancy? J. Autoimmun. 79, 91–98 (2017).
Bhaduaria, D. et al. Acute cortical necrosis in pregnancy still an important cause for end-stage renal disease in developing countries. Saudi J. Kidney Dis. Transpl. 30, 325–333 (2019).
Lameire, N. & Kellum, J. A. Contrast-induced acute kidney injury and renal support for acute kidney injury: a KDIGO summary (Part 2). Crit. Care 17, 205 (2013).
Ostermann, M., Bagshaw, S. M., Lumlertgul, N. & Wald, R. Indications for and timing of initiation of KRT. Clin. J. Am. Soc. Nephrol. 18, 113–120 (2023).
Cabiddu, G. et al. Best practices on pregnancy on dialysis: the Italian study group on kidney and pregnancy. J. Nephrol. 28, 279–288 (2015).
Hladunewich, M. A. et al. Intensive hemodialysis associates with improved pregnancy outcomes: a Canadian and United States cohort comparison. J. Am. Soc. Nephrol. 25, 1103–1109 (2014).
Piccoli, G. B. et al. Pregnancy in dialysis patients in the new millennium: a systematic review and meta-regression analysis correlating dialysis schedules and pregnancy outcomes. Nephrol. Dial. Transpl. 31, 1915–1934 (2016).
Ankawi, G., Tangirala, N., Jesudason, S. & Hladunewich, M. A. Pregnancy in patients receiving home dialysis. Clin. J. Am. Soc. Nephrol. 20, 292–300 (2024).
Asamiya, Y. et al. The importance of low blood urea nitrogen levels in pregnant patients undergoing hemodialysis to optimize birth weight and gestational age. Kidney Int. 75, 1217–1222 (2009).
Wald, R. et al. Delivering optimal renal replacement therapy to critically ill patients with acute kidney injury. Intensive Care Med. 48, 1368–1381 (2022).
World Health Organization. Breastfeeding recommendations. WHO https://www.who.int/health-topics/breastfeeding#tab=tab_2 (2025).
Shah, P. S. et al. Intention to become pregnant and low birth weight and preterm birth: a systematic review. Matern. Child Health J. 15, 205–216 (2011).
Liu, Y., Ma, X., Zheng, J., Liu, X. & Yan, T. Pregnancy outcomes in patients with acute kidney injury during pregnancy: a systematic review and meta-analysis. BMC Pregnancy Childbirth 17, 235 (2017).
Sivakumar, V. et al. Pregnancy-related acute renal failure: a ten-year experience. Saudi J. Kidney Dis. Transpl. 22, 352–353 (2011).
Sahay, M., Priyashree, Dogra, L., Ismal, K. & Vali, S. Pregnancy-related acute kidney injury in public hospital in South India: changing trends. J. Assoc. Physicians India 70, 11–12 (2022).
Mahesh, E. et al. Pregnancy-related acute kidney injury: an analysis of 165 cases. Indian J. Nephrol. 27, 113–117 (2017).
Gopalakrishnan, N. et al. Acute kidney injury in pregnancy — a single center experience. Ren. Fail. 37, 1476–1480 (2015).
Prakash, J. et al. Acute renal failure in pregnancy in a developing country: twenty years of experience. Ren. Fail. 28, 309–313 (2006).
Goplani, K. R. et al. Pregnancy-related acute renal failure: a single-center experience. Indian J. Nephrol. 18, 17–21 (2008).
Ramachandran, R. et al. Postpartum renal cortical necrosis is associated with atypical hemolytic uremic syndrome in developing countries. Kidney Int. Rep. 4, 420–424 (2019).
Prakash, J. et al. Decreasing incidence of renal cortical necrosis in patients with acute renal failure in developing countries: a single-centre experience of 22 years from Eastern India. Nephrol. Dial. Transpl. 22, 1213–1217 (2007).
Najar, M. S. et al. Pregnancy related acute kidney injury: a single center experience from the Kashmir Valley. Indian J. Nephrol. 18, 159–161 (2008).
Hildebrand, A. M. et al. Characteristics and outcomes of AKI treated with dialysis during pregnancy and the postpartum period. J. Am. Soc. Nephrol. 26, 3085–3091 (2015).
Kucirka, L. M. et al. Characteristics and outcomes of patients with pregnancy-related end-stage kidney disease. JAMA Netw. Open 6, e2346314 (2023).
Conti-Ramsden, F. I. et al. Pregnancy-related acute kidney injury in preeclampsia: risk factors and renal outcomes. Hypertension 74, 1144–1151 (2019).
Coca, S. G., Singanamala, S. & Parikh, C. R. Chronic kidney disease after acute kidney injury: a systematic review and meta-analysis. Kidney Int. 81, 442–448 (2012).
Bellamy, L., Casas, J. P., Hingorani, A. D. & Williams, D. J. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta-analysis. BMJ 335, 974 (2007).
Crump, C., Sundquist, J. & Sundquist, K. Adverse pregnancy outcomes and long-term risk of chronic kidney disease in women: national cohort and co-sibling study. Am. J. Obstet. Gynecol. 230, 563.e1–563.e20 (2023).
Srialluri, N. et al. Preeclampsia and long-term kidney outcomes: an observational cohort study. Am. J. Kidney Dis. 82, 698–705 (2023).
Inversetti, A. et al. Update on long-term cardiovascular risk after pre-eclampsia: a systematic review and meta-analysis. Eur. Heart J. Qual. Care Clin. Outcomes 10, 4–13 (2024).
Trakarnvanich, T., Ngamvichchukorn, T. & Susantitaphong, P. Incidence of acute kidney injury during pregnancy and its prognostic value for adverse clinical outcomes: a systematic review and meta-analysis. Medicine 101, e29563 (2022).
Shalaby, A. S. & Shemies, R. S. Pregnancy-related acute kidney injury in the African continent: where do we stand? A systematic review. J. Nephrol. 35, 2175–2189 (2022).
Aslan, H., Gul, A. & Cebeci, A. Neonatal outcome in pregnancies after preterm delivery for HELLP syndrome. Gynecol. Obstet. Invest. 58, 96–99 (2004).
Staley, J. R. et al. Associations of blood pressure in pregnancy with offspring blood pressure trajectories during childhood and adolescence: findings from a prospective study. J. Am. Heart Assoc. 4, e001422 (2015).
Wu, C. S. et al. Health of children born to mothers who had preeclampsia: a population-based cohort study. Am. J. Obstet. Gynecol. 201, 269.e1–269.e10 (2009).
Geelhoed, J. J. et al. Preeclampsia and gestational hypertension are associated with childhood blood pressure independently of family adiposity measures: the Avon longitudinal study of parents and children. Circulation 122, 1192–1199 (2010).
Yang, F. et al. Association of maternal preeclampsia with offspring risks of ischemic heart disease and stroke in Nordic countries. JAMA Netw. Open 5, e2242064 (2022).
UK Teratology Information Service. Best use of medicines in pregnancy. Bumps https://www.medicinesinpregnancy.org (2025).
Yildiz, P. D., Ayers, S. & Phillips, L. The prevalence of posttraumatic stress disorder in pregnancy and after birth: a systematic review and meta-analysis. J. Affect. Disord. 208, 634–645 (2017).
Goldstein, S. L. et al. Consensus-based recommendations on priority activities to address acute kidney injury in children: a modified Delphi consensus statement. JAMA Netw. Open 5, e2229442 (2022).
Author information
Authors and Affiliations
Contributions
All authors researched the data, contributed substantially to discussion of the content, wrote the article and reviewed or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
C.N.-P. has received speakers fees from UCB and Sanofi unrelated to this manuscript. R.M. is a consultant for Baxter, Vantive and Fresenius, and has received grants from the US National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) and Vantive, unrelated to this study. M.O. has received research funding from Baxter and BioMérieux. K.K. declares patents for direct GFR measurement and net ultrafiltration calculator; consulting fees and travel support from Baxter Healthcare; research grants from Google; speaker honorarium from Nikkiso Critical Care Medical Supplies (Shanghai); funding from NIDDK grant; and research grants to his institution from the following companies: Fresenius, Spectral, ExThera, Renibus, Novartis and Astute Medical. K.B. has received consulting fees from GSK, Vertex, Maze, AstraZeneca and Bayer; honoraria from AstraZeneca, Boehringer Ingelheim and Vertex; and industry grants from AstraZeneca and Roche. R.C.-D.G. has received speaker honoraria from Nova Biomedical and Baxter Healthcare. R.L.M. has received consulting fees from Am Pharma, Baxter, Fresenius, Guard Therapeutics, Abbott, Sphingotec, Mallinckrodt, Novartis, Idorsia, Renasym, Abiomed and Alexion. S.M. has received research funding from the Gerber Foundation. The other authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Nephrology thanks Gloria Ashuntantang, Giorgina Piccoli, who co-reviewed with Costanza Valentini, and the other, anonymous, reviewer for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nelson-Piercy, C., Srisawat, N., Kashani, K. et al. Pregnancy-associated acute kidney injury — consensus report of the 32nd Acute Disease Quality Initiative workgroup. Nat Rev Nephrol 21, 633–646 (2025). https://doi.org/10.1038/s41581-025-00979-6
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41581-025-00979-6
This article is cited by
-
Global incidence of acute kidney injury and renal replacement therapy among obstetric intensive care unit admissions: a systematic review and meta-analysis
Journal of Anesthesia, Analgesia and Critical Care (2026)
-
The association between intraoperative hypotension and postoperative acute kidney injury following emergent critical cesarean delivery: a retrospective cohort study
Journal of Clinical Monitoring and Computing (2026)