Abstract
Autologous haematopoietic stem cell transplantation (AHSCT) is a treatment option for relapsing forms of multiple sclerosis (MS) that are refractory to disease-modifying therapy (DMT). AHSCT after failure of high-efficacy DMT in aggressive forms of relapsing–remitting MS is a generally accepted indication, yet the optimal placement of this approach in the treatment sequence is not universally agreed upon. Uncertainties also remain with respect to other indications, such as in rapidly evolving, severe, treatment-naive MS, progressive MS, and neuromyelitis optica spectrum disorder (NMOSD). Furthermore, treatment and monitoring protocols, rehabilitation and other supportive care before and after AHSCT need to be optimized. To address these issues, we convened a European Committee for Treatment and Research in Multiple Sclerosis Focused Workshop in partnership with the European Society for Blood and Marrow Transplantation Autoimmune Diseases Working Party, in which evidence and key questions were presented and discussed by experts in these diseases and in AHSCT. Based on the workshop output and subsequent written interactions, this Consensus Statement provides practical guidance and recommendations on the use of AHSCT in MS and NMOSD. Recommendations are based on the available evidence, or on consensus when evidence was insufficient. We summarize the key evidence, report the final recommendations, and identify areas for further research.
Similar content being viewed by others
Introduction
Haematopoietic stem cell transplantation (HSCT) is a haematological procedure that has increasingly been used since the late 1990s for the treatment of autoimmune diseases that are refractory to conventional disease-modifying therapy (DMT)1,2. HSCT encompasses two procedures: autologous HSCT (AHSCT), in which the haematopoietic stem cells (HSCs) used are the patient’s own, or allogeneic HSCT, in which the HSCs are derived from a healthy donor. The most common neurological indication for AHSCT is multiple sclerosis (MS), an immune-mediated demyelinating and degenerative disease of the CNS that can cause irreversible disability3. Much less frequently, HSCT — in a few cases allogeneic HSCT — has also been used to treat other neuroinflammatory diseases, such as neuromyelitis optica spectrum disorders (NMOSD)4.
AHSCT is highly effective at stopping inflammation in the brain, as demonstrated by suppression of clinical and MRI-detected MS disease activity5. It can also stabilize or even improve function in relapsing–remitting MS, though the benefits are less clear in primary progressive MS and secondary progressive MS6. Though the safety profile of AHSCT has improved markedly over time7, the treatment involves higher acute risk than many approved DMTs for MS, so the optimal placement of AHSCT in the therapeutic algorithm for MS remains uncertain. Key questions include the criteria for patient selection, the choice of treatment protocol, the management of rehabilitation, fertility and vaccinations, and the use of DMTs after AHSCT. Long-term monitoring of adverse events and neurological outcomes all require further investigation.
In this Consensus Statement, the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS) and the European Society for Blood and Marrow Transplantation (EBMT) Autoimmune Diseases Working Party (ADWP) review current knowledge and provide recommendations for the use of AHSCT in adults with MS or NMOSD, including its indication and positioning in the treatment algorithm, candidate selection, transplant methodology and patient management. The use of HSCT in the paediatric setting was not covered; specific recommendations are provided elsewhere by the EBMT ADWP and Paediatric Diseases Working Party8.
Methods
Focused workshop
An ECTRIMS Focused Workshop to discuss the use of AHSCT for the treatment of MS and other disorders was organized by ECTRIMS in partnership with the EBMT ADWP under the leadership of the Organizing Committee (P.A.M., R.G., J.B., E.I., M.I., J.A.S., B. Stankoff and B. Sharrack) and was held as a 2-day digital event in March 2022. The aims of the workshop were: to produce practical guidance for clinicians, patients and health-care payers on the basis of expert consensus recommendations with the support of the leading subspecialist organizations; to provide a forum for the professional and scientific development of participants who, as established or emerging leaders in the neurological and haematological communities across Europe, could subsequently share their knowledge in their respective countries and further afield; and to disseminate the results with published articles and societal media with high potential to influence and improve clinical practice and health-care policy development.
As customary for ECTRIMS Focused Workshops, participation was by invitation; participants were nominated by the Organizing Committee to balance optimal expertise with equality of gender, a broad geographic distribution within Europe, and adequate societal representation from the subspecialist associations, ECTRIMS and the EBMT ADWP. The previous and current Presidents of the Americas Committee for Treatment and Research in Multiple Sclerosis (ACTRIMS; J.A.C. and M.S.F., respectively) were also invited to represent ACTRIMS. The workshop included two plenary sessions and four parallel sessions, divided into neurological and haematological streams. Presentations were delivered by 20 speakers on the current evidence and identifying key questions on the use of HSCT in MS and NMOSD, and each session was followed by round-table discussions that involved all speakers and named key discussants. Workshop chairs presented summaries of the parallel sessions and discussions to all attendees. On the basis of the agreed output, two members of the Organizing Committee (P.A.M. and B. Sharrack) led the generation of a consensus summary. The scientific programme9, recorded sessions10 and a highlights document from the workshop11 are publicly available on the ECTRIMS website.
Preparation of the Consensus Statement
After the workshop, the Organizing Committee held a debriefing meeting in which a manuscript writing plan was agreed to develop the third workshop aim. A manuscript outline including the structure and key points from the workshop was prepared by P.A.M. and B. Sharrack, circulated for comments and agreed upon within the Organizing Committee. The outline was developed into a full manuscript draft by P.A.M. and A.M. by adding detailed information and output from workshop slide decks, presentation recordings, session summaries and consensus summary. All authors reviewed the initial draft and contributed to subsequent drafts via email correspondence. During this revision process, the manuscript was updated and enriched with information obtained through structured searches to include relevant literature published up to the end of June 2024. Recommendations are based on scientific evidence from primary research, systematic reviews and meta-analyses wherever possible, and rely on consensus opinion only when the evidence was limited or unavailable. Consensus was reached through revision of the draft to address comments from all co-authors until agreement was reached. Three rounds of revision were required to establish consensus.
Stakeholders
Stakeholders interested in this Consensus Statement include people with MS or NMOSD, their families, carers and any other affected individuals; MS and NMOSD health-care professionals, including physicians, nurses, pharmacists, physician assistants, technologists, physical therapists, rehabilitation therapists, psychologists and allied professionals; researchers in neurological disease, including neuroscientists and neuroimmunologists; neurological and neuroinflammatory diseases health-care payers, insurers, commissioners and public health organizations; and MS and NMOSD patient associations and scientific societies. Representatives of all stakeholders were not included in the workshop owing to logistical limitations.
Rationale and immunological mechanisms of AHSCT
Immune reconstitution
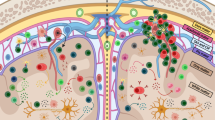
The pathogenesis of MS is initiated by unfavourable interactions between genetic and environmental risk factors1 that lead, via poorly understood mechanisms, to the activation and migration of pro-inflammatory B cells and T cells into the CNS12. The rationale for the use of AHSCT in MS and other diseases is that this treatment eradicates disease-associated adaptive and innate immune components, followed by restoration of immune tolerance through deep reconstitution of the immune system, leading to long-term suppression of new focal inflammatory activity13. After ablation of the haematolymphoid system with high-dose chemotherapy, immunological recovery usually occurs within 6 months for CD19+ B cells, CD8+ T cells and natural killer cells, but requires up to 2 years for CD4+ naive T cells and central memory T cells14,15,16,17.
Early immune reconstitution is promoted by peripheral expansion of cells that survive lymphoablative conditioning. During later reconstitution (>1 year after AHSCT), new naive T cells are generated by de novo maturation in a reactivated thymus. This process is indicated by a gradual increase in markers of recent thymic emigrants (CD31 and T cell receptor (TCR) excision circles) in the peripheral blood, and by extensive renewal of the TCR repertoire in the peripheral blood15,17,18,19,20,21 and the cerebrospinal fluid (CSF)20,21, such that the repertoire differs extensively from that before treatment.
Mechanisms of disease suppression
Changes in the immune system that have been described after AHSCT in MS include an increase in regulatory cell phenotypes (such as FoxP3+ T regulatory (Treg) cells), reduced T helper 17 (TH17) cell responses15,22, and changes in cytokine patterns and immune cell gene expression that characterize a more tolerogenic environment17,23,24,25. Re-emergence of T cells that are reactive to myelin basic protein (MBP; a component of CNS myelin) after AHSCT has been reported. Subsequent data suggest that T cell reactivity to MS-related antigens (the myelin proteins MBP, myelin oligodendrocyte glycoprotein and proteolipid protein, non-myelin autoantigens RASGRP2 and GDPLFS, and peptides from Epstein–Barr virus (EBV), cytomegalovirus (CMV) and influenza virus) is heterogeneous between individuals, but an overall decrease in specificity for MS autoantigens is seen in CD4+ effector memory T cells after AHSCT, whereas reactivity towards EBV is increased, and this increase is more pronounced in people with EBV reactivation17.
Levels of switched memory B cells are reduced after AHSCT, indicating ablation of immunoglobulin-producing B cells that take part in autoimmune processes, and the B cell receptor repertoire is less diverse early after treatment but renewed at later stages26. Reductions in the levels of mucosal associated invariant T cells with inflammatory phenotypes and increases in CD56high natural killer cells, a subset of cells with immune regulatory functions, have also been reported after AHSCT27,28,29. No data are currently available on the effects of AHSCT on the microglial compartment in vivo; microglia might not be renewed given that they are tissue-resident and slow cycling, but changes in phenotype and states of activation are possible.
In addition to these mechanisms, the effects of allogeneic HSCT might involve replacement of autoreactive cells by healthy allogeneic cells and the development of the graft-versus-autoimmunity effect30. However, the allogeneic procedure carries higher risks of morbidity from graft-versus-host disease and of mortality that curtail its use in autoimmune diseases, except as a developmental (or investigative) indication in a prospective clinical study31.
Immunological and other biomarker research and biobanking
Investigational immune monitoring after AHSCT can be done with the use of different techniques, including flow cytometry15, gene expression analyses25, mass cytometry32, deep sequencing of TCRs20, and single-cell RNA sequencing33. Monitoring of neurofilament light chain (NfL) and glial fibrillary acidic protein levels could provide insights into the effects of AHSCT on neuronal and glial pathology, similar to way that these biomarkers are being increasingly used in clinical trials of DMTs and for monitoring the effects of DMTs in patient cohorts. Further studies with novel biomarkers are needed to understand the effects of AHSCT on microglia and astroglia activation, on smouldering inflammation in the meninges and/or brain parenchyma, and on brain remyelination and other forms of functional regeneration and repair. Collection and storage of biological specimens for biobanking could contribute to routine supportive care and is essential to enable further investigation of the biological effects and mechanisms of action of AHSCT in autoimmune disease. The EBMT ADWP and Immunobiology Working Party have published recommendations for biobanking of samples and laboratory immune monitoring in people with autoimmune diseases who undergo AHSCT34.
Recommendations
-
Include objectives in clinical trials and structured treatment programmes that will provide insights into the mechanisms of AHSCT.
-
Offer informed consent for participation in mechanistic research to people who are enrolled in clinical trials or other ethically approved clinical studies or case series.
-
Plan to collect blood for studies of immune reconstitution and mechanisms of action before and after AHSCT at defined time points (for example, quarterly during the first year, then yearly) and at any relapses34.
-
Consider studying CSF biomarkers of inflammation, neuroaxonal injury and glial injury to inform prediction and assessment of treatment response.
-
Follow the relevant specialist guidelines for immune monitoring and biobanking34.
-
Harmonize sample handling and processing across sites to enable pooling of samples for multicentre collaborations.
Clinical evidence on AHSCT in MS
Case series and cohort studies
Several case series, cohort studies and prospective single-arm trials of AHSCT for MS have been published, in which different protocols have been used and patient populations have been heterogeneous35. Since the earliest studies, when AHSCT was almost exclusively used to treat people with progressive and advanced MS36,37, the selection criteria have evolved considerably. AHSCT has increasingly been used to treat relapsing–remitting MS rather than progressive forms of MS, and these developments in patient selection, along with accumulated experience at transplant centres, have improved safety5. For this reason, we focused on evidence from contemporary practice by searching the literature and reviewing studies that met the following criteria: at least ten individuals were treated with AHSCT; published in the past 5 years (1 January 2019 to 5 July 2024); listed in PubMed; written in the English language; reported objective neurological outcomes, including progression-free survival or no evidence of disease activity (NEDA); and reported transplant-related mortality. Publications that provided information obtained from self-reported questionnaires or remote interviews were not considered as evidence for our consensus and recommendations.
We identified 26 publications that met the criteria, most of which reported retrospective, single-centre or multicentre studies. Amongst these, we identified 17 studies that involved a single treatment group that underwent AHSCT16,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53 (Supplementary Table 1). In nine studies, AHSCT was compared with other therapies in two or more treatment groups54,55,56,57,58,59,60,61,–62 (Supplementary Table 2). Half of the 26 studies included only people with relapsing–remitting MS. As expected, the cohorts in these studies had lower average Expanded Disability Status Scale (EDSS) scores at baseline than the cohorts that included people with progressive MS, and their outcomes were better, with high rates of progression-free survival (80–100%) and NEDA (70–80%) (Supplementary Table 1). The average age of participants in these 26 studies ranged from 27 years to 44 years, reflecting appropriate age windows. In three studies in which people aged <45 years with relapsing–remitting MS, a short duration of disease (≤5 years from diagnosis) and recent inflammatory activity were treated with AHSCT, near-complete progression-free survival and improvement in disability were observed38,39,43; in one study, AHSCT was used as a first-line DMT43. Similar outcomes were observed in previous studies of AHSCT in people with relapsing–remitting MS63,64.
Given that long-term outcomes in MS are of particular importance, we also considered key evidence published before 2019. Long-term outcomes in a large cohort of patients treated with AHSCT for MS were first reported in a retrospective joint analysis of the EBMT and the Center for International Blood and Marrow Transplant Research (CIBMTR) databases that included 281 patients with a median follow-up period of 6.6 years65. The large majority had progressive forms of MS (78%) and only 16% had relapsing–remitting MS. Overall progression-free survival at 5 years was 46%, but progression-free survival was considerably higher in the relapsing–remitting MS subgroup (73% (95% CI 57–88%)) than in the progressive MS subgroup (33% (95% CI 24–42%)). Transplant-related mortality was high at 2.8%, explained by the large proportion of people with advanced-stage progressive MS65. In a subsequent cohort of 210 people with MS (58% with relapsing–remitting MS) reported by the Italian BMT-MS Study Group, the overall outcomes were better than in the earlier study — the overall progression-free survival was 65% at 10 years after AHSCT, the progression-free survival was higher in patients with relapsing–remitting MS than in patients with progressive MS (71% versus 57%), and the transplant-related mortality was 1.4%45. Most recently, sustained complete remission of MS has been demonstrated in two Swedish case series that included only people with relapsing–remitting MS who were followed up for up to 10 years after AHSCT38,51. Progression-free survival was 87% at 10 years, and there was no transplant-related mortality51.
The nine studies in which AHSCT was compared with standard DMTs (Supplementary Table 2) were all retrospective, non-randomized and/or non-blinded, and five were single-centre studies and four were multicentre studies (Supplementary Table 2). The most frequently used conditioning regimens were carmustine (BCNU), etoposide, cytosine arabinoside (Ara-C) and melphalan (BEAM) with anti-thymocyte globulin (ATG; BEAM–ATG) or cyclophosphamide–ATG (Supplementary Table 2), and the most common comparator was alemtuzumab, which was used in five studies. Baseline characteristics of participants were highly variable across the studies; some included only people with relapsing–remitting MS, others included only people with secondary progressive or primary progressive MS, and others included a mixture. Average age, disease duration and baseline EDSS score were also variable (Supplementary Table 2). All five studies in which AHSCT was compared with alemtuzumab showed that AHSCT has a superior effect on relapses, NEDA and MRI activity55,56,57,58,59. AHSCT was also superior to alemtuzumab in its effects on disability progression in two studies55,58, though disability outcomes were similar in the other three studies56,57,59. This discrepancy could be explained by study limitations, including relatively short observation periods and the heterogeneity of the patient populations and the assessments.
In two multicentre retrospective studies, data were collected from several centres across several countries to enable propensity score matched cohort comparisons61,62. One of these studies showed that AHSCT in highly active relapsing–remitting MS was considerably superior to fingolimod and marginally superior to natalizumab in relation to relapse-based and disability-based outcomes, but was not superior to ocrelizumab over a short duration of follow-up62. In the other study, treatment of primary progressive MS and secondary progressive MS with AHSCT was compared with treatment with natalizumab61. The ASCEND trial had previously demonstrated that natalizumab is ineffective in progressive MS66, and the comparison identified no difference in outcomes, leading to the conclusion that AHSCT is similarly ineffective61. These studies provide valuable information, but both have several limitations: a reliance on statistical methods to match patients who were selected, treated and followed up in different centres with heterogeneous criteria, treatment protocols and assessments; small numbers of individuals in the matched groups, particularly in the study of progressive MS61 and in the group that received ocrelizumab62; high dropout rates and short durations of follow-up, particularly for the group that received ocrelizumab (mean 1.52 years versus 3.78 years for the group that received AHSCT)62; and a lack of MRI data61,62.
The remaining two of the nine comparative studies included people with secondary progressive MS54,60, so are discussed below (see the section ‘AHSCT in progressive forms of MS’). No transplant-related mortality was seen in most (seven of nine) of the comparative studies (Supplementary Table 2).
AHSCT in progressive forms of MS
Most studies of AHSCT in progressive MS were performed during the early 2000s67,68,69,70,71,72,73,74 so are not included in the studies that met our search criteria (Supplementary Tables 1 and 2). Outcomes of these studies were widely variable — progression-free survival ranged from 36% at 3 years73 to 77% at 5 years after AHSCT74. Such variability could be explained, at least in part, by heterogeneity in the patient populations, the definitions of MS progression and treatment failure that were used, and in the conditioning regimens used. Overall, outcomes were worse when total body irradiation protocols were used, possibly owing to a direct neurotoxic effect75,76,77.
In large cohort studies of AHSCT in patients with secondary progressive MS, progression-free survival at 5 years ranged from 33%65 to 71%45, but the lack of a control group makes it impossible to establish whether these rates signify any reduction in disability progression. Retrospective matched studies in which AHSCT was compared with available treatments suggested some benefit in this respect in some individuals54,60. In a small study that involved 93 people with secondary progressive MS, outcomes of AHSCT with the BEAM–ATG protocol (n = 31) were compared with those of immunosuppression with cyclophosphamide (n = 62). Disability worsening over a mean follow-up period of >90 months was similar between the two groups, but a Cox regression analysis identified a trend towards better progression-free survival with AHSCT than with cyclophosphamide treatment (HR 0.65, 95% CI 0.28–1.52; P = 0.32), equivalent to a 35% reduction in the risk of progression60. This finding was probably not statistically significant owing to insufficient statistical power of the study; AHSCT was, however, superior for suppression of relapses60. A registry-based study has indicated that AHSCT (BEAM–ATG protocol in most instances) in active secondary progressive MS significantly slowed disability progression and increased the likelihood of sustained disability improvement when compared with standard immunotherapy54. As mentioned above, comparison of AHSCT and natalizumab for primary progressive and secondary progressive MS identified no differences in MS relapse or disability outcomes61 (Supplementary Table 2).
Some evidence suggests that AHSCT affects the pathogenic mechanisms that underlie progressive disease. Specifically, AHSCT reduced brain atrophy rates in a subset of individuals with secondary progressive MS40,78, and levels of serum NfL after AHSCT were similar to those in relapsing–remitting MS48,79. Levels of NfL in the CSF might be a more sensitive measure than that in the serum, and data from people with relapsing–remitting MS showed a significant reduction in these levels after AHSCT that lasted for the duration of the 5-year follow-up50. Comparisons of AHSCT with other treatments in primary progressive MS are limited, but suggest similar effects on disability outcomes as seen in secondary progressive MS, though the benefit seems to be smaller65,80.
Randomized clinical trials
Only two randomized clinical trials (RCTs) of AHSCT have been published81,82. The first, known as the ASTIMS trial, was terminated early owing to slow accrual of participants, and the primary end point was changed from confirmed EDSS progression to the cumulative number of new T2 MRI lesions over a 4-year period. When the study was closed, it included 21 people with MS (33% relapsing–remitting MS) who were randomly assigned to receive either AHSCT with the BEAM–ATG protocol or mitoxantrone81. On the basis of the MRI outcomes, AHSCT was superior to mitoxantrone (79% reduction in the number of new T2 lesions and relapse activity), but no significant difference was apparent in disability progression (57% for AHSCT versus 48% for mitoxantrone). In the second trial, known as the MIST trial, 110 people with relapsing–remitting MS were randomly assigned to receive either AHSCT with the cyclophosphamide–ATG protocol or DMTs that were approved by the FDA, excluding alemtuzumab82. Over a median follow-up of 2 years, AHSCT was superior to DMTs with respect to the primary outcome of progression-free survival at 5 years (90% versus 25%), and with respect to relapse-free survival at 5 years (85% versus 15%) and NEDA-3 (no relapses, no increase in disability, no new T2 or gadolinium-enhancing lesions on MRI) at 5 years in a post hoc analysis (78% versus 3%). One limitation of this study is that approximately only half of the control group received high-efficacy DMTs (natalizumab or mitoxantrone) and the remainder of this group received moderate-efficacy DMTs. Ongoing RCTs have been designed to overcome this limitation by including individuals receiving all current high-efficacy DMTs, including alemtuzumab, ocrelizumab, ofatumumab and cladribine in addition to natalizumab and mitoxantrone (see the section ‘Investigative indications’).
Meta-analyses
A meta-analysis published in 2017 highlighted the importance of AHSCT protocol refinement and selection of patients for optimizing safety and efficacy outcomes in MS7. The study included 764 people from 15 studies (including one RCT) published between 1995 and 2016, in which various conditioning regimens were used7. Transplant-related mortality markedly decreased over time — among 349 individuals who underwent AHSCT after 2005, transplant-related mortality was 0.3%, compared with 3.6% among 415 individuals who underwent AHSCT before 2005. The higher transplant-related mortality in the older studies was associated with a lower proportion of people with relapsing–remitting MS and a higher EDSS score at baseline among those treated. AHSCT was associated with long-term suppression of new focal inflammatory activity (clinical relapses and new T2 and gadolinium-enhancing lesions on MRI) in individuals who underwent AHSCT, but the effect on EDSS progression was highly heterogeneous across studies and mostly depended on the proportion of participants with progressive forms of MS7. Pooled rates of EDSS progression were 17.1% at 2 years and 23.3% at 5 years, and lower 2-year progression rates were associated with inclusion of a higher proportion of people with relapsing–remitting MS7. The pooled proportions of NEDA (which was reported in five studies) at 2 years and 5 years were 83% (range 70–92%) and 67% (range 59–70%), respectively. Indirect comparisons of NEDA outcomes with AHSCT and DMTs suggest that AHSCT could be more effective in selected individuals, although comparative data from RCTs are needed to determine whether this is the case5,83.
In a later meta-analysis that included 4,831 people with MS from 50 studies, the pooled estimates of progression-free survival and relapse-free survival were 73% (95% CI 69–77%) and 81% (95% CI 76–86%), respectively. The pooled proportion of people with MS in whom NEDA was maintained was 68% (95% CI 59–77%), and transplant-related mortality was 4.0% (95% CI 2–6%)84, but this overall rate is strongly influenced by high transplant-related mortality in older studies7. Taken together, the meta-analyses are useful to illustrate the evolution of the field, but their pooled estimates are influenced by historical practice and the heterogeneity of patient populations, treatment protocols and centres across studies, limiting conclusions that can be drawn about safety and efficacy.
Patient-reported outcomes and narrative studies
Patient-reported outcomes (PROs) in terms of quality of life (QoL) have not been systematically included in observational studies of AHSCT. However, PROs assessed with health-related QoL measures, including the Multiple Sclerosis Impact Scale (MSIS-29) and Short Form 36 (SF-36) scores, have been reported in some studies, usually as secondary outcomes63,64, and investigated more fully in two studies85,86. Improvements in health-related QoL have consistently been associated with sustained clinical stabilization. Physical and psychosocial health perceptions of people with MS who had undergone AHSCT have also been investigated in qualitative studies of lived experiences through the various phases of AHSCT87,88,89. In these studies, many participants described AHSCT as a second chance and an opportunity for a new life, enabling a transition from a state of illness to a state of health that countered previous profound uncertainty87. Moreover, AHSCT was seen as a life-changing event accompanied by psychological and physical stress but accompanied or followed by a feeling of regaining control and a lasting positive effect88. People had high expectations for AHSCT but felt they did not have enough information to consider it89, and people who had already had the treatment wished they could have been provided with information on and access to this treatment option earlier in their MS course88. Implementation of PROs in clinical trials and clinical practice has recently been recommended by the ADWP, Nurses Group and Patient Advocacy Committee of the EBMT to capture patient perspectives and evaluate how they are affected by AHSCT90.
Recommendations
-
Continue to collect evidence from real-world cohorts who have undergone AHSCT and report baseline and follow-up clinical data and MRI data (acquired with a standardized protocol whenever possible91) to the EBMT database (or the appropriate extra-European organization) to facilitate clinical research.
-
Collect PROs and QoL measures in cohorts and trials where possible.
-
Share and disseminate evidence with patients, health practitioners and health-care providers and payers.
-
Consider offering participation in approved clinical trials and observational studies to all eligible patients; RCTs are particularly encouraged.
-
Improve participant retention and collection of long-term data from all treated individuals, as these factors are especially important to avoid biases.
-
Harmonize the end points and data collection methodology in cohort studies and RCTs to enable future meta-analyses.
Indications for AHSCT in MS
Relapsing–remitting MS
Established indications and placement in the treatment sequence
AHSCT has been endorsed as a standard of care for the treatment of relapsing–remitting MS that is refractory to conventional DMTs by the EBMT2,31, the American Society for Blood and Marrow Transplantation92, the US National Multiple Sclerosis Society93 and the Brazilian Society of Bone Marrow Transplantation94. Compelling evidence of the need to target inflammation early in the disease course prompted a shift from stepped care to early escalation and induction strategies, as recommended by the European Academy of Neurology–ECTRIMS guidelines on the treatment of MS95. High-efficacy DMTs (usually including the monoclonal antibodies alemtuzumab, natalizumab, ocrelizumab and ofatumumab96 and, in some classifications, cladribine97) are more effective when treatment is initiated early98,99,100,101,102. Given that AHSCT is generally more effective than DMTs and that treatment at a younger age and after a lower number of previous DMTs is associated with lower rates of long-term progression65, its early use in people with highly active or aggressive MS that is not responding to high-efficacy DMTs could be beneficial.
The general principles of evaluating suitability for AHSCT are widely accepted (Fig. 1). We also provide patient selection recommendations with more specifications (Box 1). Regarding prior exposure to DMTs, AHSCT is indicated for individuals with relapsing–remitting MS and markers of aggressive disease after failure of any one high-efficacy DMT. In treatment-naive individuals, we recommend that AHSCT is considered only in those with rapidly evolving, severe MS with poor prognostic factors. However, the optimal placement of AHSCT in the treatment sequence for MS remains challenging for several reasons, including a lack of consensus on the definition of “highly active or aggressive MS” (estimated as 4–14% of cases)103. Many factors are known to be associated with aggressive forms of MS, including clinical features (for example, a high frequency of relapses and rapid accumulation of neurological dysfunction), MRI findings, neuropathological findings, immunological features in the blood, biomarker correlates and genetic markers. However, the retrospective nature of the assessments that most definitions are based on, and the high uncertainty in the prediction of disease outcomes in any given individual precluded a consensus definition in the 2018 ECTRIMS Focused Workshop on aggressive MS104. Eligibility criteria are also likely to change over time owing to the rapid evolution of the therapeutic scenario and clinical evidence.
Neurological (top) and haematological (bottom) variables on the left are associated with a positive recommendation (green profile) for autologous haematopoietic stem cell transplantation (AHSCT). The numbers (age, disease duration, Expanded Disability Status Scale (EDSS) score) are indicative to illustrate the principles but are not intended as cut-off values. The profile on the far left therefore represents the ideal candidate for AHSCT. Variables on the right are adverse factors and, when they are prevalent, AHSCT is not recommended (red profile). Specific considerations for relapsing–remitting multiple sclerosis (MS) and progressive MS are shown in the central boxes with traffic light indicators of suitability for AHSCT. DMT, disease-modifying therapy; HE-DMT, high-efficacy DMT; ME-DMT, medium-efficacy DMT. aAHSCT could be considered in older, biologically fit people on an individual basis. bWithin a clinical trial or study.
Investigative indications
Four RCTs are ongoing to compare AHSCT with high-efficacy DMTs (Table 1): RAM-MS105, STAR-MS106, BEAT-MS107 and NET-MS108. Though the inclusion criteria and transplantation protocols differ, these trials have several similarities in design, including a requirement for prior treatment failure (with limited exceptions in STAR-MS), a focus on relapsing–remitting MS, and the use of NEDA as the primary outcome, except in BEAT-MS in which the primary end point is relapses. As comparator DMTs, alemtuzumab and ocrelizumab are available options in all the RCTs; other high-efficacy DMTs (natalizumab, cladribine and other anti-CD20 monoclonal antibodies) are variably allowed. Secondary outcome measures vary, and include MRI, visual function, cognition, disability worsening and improvement, fatigue, depression, QoL and economic analysis. Blood and CSF biomarkers and mechanistic studies are also coordinated with the protocols. The results of these RCTs, which are expected in 3–5 years, should inform us about the effectiveness of AHSCT in comparison with high-efficacy DMTs.
People with a very aggressive presentation of MS and poor prognostic factors can be considered for AHSCT as an investigative treatment option even without prior treatment failure106. In this context, the individual’s risk-to-benefit profile should be accurately evaluated in a highly specialized multidisciplinary setting. As the window of therapeutic opportunity is narrower for these individuals, AHSCT as a first-line treatment could be beneficial. Indeed, in a retrospective study that included 20 people with aggressive relapsing–remitting MS with moderate to severe disability at baseline (median EDSS score 5, range 1.5–9.5), no disability progression, clinical relapses or MRI disease activity was reported, and EDSS scores improved (by a median of 2.25 points) in 95% of people at a median follow-up of 30 months (range 12–118 months) after AHSCT43.
Progressive MS
On the basis of the evidence reviewed above (see the section ‘AHSCT in progressive forms of MS’), AHSCT is only indicated for people with secondary progressive or primary progressive MS with early and inflammatory active disease (Fig. 1 and Box 1). No RCTs have been published, are ongoing or, to our knowledge, are even planned to specifically evaluate AHSCT as a treatment for progressive MS, though BEAT-MS does not exclude participants with secondary progressive MS who meet study entry criteria for disease activity107.
Recommendations
-
Consider AHSCT as an appropriate escalation therapy for people with highly active MS and in whom high-efficacy DMT has failed (Fig. 1 and Box 1); this indication should be adopted widely and with equitable access in all geographical areas.
-
Refer patients with highly active, treatment-refractory MS as early as possible for consideration of AHSCT.
-
For people with markers of aggressive disease (frequent relapses, incomplete recovery from relapses, high frequency of new MRI lesions and rapid onset of disability), AHSCT can be considered within a specialized multidisciplinary assessment pathway after failure of a single high-efficacy DMT after a meaningful period of treatment.
-
Development and adoption of risk scores and biomarkers to assist clinicians with prompt and robust selection of people who are eligible for AHSCT are encouraged.
-
AHSCT as first-line therapy should only be considered for individuals with rapidly evolving, severe MS with a poor prognosis; in this scenario, AHSCT should be offered as part of a clinical trial or an observational, longitudinal research study (if a trial is not available) without delay whenever possible.
-
AHSCT can be considered for young (<45 years) individuals with early progressive MS with a short disease duration and who have well-documented clinical and radiological evidence of inflammatory disease.
-
Offering AHSCT for progressive MS without detectable inflammatory lesion activity is not supported owing to a lack of evidence.
-
Trials to compare AHSCT with DMTs that are approved for treatment of progressive forms of MS are encouraged.
-
Owing to a high risk and low or no benefit, AHSCT is not recommended for treatment of individuals with long-standing, advanced forms of MS with severe disability.
HSCT in NMOSD
AHSCT and allogeneic HSCT are endorsed by the EBMT as clinical options and developmental indications for the treatment of NMOSD that is refractory to conventional treatment2,31. The indication has reduced in recent years, however, owing to the availability of highly effective pharmacological treatments, including B cell-depleting, anti-IL-6 receptor and complement-inhibiting monoclonal antibodies, which effectively suppressed disease activity in RCTs109.
The role of HSCT in NMOSD has been explored in only a few studies, and outcomes have been mixed110. In a registry analysis by the EBMT ADWP that included 16 people with NMOSD who underwent AHSCT with different protocols (BEAM–ATG in nine, thiotepa–cyclophosphamide in three and cyclophosphamide–ATG in four), progression-free survival at years 3–5 was 48%, but 81% experienced a relapse at a median of 7 months after AHSCT111. Transplant-related mortality was zero. At long-term follow-up (median 47 months), one person had died of disease progression and four had undergone HSCT a second time; three had undergone allogeneic HSCT. In eight individuals assessed, aquaporin 4 (AQP4) antibodies remained positive at follow-up but these antibodies became undetectable in two who subsequently underwent allogeneic HSCT, and their absence was associated with durable disease remission.
A prospective open-label cohort study in which 13 people with NMOSD were treated with a complex cyclophosphamide-based protocol (including plasmapheresis the day before hospital admission and two doses of rituximab) produced more impressive results, with progression-free survival of 90% at year 5 (ref. 112). Median EDSS scores improved from 4.4 to 3.3, and 80% of individuals were free from relapses and immunosuppressive treatment after 5 years. AQP4 antibodies became negative in 9 of 11 individuals tested, and clearance of autoantibodies was associated with durable disease remission, suggesting that elimination of AQP4 antibodies could be a biomarker of treatment response. No grade 4 adverse events or transplant-related mortality occurred.
In a retrospective study of allogeneic HSCT, long-term disease control was seen in a large proportion of individuals with refractory autoimmune diseases, including five individuals with NMOSD, suggesting that this treatment has an acceptable toxicity profile and transplant-related mortality113. Durable disease remission for up to 10 years with no detectable AQP4 antibodies was reported in two individuals who were treated with allogeneic HSCT even after failure of AHSCT114. Allogeneic HSCT has also been explored in paediatric NMOSD — four cases have been logged in the EBMT database, and outcomes have been reported in only one. In this individual, control and improvement of disease was observed after 2 years of follow-up8.
Recommendations
-
Evidence is insufficient to indicate the use of HSCT in NMOSD outside clinical trials, mostly owing to the availability of highly effective treatments.
-
AHSCT could be considered as a rescue therapy for NMOSD that does not respond to treatment, or as an induction therapy for aggressive disease, especially with the use of conditioning regimens that include anti-CD20 or antibody-depleting strategies.
-
Allogeneic HSCT should only be considered for individuals in whom AHSCT has failed and no other treatment options are available.
Development of AHSCT services
Neurology and haematology specialists should be involved in the selection of candidates for AHSCT, and an effective AHSCT service requires multidisciplinary expertise and coordination across the areas of neurology, haematology, neuroradiology, physiotherapy, laboratory medicine and reproductive medicine (Table 2). A neurology unit that aspires to offer AHSCT should have good expertise in the management of MS and/or NMOSD, and experience of AHSCT should be developed through participation in clinical trials or service provision programmes led by neurologists with experience in AHSCT and haematologists with experience in MS in units that comply with the standards set by the Foundation for the Accreditation of Cellular Therapy (FACT) and the Joint Accreditation Committee ISCT–Europe and EBMT (JACIE)115. Given the high costs of DMTs, particularly monoclonal antibodies, the time-limited, one-off cost of AHSCT is likely to be a more cost-effective use of resources for the treatment of highly active forms of relapsing–remitting MS, as found in three studies completed in the USA116, UK117 and Norwegian118 health-care systems. Appropriate, up-to-date evaluations are needed to inform health-care payers about AHSCT access and commissioning or repayment policies.
Recommendations
-
Multidisciplinary expertise and facilities are required for development of an AHSCT service (Table 2).
-
Build experience of AHSCT locally through participation in clinical trials or service provision programmes led by neurologists with experience of AHSCT and haematologists with experience of MS.
-
For HSCT units, FACT–JACIE or equivalent accreditation is recommended.
-
Develop high-quality multidisciplinary regional and national programmes.
-
Promote economic evaluations of AHSCT versus licensed therapeutics and appropriate updates in access and funding by health-care payers.
Haematological and other specialist assessments
Assessment of fitness to undergo AHSCT
Assessment of the indication to treat with AHSCT requires detailed neurological assessment with disease history, disability status and MRI examination. Once the indication is established, haematological pretransplant assessment is required to confirm eligibility and screen for comorbidities that contraindicate the procedure. Standard screening for comorbidities includes liver, bone and viral profiles, measurement of glomerular filtration rate, a lung function test and plain radiography of the chest, cardiac assessment with electrocardiography and echocardiography, a dental check-up, identification of fertility needs and assessment of performance status; an HSCT comorbidity index can be used (Box 1). For individuals whose standard lung function tests are out of range, additional respiratory work-up, including chest CT and referral to a respiratory consultant for further assessment, is needed to rule out ventilatory defects. Additional cardiological work-up should be done for individuals with considerable cardiac risk factors or those aged >40 years; if any results are abnormal, they should be referred for cardiological review before proceeding to AHSCT. Likewise, any psychological or psychiatric concerns should be evaluated by the appropriate mental health specialist.
The impact of previous DMTs on safety should also be considered, as carryover effects can complicate mobilization, conditioning and immune reconstitution, particularly after treatment with long-acting lymphodepleting agents, such as alemtuzumab, after any cytotoxic treatment, or after multiple lines of therapy. A washout period that is appropriate for previous treatment and host factors is warranted to balance the risks of an MS relapse during DMT withdrawal against that of complications from the sequence of treatments. DMT withdrawal should generally be kept as short as possible to avoid MS disease activity. Specific recommendations for washout periods before leukapheresis and lymphodepleting conditioning treatment have been published by the EBMT119; however, given that clinical and medication histories are often complex for individuals considering AHSCT, we recommend discussion and decision-making among a multidisciplinary expert group on an individual case basis.
Management of fertility
MS is prevalent in young adults and especially in women of childbearing age. Furthermore, demographic shifts mean that the age of women at childbirth is increasing in developed countries, suggesting that an increasing proportion of individuals with MS who are referred for AHSCT will still hope to become pregnant after the procedure. Successful pregnancies after AHSCT (mostly through natural conception) have been reported in the retrospective EBMT survey of AHSCT in autoimmune diseases without any apparent effects of conditioning regimens or increased risk of disease reactivation after delivery, though the numbers were small and the data were not corrected for the desire for pregnancy120. In retrospective studies of people who have undergone AHSCT for MS, the rate of menses recovery was 57% after the use of the cyclophosphamide–ATG protocol in one study 39 and 70% after the use of the BEAM–ATG or cyclophosphamide–ATG protocol in another report121. In the latter study, older age and prior use of cyclophosphamide were associated with persistent amenorrhoea after AHSCT121. Evidence from large, well-designed prospective studies is lacking.
Importantly, however, spontaneous resumption of menses might not be an accurate marker of fertility in this context, as anti-Mullerian hormone (AMH) was low even in individuals in whom menses resumed121, and natural conception has been reported despite post-transplant amenorrhoea122 and low AMH levels123. Hence, contraception is not only mandatory before starting cytotoxic chemotherapy or any other agent that is teratogenic or contraindicated in pregnancy, but also recommended in the early post-transplantation period and thereafter if pregnancy is not desired, even in those with amenorrhoea. Hormonal replacement therapy should be considered if premature ovarian failure is diagnosed124. In addition, autoimmune diseases that warrant AHSCT can be associated with reduced fertility at baseline125,126 (which may be undiagnosed), possibly increasing the risk of permanent amenorrhoea after the procedure125. Evidence indicates that treatment with a gonadotropin-releasing hormone agonist before AHSCT protects against chemotherapy-related premature ovarian failure and helps to maintain ovulation124,127; yet evidence that this treatment helps to preserve fertility was considered insufficient and further investigation is required127.
Impairment of fertility has been reported in men who have undergone HSCT for haemato-oncological indications at rates of 20–90%, depending on the conditioning regimen128. However, few data are available about the effects in men with autoimmune disease; the data that exist show a reduction in testosterone levels after AHSCT, though levels remained above the defined threshold in three of four individuals tested125. In men with MS, the incidence of disorders of the reproductive organs and fertility after AHSCT with BEAM–ATG or cyclophosphamide–ATG protocols has been reported to be ~28 per 1,000 person-years129. Sporadic cases of unassisted fertilization resulting in conception after men have undergone AHSCT for MS have been reported53,122.
Recommendations
-
Perform an accurate haematological assessment before AHSCT to confirm eligibility and to screen for comorbidities (Box 1).
-
Manage the risks of toxicity and carryover effects from prior treatments with an appropriate washout period; this period should not be longer than necessary because withdrawal of DMTs increases the risk of MS activity and neurological deterioration.
-
Assess, counsel and refer individuals for provision of personalized information and management of their reproductive needs, fertility risk and contraception before initiation of treatment.
-
Emphasize to patients that use of contraception in the pre-transplantation to early post-transplantation period is essential, even for those who are expected to have reduced fertility.
-
Facilitate access to reproductive endocrinology or gynaecology services before AHSCT for counselling and preservation of fertility for both male and female candidates, and after AHSCT for treatment of premature menopause in women and of subfertility and hypogonadism in men.
-
Reproductive specialists are encouraged to include measurement of follicle-stimulating hormone, luteinizing hormone, oestradiol, anti-Mullerian hormone (in women) and testosterone (in men) in the endocrine work-up before AHSCT.
-
When appropriate, specialists should consider treatment with a gonadotropin-releasing hormone agonist to attenuate the risk of premature menopause.
AHSCT treatment methodology
Treatment protocols in MS
Given that lymphoablative conditioning has a key role in the mechanism of AHSCT, a correlation between the intensity of the regimen and neurological outcomes has been postulated63,64,130. Though low-intensity regimens (for example, lower-dose cyclophosphamide without serotherapy) were ineffective in one study131, evidence for the proposed correlation is lacking. Intensive conditioning protocols (for example, cylophosphamide–total body irradiation–ATG or busulfan–cyclophosphamide–ATG) are likely to be more effective but also to be associated with a higher risk of toxicity130. For these reasons, intermediate-intensity conditioning protocols, such as BEAM–ATG or cyclophosphamide–ATG, have been widely adopted for AHSCT treatment of MS; the latter has become the most commonly used over the past 10 years owing to easier inpatient management and the influence of the MIST trial82, amongst other factors. The current EBMT guidelines advocate the use of either the cyclophosphamide–ATG or BEAM–ATG regimens delivered in transplant units that provide high-quality care and are accredited by JACIE or equivalent organizations31.
The efficacy and safety of BEAM–ATG and cyclophosphamide–ATG regimens have been compared only in retrospective studies. In one such comparison in relapsing–remitting MS, the use of the BEAM–ATG conditioning protocol was independently associated with a higher chance of NEDA-3 maintenance than other intermediate-intensity or low-intensity regimens, though the number of individuals who were treated with the standard cyclophosphamide–ATG conditioning protocol was very low (27 people)45. More evidence is expected from a retrospective analysis of the EBMT database to compare efficacy and safety outcomes in a larger cohort (n = 1,114) of people with MS who were treated with either BEAM–ATG (n = 442) or cyclophosphamide–ATG (n = 672) regimens between 1985 and 2023. From a preliminary report of this analysis132, no statistically significant differences were detected in either the effectiveness or the toxicities of the two regimens when adjusted for disease type (progressive versus relapsing–remitting), EDSS score at baseline and year of the procedure.
The ECTRIMS Focused Workshop attendees agreed that a personalized medicine strategy in which the AHSCT protocol is tailored to individuals according to disease activity and risk profile is worth exploring. When assessing the treatment intensity required, the use of chemotherapy in the mobilization regimen, graft manipulation (that is, CD34 selection to enrich for HSCs) and the use and type of serotherapy should also be considered in addition to the conditioning regimen used. Differences in the ability of chemotherapy drugs and immunosuppressive treatments to penetrate the CNS, which may affect their ability to suppress the immune attack in the target organ, should also be considered in the choice of conditioning regimen. Any previous treatments, particularly cytotoxic drugs, should also be considered, as cumulative toxicities can increase the risk of AHSCT.
Treatment protocols in NMOSD
Evidence in NMOSD is limited because only a small number of individuals have been treated with heterogeneous treatment protocols and comparative studies are lacking. A retrospective study by the EBMT showed that the majority of people who underwent AHSCT for NMOSD with various conditioning regimens experienced subsequent relapses and neurological deterioration in the long term111. Evidence from a single-centre study suggests that addition of rituximab and/or plasmapheresis to the conditioning regimen improves outcomes after AHSCT for NMOSD — the use of a complex protocol that included rituximab led to markedly better outcomes than in previous studies, inducing disease remission and clearance of AQP4 antibodies in nine of 11 participants over a median follow-up period of 5 years112. However, further evidence is needed to confirm this finding.
Allogeneic HSCT for NMOSD mainly involves the use of HSCs from HLA-matched donors, and myeloablative conditioning regimens that include serotherapy with ATG or alemtuzumab. Safety has improved over time, yet complications and transplant-related mortality remain higher than with AHSCT133,134. In the EBMT registry study, factors associated with improved progression-free survival were age <18 years, male sex and undergoing the procedure more recently113. Accordingly, allogeneic HSCT could be a treatment option only when conventional treatment has failed and relapses continue after AHSCT, but further studies are needed to determine the optimal approach. In this context, future strategies to reduce the risks include exploring new conditioning regimens with lower toxicity and/or different approaches to graft-versus-host disease prophylaxis, such as post-HSCT cyclophosphamide.
Recommendations
-
For the treatment of MS, intermediate-intensity conditioning protocols, such as BEAM–ATG or cyclophosphamide–ATG, are recommended to achieve the best balance of efficacy and risk in most settings, according to EBMT guidelines.
-
Use of low-intensity regimens (for example, low-dose cyclophosphamide without serotherapy) is not recommended outside clinical trials owing to poor evidence of efficacy.
-
Use of high-intensity, myeloablative conditioning protocols (for example busulfan–cyclophosphamide–ATG) is not recommended outside clinical trials owing to a higher risk of toxicity, but can be considered at a centre with the specific expertise.
-
For the treatment of NMOSD, when indicated, cyclophosphamide-based conditioning protocols, possibly associated with rituximab, are appropriate; the role of allogeneic HSCT is confined to a rescue treatment option for when NMOSD does not respond to approved biological therapy and relapses continue after AHSCT.
Neurological care after AHSCT
Rehabilitation
Rehabilitation for individuals with MS in whom AHSCT completely suppresses inflammation is a unique opportunity to exploit the reorganizational capacity of the brain and achieve maximal clinical recovery. Recommendations for rehabilitation in people with MS who undergo AHSCT135 include four phases (Table 3). ECTRIMS Focused Workshop attendees agreed on the need for further research in this field to clarify issues such as the optimal timing and setting of treatment, the type and intensity of exercises during the acute phase, and the potential additive effects of rehabilitation on neurological outcomes.
Clinical monitoring
In MS, disability outcomes are mostly based on changes in EDSS scores, but the low sensitivity of this scale to changes, especially for baseline scores close to six, makes it suboptimal for assessment of treatment effects136. Combination of the EDSS with other disability measures, such as the Multiple Sclerosis Functional Composite (MSFC)137, is therefore warranted; this combination has already been implemented in some studies of AHSCT64,82. The use of more sensitive tools should also be explored; for example, longitudinal changes in accelerometry data138 could be useful for assessing disability worsening beyond an EDSS score of 4.0. In order to better define the main driver of disability accrual after AHSCT, we suggest separation of confirmed disability accrual into relapse-associated worsening and progression independent of relapse activity (PIRA)139. In people with relapsing–remitting MS, prevention of conversion to secondary progressive MS would be a highly relevant end point but can only be evaluated in long-term studies. Cognitive outcomes should also be systematically assessed with the most appropriate instruments in the clinical setting, as such assessments could provide the most sensitive measure of overall brain function. Validated and standardized PROs, including fatigue and QoL measures, should be collected in prospective studies, and the use of new technologies, such as smartphones, wearable devices and sensors, for data collection should be explored90.
MRI monitoring
The MRI metrics that have been most commonly reported in studies of AHSCT are the numbers of new T2 and gadolinium-enhancing lesions. Across multiple studies, suppression of MRI inflammatory activity for at least 3–5 years was observed in most people who were treated with AHSCT72,140, with complete suppression of gadolinium-enhancing lesions for up to 12.7 years after high-intensity regimen AHSCT in one study130. Reductions in T2 lesion load have also been reported16,63. In both published RCTs in which AHSCT was compared with DMTs, MRI outcomes were superior with AHSCT81,82.
Brain volume changes have been explored in a few studies, but these studies have indicated that brain volume loss slows in the mid-to-long term after AHSCT to rates that are comparable with those in healthy individuals64,130. This slowing usually follows a transient increase in the rate of loss in the first 1–2 years after AHSCT, which could result from a combination of pseudo-atrophy and neurotoxic effects related to the intensity of the conditioning regimen78.
The ECTRIMS Focused Workshop attendees agreed that evaluation of MRI outcomes after AHSCT requires dedicated protocols, and that the Magnetic Resonance Imaging in Multiple Sclerosis (MAGNIMS) guidelines91 could be applied for standardization in this setting. A so-called re-baseline MRI should be acquired 6 months after AHSCT to serve as a new reference for assessment of post-therapy MRI lesion-based outcomes, while a later re-baseline MRI is required for assessment of brain atrophy to account for the pseudo-atrophy effects described above. Advanced MRI measures, including structural and functional connectivity, remyelination metrics and emerging biomarkers such as paramagnetic rim lesions, could provide new insights into the effects of AHSCT in MS in future studies.
Management of MS reactivation and DMTs
Information that is useful for the management of MS reactivation after AHSCT is sparse because the rate of events has been low. Even when MS reactivations have been reported141, few details have been provided about the criteria used for reintroduction of DMTs, neurological outcomes after follow-up and the safety of further treatment with DMTs. For these reasons, evidence-based recommendations for this scenario cannot be provided.
Attendees of the ECTRIMS Focused Workshop were in consensus that MS reactivations that occur between mobilization and conditioning (the occurrence of which is usually related to the time between these steps and previous treatment received) do not require resumption of DMTs. Reactivations that occur after completion of the AHSCT protocol should be managed on an individual case basis. In studies of AHSCT with follow-up periods of >5 years, DMTs were reintroduced in 11–35% of individuals51,142. In one study of long-term clinical outcomes after AHSCT, 15% of people who underwent AHSCT were retreated with DMTs, and the retreatment started after a median of 2 years (range 0.5–13 years). Among the retreated subgroup, moderate-efficacy DMTs were prescribed in 60% and high-efficacy DMTs in 40%45. Reintroduction of DMTs followed MS relapses in most cases, but also after detection of MRI activity alone in some16,140. DMTs were usually not reintroduced in individuals with PIRA, as their benefits in this context are currently unknown.
When reintroducing a DMT after AHSCT, the safety of the treatment should be considered particularly carefully. Though one study showed that the risk of infections at 12 months was comparable in people who had undergone AHSCT and people who received non-induction DMTs129, the risk of adverse events might be increased by previous exposure to high-dose immunosuppression owing to a cumulative lower level of immune competence. Furthermore, MS inflammatory activity can occur after reintroduction of DMTs, mostly when using first-line DMTs and when reintroduction was due to an MS relapse rather than MRI activity. The role of a second AHSCT, including for those who have had a prolonged response to a first AHSCT, is currently under evaluation by the EBMT ADWP. Given that evidence is lacking, neurological and safety outcomes after MS reactivation require further investigation, preferably in large collaborative studies.
Recommendations
-
Facilitate access to rehabilitation services that cover the four recommended phases (Table 3).
-
After AHSCT, monitor neurological outcomes, including relapses and disability metrics; to assess disability, use the EDSS, MSFC and other established rating scales, as well as more advanced instruments where available.
-
Consider collecting measures of cognitive function, fatigue and QoL.
-
Explore new technologies, such as wearable electronic devices and biosensors, for collecting PROs.
-
Monitor MRI outcomes according to MAGNIMS guidelines; acquire images before HSC mobilization, a re-baseline scan 6 months after AHSCT, and yearly scans thereafter, or as clinically required.
-
Consider reintroducing DMT if a relapse occurs after AHSCT on an individual case basis, paying special attention to additional risks from all previous treatment exposures.
Prophylaxis and care of complications
Risk of infection and vaccinations
In addition to the extent of experience at the centre, several factors can influence the risk of infection in people who have undergone AHSCT, including epidemiological factors (for example, influenza season or the presence of small children in the household), previous disease (such as recurrent urinary or respiratory infections), immunosuppressive treatment received before AHSCT, immunization history before AHSCT, transplantation-related factors (for example, the type of chemotherapy used, use of irradiation, HSC purification or T cell depletion of the haematopoietic graft), and the use of B cell-depleting antibodies either as DMT before AHSCT or after AHSCT for treatment of post-transplantation reactivation of EBV.
Published guidance recommends that people who have undergone HSCT are considered as ‘never vaccinated’ and offered revaccination143. Vaccination planning after AHSCT should follow national143 and international recommendations144 and be adapted to local practice. Vaccination can follow a routine schedule or flexible time points based on immunity milestones; the latter maximizes the likelihood of response but also carries a higher risk of missing vaccinations. No evidence suggests a major risk of direct adverse effects from inactivated vaccines in immunocompromised individuals, and existing data indicate only very low risks of complications associated with immune activation, such as rejections or disease exacerbation144. By contrast, vaccine-induced infectious disease has been associated with administration of live vaccines, especially in people with suppressed T cell immunity, and outcomes can be severe144. The ECTRIMS Focused Workshop attendees recommended harmonization of vaccination protocols within regional and national AHSCT programmes, and regular (for example, annual) review and updates of protocols as necessary to ensure coverage of emerging indications, as required for disease outbreaks from new pathogens or variants (for example, COVID-19).
The main infections to be considered in people who have undergone AHSCT for MS include pneumococcal disease, influenza virus infection, varicella zoster virus (VZV)-related infections, and COVID-19. In a meta-analysis of invasive pneumococcal disease in immunocompromised individuals, the risk of severe invasive pneumococcal disease was increased in recipients of AHSCT compared with that in healthy controls, though the data were not stratified according to the underlying disease145. The 2017 European Conference on Infections in Leukaemia (ECIL7) guidelines144 suggest that recipients of AHSCT should receive three doses of conjugated anti-pneumococcal polysaccharide vaccine administered at 1-month intervals starting from 3–6 months after transplantation, followed by one dose at 12 months. One dose of annual seasonal inactivated influenza vaccination is recommended at the beginning of the influenza season in all recipients of AHSCT at 3–6 months after transplantation, particularly in those who are considered to be immunosuppressed144. In case of an influenza outbreak in the community, vaccination could be administered before 6 months, but should not be administered less than 3 months after transplantation in any case143. In a clinical trial, two doses of the recombinant VZV vaccine effectively prevented herpes zoster in people who had undergone AHSCT146. Given the high risk of herpes zoster virus infection in the first 2–3 years after HSCT, published guidance recommends vaccination with the recombinant VZV vaccine to commence 6 months after transplantation, with specified schedules, cautions and contraindications143. With respect to SARS-CoV-2 vaccination, the effects of DMTs on antibody-mediated responses in MS have been extensively studied147, but few data are available in people who have undergone AHSCT for MS, so a standard schedule should be adopted according to national and international guidelines148.
Viral reactivations
Comprehensive data on viral infection and reactivation after AHSCT for autoimmune diseases, including MS, are lacking. CMV reactivation has been reported in 11–35% of people who have undergone AHSCT for MS46,149,150, and EBV reactivation after AHSCT for treatment of MS has been reported in 34–100%51,149,150. The discrepancies between studies could be attributed to differences in treatment protocols, the methodology used for testing, the frequency of testing, the definitions of reactivation used and/or differences in the patient populations, which could also be influenced by previous treatment. The risk of EBV reactivation is increased by addition of T cell-depleting strategies (for example, alemtuzumab or ATG, especially at higher doses)151, the use of a high-intensity conditioning regimen and the MS disease itself, as the prevalence of EBV is high among people with MS152, yet the occurrence of EBV disease or EBV-associated post-transplant lymphoproliferative disorder (EBV–PTLD) is rare and can be managed with current monitoring and pre-emptive strategies153. The use of B cell-depleting CD20 antibody therapy in the period before AHSCT could, in theory, protect against EBV reactivation by eliminating EBV-infected B cells, which are the main reservoir of the virus, but, to our knowledge, this hypothesis is yet to be tested. More research is needed on reactivation of CMV and EBV, their management and outcomes after AHSCT; an ADWP survey on this topic is underway.
Nevertheless, current EBMT guidelines recommend screening for CMV, herpes simplex virus (HSV), VZV, EBV, HIV, human T-lymphotropic virus types 1 and 2, and hepatitis viruses as part of the pre-transplantation work-up154. Positivity for HIV, HSV, HTLV-1 or hepatitis viruses is not in itself a contraindication to AHSCT, but the associated diseases and treatments should be considered when evaluating the risk of AHSCT and planning the management of the individual. For individuals who are positive for antibodies against CMV and EBV and who receive ATG, other serotherapy or manipulated autografts, the same guidelines recommend monitoring for reactivation of these viruses for the first 100 days154. To monitor for CMV and EBV reactivation, standardized PCR assays are recommended, at least during the highest risk period (days 15–60), with weekly testing in the first 2 months, then fortnightly until day 100 (ref. 154).
For CMV, pre-emptive treatment of laboratory-detected viral reactivation with valganciclovir or ganciclovir should follow local or national guidelines, and treatment of CMV-related disease, which is exceedingly rare, is always recommended. EBV reactivation associated with monoclonal paraproteinaemia has been associated with adverse neurological events and lymphoproliferative disease44,155,156. To mitigate the risks, active surveillance for post-transplantation lymphoproliferative disease according to local practice is recommended after EBV reactivation154. Pre-emptive treatment with rituximab should be considered for people who are at high risk of EBV–PTLD and impaired immune reconstitution, such as those with a high peak EBV viral load after AHSCT155.
Another important virus that must be considered is John Cunningham virus (JCV), as failure to control latent infection of JCV in the brain can cause progressive multifocal leukoencephalopathy (PML), which is a known risk of treatment with natalizumab157 and, less commonly, other MS DMTs158,159 that can cause long-lasting CNS injury and, in severe cases, can be fatal. PML has been reported as a rare complication after AHSCT for the treatment of haematological malignancies, but only 11 cases were reported up to 2017 (ref. 160), and, to our knowledge, no cases of PML have been reported after AHSCT for the treatment of MS.
Secondary autoimmunity
Autoimmune complications that can occur after AHSCT include organ-specific involvement and systemic diseases, but the incidence, risk factors, treatment and outcomes of these complications are not well characterized. So-called secondary autoimmune diseases have been described in <1–18% of people who have undergone AHSCT for MS65,161,162, with some differences between transplantation regimens, but these complications are thought to be under-reported. The main secondary autoimmune diseases that have occurred in people with MS are thyroiditis and, less frequently, idiopathic thrombocytopenic purpura (ITP), but other disorders that have been described include Crohn’s disease, acquired autoimmune factor VIII deficiency and alopecia areata162.
A review of the available literature published in 2021 determined that a high risk of secondary autoimmune diseases was associated with the use of high-intensity myeloablative conditioning regimens that involve the use of busulfan, after which the overall incidence was 18% across multiple studies58. By contrast, intermediate-intensity non-myeloablative conditioning regimens were associated with a lower incidence (7.7%) overall, though regimens that involved the use of alemtuzumab were associated with an incidence of 14% in one study162, and with a higher risk of ITP (incidence 11.5%) when compared with regimens that used ATG in another study46. Secondary autoimmunity is a known complication of alemtuzumab treatment in MS, and in a comparison of alemtuzumab treatment with AHSCT for MS, the risk of thyroid disease was higher with alemtuzumab55,129, though the incidence of thyroid disease was higher in both groups than in those who received non-induction therapies129. These observations suggest that higher vigilance for secondary autoimmunity could be warranted in people who had received alemtuzumab before AHSCT.
In the same review, pooled rates of secondary autoimmune diseases were <1% after the use of BEAM regimens162, though this low rate could have been due to under-reporting. Indeed, in a retrospective study, AHSCT with the use of either BEAM–ATG or cyclophosphamide–ATG regimens129 was associated with an 11% incidence of autoimmune thyroiditis in the first 3 years, almost sixfold the incidence in a reference group that were treated with any of rituximab, fingolimod, natalizumab or dimethyl fumarate129. One possible strategy to decrease the risk of secondary autoimmunity after AHSCT is post-transplantation B cell depletion; this approach has been tested in a small group of people who were receiving alemtuzumab treatment163, in whom rituximab therapy seemed to prevent secondary autoimmunity, so the use of this approach in the context of AHSCT warrants further investigation.
Late adverse events
Besides secondary autoimmune diseases and effects on fertility, other delayed adverse events of AHSCT mainly include risk of infection and malignancies. Data on the frequency of these events after AHSCT for autoimmune diseases and how strongly they are related to the treatment are sparse, and limited information is available on other potential long-term complications, such as cardiovascular and bone mineral diseases. The risk of infections (mainly pneumonia and VZV reactivation) is considered highest during the first 2 years after AHSCT, but systematic evidence is lacking. Standard management of such infections includes antibiotic prophylaxis to cover invasive fungal infections for the first 3–4 months after AHSCT and herpesvirus and pneumocystis infection for 6 months, alongside immune monitoring for T cell and B cell subsets and immunoglobulin electrophoresis (on a 3-month basis in the first year and then annually) to guide infection prophylaxis31.
Though data from the oncology field have raised the concern that chemotherapy can be associated with an increased lifetime risk of malignancy164, no current evidence suggests this to be the case in a non-malignant (that is, autoimmune) primary disease setting. In an ongoing retrospective study of the EBMT–ADWP Registry that includes ~500 individuals who have been treated with AHSCT for various autoimmune diseases (47% MS) at 27 participating centres in 11 countries during the period 1997–2016, predictive cumulative incidence of malignancies, endocrine or bone complications and cardiac complications at year 10 were 3.5%, 20.3% and 13.1%, respectively165. A similar risk of malignancies was reported among people with MS in a previous EBMT–CIBMTR Registry study65. However, the low numbers of events and possible contributions of previous exposure to immunosuppressive treatments prevents accurate estimation of the risk of malignancy after AHSCT.
Recommendations
-
Offer revaccination after AHSCT according to local, national and international (ECIL7) recommendations.
-
Monitor for CMV and EBV reactivation with standardized PCR assays, at least over the highest risk period (days 15–60), with a weekly schedule in the first 2 months, and then fortnightly until day 100.
-
Watch and treat or refer promptly for secondary autoimmune disease; these mainly present as thyroiditis or ITP, but be aware of less common diseases, such as autoimmune haemolytic anaemia, acquired haemophilia, antiphospholipid syndrome and myasthenia gravis.
-
Collect long-term survival data and use standardized surveillance tools to capture and report late adverse events, with particular attention to late infections and malignancies.
Conclusions
Immunological studies provide increasing support to the hypothesis that ‘immune resetting’ is the mechanism of action of AHSCT in MS. Refinement of treatment protocols and patient selection has improved the efficacy and safety of the procedure. Uncontrolled cohort studies and meta-analyses have shown that among people with relapsing–remitting MS in whom standard treatment has failed, AHSCT has high effectiveness with acceptable safety, and two RCTs have shown that its efficacy is greater than that of moderate-efficacy and some high-efficacy DMTs (mitoxantrone and natalizumab).
In this Consensus Statement, ECTRIMS and the EBMT, as well as lead representatives of ACTRIMS, endorse AHSCT for selected indications. In relapsing–remitting MS, AHSCT should be offered to appropriate candidates, normally after failure of high-efficacy DMT but within the window of opportunity before the development of irreversible disability. More evidence to inform the optimal positioning of AHSCT in MS care is awaited from ongoing RCTs in which AHSCT is being compared with high-efficacy DMTs in relapsing–remitting MS. AHSCT is not recommended in any late-stage forms of MS that are typically progressive, although AHSCT could have a role in early progressive disease with clear clinical and/or radiological evidence of inflammation. AHSCT with adapted protocols can be considered for treatment-refractory NMOSD.
Improved outcome measures that sensitively and accurately capture all domains that are relevant to patients are needed in future studies of AHSCT. We recommend that long-term objective neurological assessments, MRI data and PROs are systematically collected from all individuals who undergo AHSCT at all centres worldwide, including those that offer low-intensity protocols in an outpatient setting, so that adequate evidence can be assessed and included in meta-analyses. To advance knowledge on the biological effects and mechanisms of action of AHSCT, further studies of immune reconstitution and immune function are needed, making use of inflammatory, neuroaxonal and glial biomarkers. Advances in knowledge will be maximized through collaborative research in registry-based studies, large cohort studies and multicentre trials.
References
Muraro, P. A. Resetting tolerance in autoimmune disease. Science 380, 470–471 (2023).
Snowden, J. A. et al. Indications for haematopoietic cell transplantation for haematological diseases, solid tumours and immune disorders: current practice in Europe, 2022. Bone Marrow Transpl. 57, 1217–1239 (2022).
Thompson, A. J., Baranzini, S. E., Geurts, J., Hemmer, B. & Ciccarelli, O. Multiple sclerosis. Lancet 391, 1622–1636 (2018).
Wingerchuk, D. M. et al. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology 85, 177–189 (2015).
Muraro, P. A. et al. Autologous haematopoietic stem cell transplantation for treatment of multiple sclerosis. Nat. Rev. Neurol. 13, 391–405 (2017).
Sharrack, B., Petrie, J., Coles, A. & Snowden, J. A. Is stem cell transplantation safe and effective in multiple sclerosis. BMJ https://doi.org/10.1136/bmj-2020-061514 (2022).
Sormani, M. P. et al. Autologous hematopoietic stem cell transplantation in multiple sclerosis: a meta-analysis. Neurology 88, 2115–2122 (2017).
Achini-Gutzwiller, F. R. et al. Haematopoietic stem cell transplantation for severe autoimmune diseases in children: a review of current literature, registry activity and future directions on behalf of the autoimmune diseases and paediatric diseases working parties of the European Society for Blood and Marrow Transplantation. Br. J. Haematol. 198, 24–45 (2022).
European Committee for Treatment and Research in Multiple Sclerosis. 9th ECTRIMS Focused Workshop: Autologous Haematopoietic Stem Cell Transplantation for Treatment of MS and Related Diseases. ECTRIMS ectrims.eu/ongoing-educational-programmes/focused-workshops/ (2022).
European Committee for Treatment and Research in Multiple Sclerosis. 9th ECTRIMS Focused Workshop: Autologous Haematopoietic Stem Cell Transplantation for Treatment of MS and Related Diseases. ECTRIMS https://ectrims.conference2web.com/filter?event_groups=Focused+Workshop&starts_at_years=2022 (2022).
European Committee for Treatment and Research in Multiple Sclerosis. Highlights from the 9th ECTRIMS Focused Workshop, 2022. ECTRIMS https://ectrims.eu/app/uploads/2022/03/9th-ECTRIMS-Focused-Workshop-2022.pdf (2022).
Jelcic, I. et al. Memory B cells activate brain-homing, autoreactive CD4+ T cells in multiple sclerosis. Cell 175, 85–100.e23 (2018).
Cencioni, M. T. et al. Immune reconstitution following autologous hematopoietic stem cell transplantation for multiple sclerosis: a review on behalf of the EBMT Autoimmune Diseases Working Party. Front. Immunol. 12, 813957 (2021).
Nash, R. A. et al. High-dose immunosuppressive therapy and autologous hematopoietic cell transplantation for relapsing-remitting multiple sclerosis (HALT-MS): a 3-year interim report. JAMA Neurol. 72, 159–169 (2015).
Darlington, P. J. et al. Diminished Th17 (not Th1) responses underlie multiple sclerosis disease abrogation after hematopoietic stem cell transplantation. Ann. Neurol. 73, 341–354 (2013).
Moore, J. J. et al. Prospective phase II clinical trial of autologous haematopoietic stem cell transplant for treatment refractory multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 90, 514–521 (2019).
Ruder, J. et al. Dynamics of T cell repertoire renewal following autologous hematopoietic stem cell transplantation in multiple sclerosis. Sci. Transl. Med. 14, eabq1693 (2022).
Muraro, P. A. et al. Thymic output generates a new and diverse TCR repertoire after autologous stem cell transplantation in multiple sclerosis patients. J. Exp. Med. 201, 805–816 (2005).
Muraro, P. A. et al. T cell repertoire following autologous stem cell transplantation for multiple sclerosis. J. Clin. Invest. 124, 1168–1172 (2014).
Harris, K. M. et al. Extensive intrathecal T cell renewal following hematopoietic transplantation for multiple sclerosis. JCI Insight 5, e127655 (2020).
Amoriello, R. et al. TCR repertoire diversity in multiple sclerosis: high-dimensional bioinformatics analysis of sequences from brain, cerebrospinal fluid and peripheral blood. EBioMedicine 68, 103429 (2021).
Darlington, P. J. et al. Natural killer cells regulate Th17 cells after autologous hematopoietic stem cell transplantation for relapsing remitting multiple sclerosis. Front. Immunol. 9, 834 (2018).
Arruda, L. C. et al. Autologous hematopoietic SCT normalizes miR-16, -155 and -142-3p expression in multiple sclerosis patients. Bone Marrow Transpl. 50, 380–389 (2015).
de Oliveira, G. et al. Defective expression of apoptosis‐related molecules in multiple sclerosis patients is normalized early after autologous haematopoietic stem cell transplantation. Clin. Exp. Immunol. 187, 383–398 (2017).
de Paula A Sousa, A. et al. Autologous haematopoietic stem cell transplantation reduces abnormalities in the expression of immune genes in multiple sclerosis. Clin. Sci. 128, 111–120 (2015).
von Niederhäusern, V. et al. B-cell reconstitution after autologous hematopoietic stem cell transplantation in multiple sclerosis. Neurol. Neuroimmunol. Neuroinflamm. 9, e200027 (2022).
Abrahamsson, S. V. et al. Non-myeloablative autologous haematopoietic stem cell transplantation expands regulatory cells and depletes IL-17 producing mucosal-associated invariant T cells in multiple sclerosis. Brain 136, 2888–2903 (2013).
Visweswaran, M. et al. Sustained immunotolerance in multupe sclerosis after stem cell transplant. Ann. Clin. Transl. Neurol. 9, 206–220 (2022).
Ruder, J. et al. NK cells and innate-like T cells after autologous hematopoietic stem cell transplantation in multiple sclerosis. Front. Immunol. 12, 5398 (2021).
Van Wijmeersch, B. et al. Allogeneic bone marrow transplantation in models of experimental autoimmune encephalomyelitis: evidence for a graft-versus-autoimmunity effect. Biol. Blood Marrow Transpl. 13, 627–637 (2007).
Sharrack, B. et al. Autologous haematopoietic stem cell transplantation and other cellular therapy in multiple sclerosis and immune-mediated neurological diseases: updated guidelines and recommendations from the EBMT Autoimmune Diseases Working Party (ADWP) and the Joint Accreditation Committee of EBMT and ISCT (JACIE). Bone Marrow Transpl. 55, 283–306 (2020).
Karnell, F. G. et al. Reconstitution of immune cell populations in multiple sclerosis patients after autologous stem cell transplantation. Clin. Exp. Immunol. 189, 268–278 (2017).
Schafflick, D. et al. Integrated single cell analysis of blood and cerebrospinal fluid leukocytes in multiple sclerosis. Nat. Commun. 11, 247 (2020).
Alexander, T. et al. SCT for severe autoimmune diseases: consensus guidelines of the European Society for Blood and Marrow Transplantation for immune monitoring and biobanking. Bone Marrow Transpl. 50, 173–180 (2015).
Willison, A. G., Ruck, T., Lenz, G., Hartung, H. P. & Meuth, S. G. The current standing of autologous haematopoietic stem cell transplantation for the treatment of multiple sclerosis. J. Neurol. 269, 3937–3958 (2022).
Fassas, A. et al. Peripheral blood stem cell transplantation in the treatment of progressive multiple sclerosis: first results of a pilot study. Bone Marrow Transpl. 20, 631–638 (1997).
Burt, R. et al. T cell-depleted autologous hematopoietic stem cell transplantation for multiple sclerosis: report on the first three patients. Bone marrow Transplant. 21, 537–541 (1998).
Tolf, A. et al. Sustained remission in multiple sclerosis after hematopoietic stem cell transplantation. Acta Neurol. Scand. 140, 320–327 (2019).
Kvistad, S. A. S. et al. Safety and efficacy of autologous hematopoietic stem cell transplantation for multiple sclerosis in Norway. Mult. Scler. 26, 1889–1897 (2019).
Mariottini, A. et al. Impact of autologous haematopoietic stem cell transplantation on disability and brain atrophy in secondary progressive multiple sclerosis. Mult. Scler. 27, 61–70 (2021).
Giedraitiene, N., Kizlaitiene, R., Peceliunas, V., Griskevicius, L. & Kaubrys, G. Selective cognitive dysfunction and physical disability improvement after autologous hematopoietic stem cell transplantation in highly active multiple sclerosis. Sci. Rep. 10, 21286 (2020).
Dayama, A., Bhargava, R., Kurmi, S. R., Jain, S. & Dua, V. Autologous stem cell transplant in adult multiple sclerosis patients: a study from North India. Neurol. India 68, 454–457 (2020).
Das, J. et al. Autologous haematopoietic stem cell transplantation as a first-line disease-modifying therapy in patients with ‘aggressive’ multiple sclerosis. Mult. Scler. J. 27, 1198–1204 (2021).
Nicholas, R. S. et al. Autologous hematopoietic stem cell transplantation in active multiple sclerosis: a real-world case series. Neurology 97, e890–e901 (2021).
Boffa, G. et al. Long-term clinical outcomes of hematopoietic stem cell transplantation in multiple sclerosis. Neurology 96, e1215–e1226 (2021).
Burt, R. K., Han, X., Quigley, K., Helenowski, I. B. & Balabanov, R. Real-world application of autologous hematopoietic stem cell transplantation in 507 patients with multiple sclerosis. J. Neurol. 269, 2513–2526 (2022).
Kvistad, S. A. S. et al. Impact of previous disease-modifying treatment on safety and efficacy in patients with MS treated with AHSCT. J. Neurol. Neurosurg. Psychiatry 93, 844–848 (2022).
Mariottini, A. et al. Intermediate-intensity autologous hematopoietic stem cell transplantation reduces serum neurofilament light chains and brain atrophy in aggressive multiple sclerosis. Front. Neurol. 13, 820256 (2022).
Ruder, J. et al. Dynamics of inflammatory and neurodegenerative biomarkers after autologous hematopoietic stem cell transplantation in multiple sclerosis. Int. J. Mol. Sci. 23, 10946 (2022).
Zjukovskaja, C., Larsson, A., Cherif, H., Kultima, K. & Burman, J. Biomarkers of demyelination and axonal damage are decreased after autologous hematopoietic stem cell transplantation for multiple sclerosis. Mult. Scler. Relat. Disord. 68, 104210 (2022).
Silfverberg, T. et al. Haematopoietic stem cell transplantation for treatment of relapsing-remitting multiple sclerosis in Sweden: an observational cohort study. J. Neurol. Neurosurg. Psychiatry 95, 125–133 (2023).
Jespersen, F. et al. Autologous hematopoietic stem cell transplantation of patients with aggressive relapsing-remitting multiple sclerosis: Danish nation-wide experience. Mult. Scler. Relat. Disord. 76, 104829 (2023).
Kvistad, C. E. et al. Autologous hematopoietic stem cell transplantation for multiple sclerosis: long-term follow-up data from Norway. Mult. Scler. 30, 751–754 (2024).
Boffa, G. et al. Hematopoietic stem cell transplantation in people with active secondary progressive multiple sclerosis. Neurology 22, e1215–e1226 (2022).
Zhukovsky, C. et al. Autologous haematopoietic stem cell transplantation compared with alemtuzumab for relapsing–remitting multiple sclerosis: an observational study. J. Neurol. Neurosurg. Psychiatry 92, 189–194 (2021).
Boffa, G. et al. Aggressive multiple sclerosis: a single‐centre, real‐world treatment experience with autologous haematopoietic stem cell transplantation and alemtuzumab. Eur. J. Neurol. 27, 2047–2055 (2020).
Häußler, V. et al. aHSCT is superior to alemtuzumab in maintaining NEDA and improving cognition in multiple sclerosis. Ann. Clin. Transl. Neurol. 8, 1269–1278 (2021).
Vaisvilas, M., Kaubrys, G., Kizlaitiene, R., Taluntiene, V. & Giedraitiene, N. Autologous hematopoietic stem cell transplantation is superior to alemtuzumab in patients with highly active relapsing multiple sclerosis and severe disability. Mult. Scler. Relat. Disord. 80, 105096 (2023).
Braun, B. et al. Benefits of aHSCT over alemtuzumab in patients with multiple sclerosis besides disability and relapses: sustained improvement in cognition and quality of life. Mult. Scler. Relat. Disord. 82, 105414 (2023).
Mariottini, A. et al. Autologous haematopoietic stem cell transplantation versus low-dose immunosuppression in secondary-progressive multiple sclerosis. Eur. J. Neurol. 29, 1708–1718 (2022).
Kalincik, T. et al. Effectiveness of autologous haematopoietic stem cell transplantation versus natalizumab in progressive multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 95, 775–783 (2024).
Kalincik, T. et al. Comparative effectiveness of autologous hematopoietic stem cell transplant vs fingolimod, natalizumab, and ocrelizumab in highly active relapsing-remitting multiple sclerosis. JAMA Neurol. 80, 702–713 (2023).
Burt, R. K. et al. Association of nonmyeloablative hematopoietic stem cell transplantation with neurological disability in patients with relapsing-remitting multiple sclerosis. JAMA 313, 275–284 (2015).
Nash, R. A. et al. High-dose immunosuppressive therapy and autologous HCT for relapsing-remitting MS. Neurology 88, 842–852 (2017).
Muraro, P. A. et al. Long-term outcomes after autologous hematopoietic stem cell transplantation for multiple sclerosis. JAMA Neurol. 74, 459–469 (2017).
Kapoor, R. et al. Effect of natalizumab on disease progression in secondary progressive multiple sclerosis (ASCEND): a phase 3, randomised, double-blind, placebo-controlled trial with an open-label extension. Lancet Neurol. 17, 405–415 (2018).
Burt, R. K. et al. Hematopoietic stem cell transplantation for progressive multiple sclerosis: failure of a total body irradiation-based conditioning regimen to prevent disease progression in patients with high disability scores. Blood 102, 2373–2378 (2003).
Krasulová, E. et al. High-dose immunoablation with autologous haematopoietic stem cell transplantation in aggressive multiple sclerosis: a single centre 10-year experience. Mult. Scler. J. 16, 685–693 (2010).
Chen, B. et al. Long-term efficacy of autologous haematopoietic stem cell transplantation in multiple sclerosis at a single institution in China. Neurol. Sci. 33, 881–886 (2012).
Fassas, A. et al. Long-term results of stem cell transplantation for MS: a single-center experience. Neurology 76, 1066–1070 (2011).
Bowen, J. D. et al. Autologous hematopoietic cell transplantation following high-dose immunosuppressive therapy for advanced multiple sclerosis: long-term results. Bone Marrow Transplant. 47, 946–951 (2012).
Mancardi, G. et al. Autologous haematopoietic stem cell transplantation with an intermediate intensity conditioning regimen in multiple sclerosis: the Italian multi-centre experience. Mult. Scler. J. 18, 835–842 (2012).
Samijn, J. P. et al. Intense T cell depletion followed by autologous bone marrow transplantation for severe multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 77, 46–50 (2006).
Xu, J. et al. Clinical outcomes after autologous haematopoietic stem cell transplantation in patients with progressive multiple sclerosis. Chin. Med. J. 119, 1851–1855 (2006).
Monje, M. L., Mizumatsu, S., Fike, J. R. & Palmer, T. D. Irradiation induces neural precursor-cell dysfunction. Nat. Med. 8, 955–962 (2002).
Peterson, K. et al. Effect of brain irradiation on demyelinating lesions. Neurology 43, 2105–2105 (1993).
Cook, S. D. et al. Total lymphoid irradiation in multiple sclerosis: blood lymphocytes and clinical course. Ann. Neurol. 22, 634–638 (1987).
Lee, H. et al. Brain atrophy after bone marrow transplantation for treatment of multiple sclerosis. Mult. Scler. 23, 420–431 (2017).
Thebault, S. et al. High serum neurofilament light chain normalizes after hematopoietic stem cell transplantation for MS. Neurol. Neuroimmunol. Neuroinflamm 6, e598 (2019).
Fassas, A. et al. Autologous stem cell transplantation in progressive multiple sclerosis – an interim analysis of efficacy. J. Clin. Immunol. 20, 24–30 (2000).
Mancardi, G. L. et al. Autologous hematopoietic stem cell transplantation in multiple sclerosis: a phase II trial. Neurology 84, 981–988 (2015).
Burt, R. K. et al. Effect of nonmyeloablative hematopoietic stem cell transplantation vs continued disease-modifying therapy on disease progression in patients with relapsing-remitting multiple sclerosis: a randomized clinical trial. JAMA 321, 165–174 (2019).
Sormani, M. P., Muraro, P. A., Saccardi, R. & Mancardi, G. NEDA status in highly active MS can be more easily obtained with autologous hematopoietic stem cell transplantation than other drugs. Mult. Scler. 23, 201–204 (2017).
Nabizadeh, F. et al. Autologous hematopoietic stem-cell transplantation in multiple sclerosis: a systematic review and meta-analysis. Neurol. Ther. 11, 1553–1569 (2022).
Saccardi, R. et al. Autologous HSCT for severe progressive multiple sclerosis in a multicenter trial: impact on disease activity and quality of life. Blood 105, 2601–2607 (2005).
Giedraitiene, N., Gasciauskaite, G. & Kaubrys, G. Impact of autologous HSCT on the quality of life and fatigue in patients with relapsing multiple sclerosis. Sci. Rep. 12, 15404 (2022).
Tolf, A., Gauffin, H., Burman, J., Landtblom, A. M. & Flensner, G. Experiences of being treated with autologous haematopoietic stem cell transplantation for aggressive multiple sclerosis: a qualitative interview study. PLoS ONE 19, e0297573 (2024).
Volz, T. et al. “A second birthday”? Experiences of persons with multiple sclerosis treated with autologous hematopoietic stem cell transplantation – a qualitative interview study. Front. Neurol. 15, 1384551 (2024).
De Kleermaeker, F., Uitdehaag, B. M. J. & van Oosten, B. W. Patients’ expectations of autologous hematopoietic stem cell transplantation as a treatment for MS. Mult. Scler. Relat. Disord. 37, 101467 (2020).
Alexander, T. et al. Patient-reported-outcomes in HSCT for autoimmune diseases: considerations on behalf of the EBMT ADWP, PAC and Nurses Group. J. Allergy Clin. Immunol.: Glob. 3, 100283 (2024).
Wattjes, M. P. et al. 2021 MAGNIMS-CMSC-NAIMS consensus recommendations on the use of MRI in patients with multiple sclerosis. Lancet Neurol. 20, 653–670 (2021).
Cohen, J. A. et al. Autologous hematopoietic cell transplantation for treatment-refractory relapsing multiple sclerosis: position statement from the American Society for Blood and Marrow Transplantation. Biol. Blood Marrow Transpl. 25, 845–854 (2019).
Miller, A. E. et al. Autologous hematopoietic stem cell transplant in multiple sclerosis: recommendations of the National Multiple Sclerosis Society. JAMA Neurol. 78, 241–246 (2021).
Oliveira, M. C. et al. A review of hematopoietic stem cell transplantation for autoimmune diseases: multiple sclerosis, systemic sclerosis and Crohn’s disease. Position paper of the Brazilian Society of Bone Marrow Transplantation. Hematol. Transfus. Cell Ther. 43, 65–86 (2021).
Montalban, X. et al. ECTRIMS/EAN guideline on the pharmacological treatment of people with multiple sclerosis. Eur. J. Neurol. 25, 215–237 (2018).
Samjoo, I. A. et al. Comparative efficacy of therapies for relapsing multiple sclerosis: a systematic review and network meta-analysis. J. Comp. Eff. Res. 12, e230016 (2023).
Samjoo, I. A. et al. Efficacy classification of modern therapies in multiple sclerosis. J. Comp. Eff. Res. 10, 495–507 (2021).
Brown, J. W. L. et al. Association of initial disease-modifying therapy with later conversion to secondary progressive multiple sclerosis. JAMA 321, 175–187 (2019).
Harding, K. et al. Clinical outcomes of escalation vs early intensive disease-modifying therapy in patients with multiple sclerosis. JAMA Neurol. 76, 536–541 (2019).
Buron, M. D. et al. Initial high-efficacy disease-modifying therapy in multiple sclerosis: a nationwide cohort study. Neurology 95, e1041–e1051 (2020).
He, A. et al. Timing of high-efficacy therapy for multiple sclerosis: a retrospective observational cohort study. Lancet Neurol. 19, 307–316 (2020).
Iaffaldano, P. et al. Long-term disability trajectories in relapsing multiple sclerosis patients treated with early intensive or escalation treatment strategies. Ther. Adv. Neurol. Disord. 14, 17562864211019574 (2021).
Rush, C. A., MacLean, H. J. & Freedman, M. S. Aggressive multiple sclerosis: proposed definition and treatment algorithm. Nat. Rev. Neurol. 11, 379–389 (2015).
Iacobaeus, E. et al. Aggressive multiple sclerosis (1): towards a definition of the phenotype. Mult. Scler. J. 26, 1031–1044 (2020).
US National Library of Medicine. ClinicalTrials.gov clinicaltrials.gov/ct2/show/study/NCT03477500?term=NCT03477500&cond=Multiple+Sclerosis&draw=2&rank=1 (2024).
European Medicines Agency. EudraCT www.clinicaltrialsregister.eu/ctr-search/trial/2019-001549-42/GB (2019).
US National Library of Medicine. ClinicalTrials.gov clinicaltrials.gov/ct2/show/NCT04047628?term=NCT04047628&draw=2&rank=1 (2024).
European Medicines Agency. EudraCT www.clinicaltrialsregister.eu/ctr-search/trial/2022-002654-95/IT (2023).
Kümpfel, T. et al. Update on the diagnosis and treatment of neuromyelitis optica spectrum disorders (NMOSD) – revised recommendations of the Neuromyelitis Optica Study Group (NEMOS). Part II: attack therapy and long-term management. J. Neurol. 271, 141–176 (2024).
Nabizadeh, F. et al. Autologous hematopoietic stem cell transplantation in neuromyelitis optica spectrum disorder: a systematic review and meta-analysis. J. Clin. Neurosci. 105, 37–44 (2022).
Greco, R. et al. Autologous hematopoietic stem cell transplantation in neuromyelitis optica: a registry study of the EBMT Autoimmune Diseases Working Party. Mult. Scler. 21, 189–197 (2015).
Burt, R. K. et al. Autologous nonmyeloablative hematopoietic stem cell transplantation for neuromyelitis optica. Neurology 93, e1732–e1741 (2019).
Greco, R. et al. Allogeneic HSCT for autoimmune diseases: a retrospective study from the EBMT ADWP, IEWP, and PDWP working parties. Front. Immunol. 10, 1570 (2019).
Greco, R. et al. Allogeneic hematopoietic stem cell transplantation for neuromyelitis optica. Ann. Neurol. 75, 447–453 (2014).
Snowden, J. A. et al. Evolution, trends, outcomes, and economics of hematopoietic stem cell transplantation in severe autoimmune diseases. Blood Adv. 1, 2742–2755 (2017).
Burt, R. K. et al. Health economics and patient outcomes of hematopoietic stem cell transplantation versus disease-modifying therapies for relapsing remitting multiple sclerosis in the United States of America. Mult. Scler. Relat. Disord. 45, 102404 (2020).
Hughes, S. L., Prettyjohns, M. J., Snowden, J. A. & Sharrack, B. in Handbook of Clinical Neurology, Vol. 202 (eds Inglese, M. & Mancardi, G. L.) Ch. 18, 279–294 (Elsevier, 2024).
Gottschlich, K. N. et al. Healthcare utilization and costs associated with autologous haematopoietic stem cell transplantation in Norwegian patients with relapsing remitting multiple sclerosis. Mult. Scler. Relat. Disord. 84, 105507 (2024).
Greco, R. et al. Innovative cellular therapies for autoimmune diseases: expert-based position statement and clinical practice recommendations from the EBMT practice harmonization and guidelines committee. eClinicalMedicine 69, 102476 (2024).
Snarski, E. et al. Onset and outcome of pregnancy after autologous haematopoietic SCT (AHSCT) for autoimmune diseases: a retrospective study of the EBMT Autoimmune Diseases Working Party (ADWP). Bone Marrow Transpl. 50, 216–220 (2015).
Massarotti, C. et al. Menstrual cycle resumption and female fertility after autologous hematopoietic stem cell transplantation for multiple sclerosis. Mult. Scler. 27, 2103–2107 (2021).
Chatterton, S. et al. Pregnancy post autologous stem cell transplant with BEAM conditioning for multiple sclerosis. Mult. Scler. 27, 2112–2115 (2021).
Zafeiri, L. et al. Anti-Müllerian hormone and pregnancy after autologous hematopoietic stem cell transplantation for multiple sclerosis. PLoS ONE 18, e0284288 (2023).
Murphy, J., McKenna, M., Abdelazim, S., Battiwalla, M. & Stratton, P. A practical guide to gynecologic and reproductive health in women undergoing hematopoietic stem cell transplant. Biol. Blood Marrow Transpl. 25, e331–e343 (2019).
Massenkeil, G. et al. Long-term follow-up of fertility and pregnancy in autoimmune diseases after autologous haematopoietic stem cell transplantation. Rheumatol. Int. 36, 1563–1568 (2016).
Khizroeva, J. et al. Infertility in women with systemic autoimmune diseases. Best. Pract. Res. Clin. Endocrinol. Metab. 33, 101369 (2019).
Chen, H., Xiao, L., Li, J., Cui, L. & Huang, W. Adjuvant gonadotropin-releasing hormone analogues for the prevention of chemotherapy-induced premature ovarian failure in premenopausal women. Cochrane Database Syst. Rev. 3, CD008018 (2019).
Phelan, R. et al. Male-specific late effects in adult hematopoietic cell transplantation recipients: a systematic review from the Late Effects and Quality of Life Working Committee of the Center for International Blood and Marrow Transplant Research and Transplant Complications Working Party of the European Society of Blood and Marrow Transplantation. Bone Marrow Transpl. 57, 1150–1163 (2022).
Alping, P., Burman, J., Lycke, J., Frisell, T. & Piehl, F. Safety of alemtuzumab and autologous hematopoietic stem cell transplantation compared to noninduction therapies for multiple sclerosis. Neurology 96, e1574–e1584 (2021).
Atkins, H. L. et al. Immunoablation and autologous haemopoietic stem-cell transplantation for aggressive multiple sclerosis: a multicentre single-group phase 2 trial. Lancet 388, 576–585 (2016).
Curro, D. et al. Low intensity lympho-ablative regimen followed by autologous hematopoietic stem cell transplantation in severe forms of multiple sclerosis: a MRI-based clinical study. Mult. Scler. 21, 1423–1430 (2015).
Saccardi, R. et al. BEAM/ATG or cyclophosphamide/ATG as conditioning regimen in autologous transplantation for multiple sclerosis: a retrospective analysis of the EBMT Autoimmune Diseases Working Party [abstract O036]. Bone Marrow Transpl. 59 (Suppl. 1), 41 (2024).
Daikeler, T. et al. Allogeneic hematopoietic SCT for patients with autoimmune diseases. Bone Marrow Transpl. 44, 27–33 (2009).
Sullivan, K. M. & Sarantopoulos, S. Allogeneic HSCT for autoimmune disease: a shared decision. Nat. Rev. Rheumatol. 15, 701–702 (2019).
Roberts, F. et al. Rehabilitation before and after autologous haematopoietic stem cell transplantation (AHSCT) for patients with multiple sclerosis (MS): consensus guidelines and recommendations for best clinical practice on behalf of the Autoimmune Diseases Working Party, Nurses Group, and Patient Advocacy Committee of the European Society for Blood and Marrow Transplantation (EBMT). Front. Neurol. 11, 1602 (2020).
Liu, C. & Blumhardt, L. D. Disability outcome measures in therapeutic trials of relapsing-remitting multiple sclerosis: effects of heterogeneity of disease course in placebo cohorts. J. Neurol. Neurosurg. Psychiatry 68, 450–457 (2000).
Fischer, J., Rudick, R., Cutter, G. & Reingold, S. National MS Society Clinical Outcomes Assessment Task Force The Multiple Sclerosis Functional Composite measure (MSFC): an integrated approach to MS clinical outcome assessment. Mult. Scler. J. 5, 244–250 (1999).
Motl, R. et al. Accelerometry as a measure of walking behavior in multiple sclerosis. Acta Neurol. Scand. 127, 384–390 (2013).
Kappos, L. et al. Contribution of relapse-independent progression vs relapse-associated worsening to overall confirmed disability accumulation in typical relapsing multiple sclerosis in a pooled analysis of 2 randomized clinical trials. JAMA Neurol. 77, 1132–1140 (2020).
Burman, J. et al. Autologous haematopoietic stem cell transplantation for aggressive multiple sclerosis: the Swedish experience. J. Neurol. Neurosurg. Psychiatry 85, 1116–1121 (2014).
Manzano, G. S. et al. Disease modifying therapy management of multiple sclerosis after stem cell therapies: a retrospective case series. Mult. Scler. Relat. Disord. 63, 103861 (2022).
Casanova, B. et al. Autologous hematopoietic stem cell transplantation in relapsing-remitting multiple sclerosis: comparison with secondary progressive multiple sclerosis. Neurol. Sci. 38, 1213–1221 (2017).
Miller, P. et al. Joint consensus statement on the vaccination of adult and paediatric haematopoietic stem cell transplant recipients: prepared on behalf of the British Society of Blood and Marrow Transplantation and Cellular Therapy (BSBMTCT), the Children’s Cancer and Leukaemia Group (CCLG), and British Infection Association (BIA). J. Infect. 86, 1–8 (2023).
Cordonnier, C. et al. Vaccination of haemopoietic stem cell transplant recipients: guidelines of the 2017 European Conference on Infections in Leukaemia (ECIL 7). Lancet Infect. Dis. 19, e200–e212 (2019).
van Aalst, M. et al. Incidence of invasive pneumococcal disease in immunocompromised patients: a systematic review and meta-analysis. Travel. Med. Infect. Dis. 24, 89–100 (2018).
Bastidas, A. et al. Effect of recombinant zoster vaccine on incidence of herpes zoster after autologous stem cell transplantation: a randomized clinical trial. JAMA 322, 123–133 (2019).
Wu, X., Wang, L., Shen, L. & Tang, K. Response of COVID-19 vaccination in multiple sclerosis patients following disease-modifying therapies: a meta-analysis. EBioMedicine 81, 104102 (2022).
Greco, R. et al. Hematopoietic stem cell transplantation for autoimmune diseases in the time of COVID-19: EBMT guidelines and recommendations. Bone Marrow Transpl. 56, 1493–1508 (2021).
Mehra, V. et al. Early Morbidity of Autologous Haematopoietic Stem Cell Transplantation in Multiple Sclerosis-A Real World Experience. In: The 47th Annual Meeting of the European Society for Blood and Marrow Transplantation: Physicians - Oral Sessions (O010 – O169). Bone Marrow Transplantation 56, 33–34 (2021).
Saccardi, R. et al. Cytomegalovirus (CMV) and Epstein-Barr virus (EBV) reactivation In autologous hematopoietic stem cell transplantation (HSCT) for severe multiple sclerosis (MS) [abstract]. Blood 116(21), 4537 (2010).
Van Esser, J. W. et al. Epstein-Barr virus (EBV) reactivation is a frequent event after allogeneic stem cell transplantation (SCT) and quantitatively predicts EBV-lymphoproliferative disease following T-cell–depleted SCT. Blood J. Am. Soc. Hematol. 98, 972–978 (2001).
Bjornevik, K. et al. Longitudinal analysis reveals high prevalence of Epstein-Barr virus associated with multiple sclerosis. Science 375, 296–301 (2022).
Styczynski, J. et al. Management of Epstein-Barr virus infections and post-transplant lymphoproliferative disorders in patients after allogeneic hematopoietic stem cell transplantation: Sixth European Conference on Infections in Leukemia (ECIL-6) guidelines. Haematologica 101, 803–811 (2016).
Snowden, J. A. et al. Haematopoietic SCT in severe autoimmune diseases: updated guidelines of the European Group for Blood and Marrow Transplantation. Bone Marrow Transpl. 47, 770–790 (2012).
Mehra, V. et al. Epstein-Barr virus and monoclonal gammopathy of clinical significance in autologous stem cell transplantation for multiple sclerosis. Clin. Infect. Dis. 69, 1757–1763 (2019).
Meier, U.-C., Cipian, R. C., Karimi, A., Ramasamy, R. & Middeldorp, J. M. Cumulative roles for Epstein-Barr virus, human endogenous retroviruses, and human herpes virus-6 in driving an inflammatory cascade underlying MS pathogenesis. Front. Immunol. 12, 757302 (2021).
Schwab, N., Schneider-Hohendorf, T., Melzer, N., Cutter, G. & Wiendl, H. Natalizumab-associated PML: challenges with incidence, resulting risk, and risk stratification. Neurology 88, 1197–1205 (2017).
Berger, J. R. Classifying PML risk with disease modifying therapies. Mult. Scler. Relat. Disord. 12, 59–63 (2017).
Moiola, L. et al. The risk of infection in patients with multiple sclerosis treated with disease-modifying therapies: a Delphi consensus statement. Mult. Scler. J. 27, 331–346 (2021).
Adrianzen Herrera, D. et al. Characteristics and outcomes of progressive multifocal leukoencephalopathy in hematologic malignancies and stem cell transplant – a case series. Leuk. Lymphoma 60, 395–401 (2019).
Daikeler, T. et al. Secondary autoimmune diseases occurring after HSCT for an autoimmune disease: a retrospective study of the EBMT Autoimmune Disease Working Party. Blood 118, 1693–1698 (2011).
Burt, R. K. et al. New autoimmune diseases after autologous hematopoietic stem cell transplantation for multiple sclerosis. Bone Marrow Transpl. 56, 1509–1517 (2021).
Meltzer, E. et al. Mitigating alemtuzumab-associated autoimmunity in MS: a “whack-a-mole” B-cell depletion strategy. Neurol. Neuroimmunol. Neuroinflamm. 7, e868 (2020).
Demoor-Goldschmidt, C. & de Vathaire, F. Review of risk factors of secondary cancers among cancer survivors. Br. J. Radiol. 92, 20180390 (2019).
Kirgizov, K. et al. Late complications after autologous HSCT for autoimmune diseases: a retrospective study from the EBMT autoimmune diseases, transplant complications and paediatric diseases working parties [abstract O025]. Bone Marrow Transplant. 57 (Suppl. 1), 25–26 (2022).
Acknowledgements
The workshop on which this manuscript is based was supported by the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS) entirely by support in kind. The authors thank all workshop attendees for their participation. The authors are also grateful to M. Trpkoski, ECTRIMS Meeting Planning Coordinator, for her assistance in coordinating the workshop, and to I. S. Ortega, EBMT Medical Officer, for her liaison role within the organization. The authors are grateful to the EBMT and ADWP.
Author information
Authors and Affiliations
Consortia
Contributions
P.A.M. and A.M. wrote the manuscript. P.A.M., A.M., R.G., J.B., E.I., M.I., J.A.S., T.A., M.P.A., L.B., G.B., O.C., M.G., C.H., M.K., P.L., R.M., V.M., L.M. and R.S. researched data for the article. All authors made substantial contributions to discussion of content and reviewed and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
P.A.M. has received fees from consulting for Cellerys, Jasper Therapeutics and Magenta Therapeutics, all outside the submitted work. A.M. has received speaking honoraria from Biogen, Janssen, Novartis, Sanofi and Viatris, all outside the submitted work. R.G. has received speaker honoraria from Biotest, Magenta, Medac and Pfizer, all outside the submitted work. E.I. has received speaker fees and honoraria for advisory boards from Biogen, Merck and Sanofi-Genzyme, and an unrestricted research grant from Sanofi-Genzyme. M.I. is co-Editor of Multiple Sclerosis Journal and she has received honoraria for participating in educational activities or advisory boards for Biogen, Janssen, Merck, Novartis, Roche and Sanofi. T.A. has received honoraria and/or travel grants from Amgen, AstraZeneca, GSK and Neovii, and study support from Amgen, Janssen-Cilag and Miltenyi. M.P.A. has served on scientific advisory boards for Biogen, Merck, Novartis, Roche, Sanofi-Genzyme and Teva; has received speaker honoraria from Biogen, Merck, Novartis, Roche, Sanofi-Genzyme and Teva; has received research grants for her Institution from Biogen, Merck, Novartis, Roche and Sanofi-Genzyme. She is co-Editor of Multiple Sclerosis Journal and Associate Editor of Frontiers in Neurology. G.B. was supported by a research fellowship FISM – Fondazione Italiana Sclerosi Multipla 019/BR/016, and financed or co-financed with the ‘5 per mille’ public funding. O.C. has received personal compensation for consulting for Biogen, Merck and Novartis, and she serves as deputy Editor of Neurology. J.A.C. has received personal compensation for consulting for Astoria, Bristol-Myers Squibb, Convelo, EMD Serono, FiND Therapeutics, INMune, and Sandoz, and serves as an Editor of Multiple Sclerosis Journal. T.D. has received speaker fees, research support, travel support, and/or served on advisory boards or steering committees of Alexion, Biogen, Celgene, GeNeuro, MedDay, Merck, Novartis, Roche and Sanofi-Genzyme; he has received research support from Swiss National Research Foundation, University of Basel, and the Swiss MS Society. M.G. has received educational support from Novartis and has an advisory board role for Merck. C.H. has received funding support, speaker honoraria and travel grants from Merck, Novartis and Roche. R.M. has received unrestricted grants from Biogen, Novartis, Roche and Third Rock; has advisory roles and has given lectures for Biogen, CellProtect, Genzyme, Neuway, Novartis, Roche, Swiss Rockets and Third Rock; is a patent holder and co-holder on patents for daclizumab in MS, JCV VP1 for vaccination against PML, JCV-specific neutralizing antibodies to treat PML, and antigen-specific tolerization with peptide-coupled cells and novel autoantigens in MS; is a co-founder of Abata, Cambridge, MA, USA (adoptive Treg therapy); and is a co-founder and employee of Cellerys. L.M. has received compensation for speaking activities and/or consulting services from Alexion, Biogen, Celgene, Merck, Novartis, Roche and Sanofi. M.T. has received compensation for consulting services, speaking honoraria and research support from Almirall, Bayer Schering Pharma, Biogen-Idec, Genzyme, Immunic Therapeutics, Janssen, Merck-Serono, Novartis, Roche, Sanofi-Aventis, Teva and Viela Bio; is on data safety monitoring boards for Parexel and UCB Biopharma; and is on the Relapse Adjudication Committee for Imcyse. B. Stankoff has received research support (to the institution) from Merck, Novartis and Roche, and personal speaker fees from Biogen, Janssen, Merck, Novartis and Sanofi. The other authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Neurology thanks S. Meuth; J. Moore, who co-reviewed with J. Massey; R. Nash; G. Ruiz-Arguelles; and B. Yamout for their contribution to the peer review of this work.
Additional information
We wish to dedicate this article to the memory of Riccardo Saccardi, who passed away on 19th February 2024 after a long battle with cancer. His relentless commitment to advancing the application of haematopoietic stem cell transplantation in autoimmune diseases will continue to influence our work for many years to come. Together with his knowledge, his kindness and humility made him a uniquely collaborative individual, and we and his many other friends and colleagues will deeply miss him.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Glossary
- Anti-Mullerian hormone
-
A hormone produced by the granulosa cells of growing ovarian follicles in women and the testicles in men; in women, levels in the serum are considered to be a marker of ovarian reserve.
- Progression-free survival
-
Survival in the absence of neurological deterioration, as measured with the Expanded Disability Status Scale score determined via a physical examination by a neurologist.
- Switched memory B cells
-
Long-lived B cells that have undergone class switch recombination, enabling them to produce antibodies of different isotypes (such as IgG or IgA) while retaining memory of a specific antigen.
- Transplant-related mortality
-
Death from any cause during the first 100 days from autologous graft infusion.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Muraro, P.A., Mariottini, A., Greco, R. et al. Autologous haematopoietic stem cell transplantation for treatment of multiple sclerosis and neuromyelitis optica spectrum disorder — recommendations from ECTRIMS and the EBMT. Nat Rev Neurol 21, 140–158 (2025). https://doi.org/10.1038/s41582-024-01050-x
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41582-024-01050-x
This article is cited by
-
Comparison of anti-human T cell globulins on immune reconstitution and early infections after autologous transplant in patients with multiple sclerosis
Bone Marrow Transplantation (2025)
-
Multiple sclerosis: molecular pathogenesis and therapeutic intervention
Signal Transduction and Targeted Therapy (2025)
-
Indications for haematopoietic cell transplantation and CAR-T for haematological diseases, solid tumours and immune disorders: 2025 EBMT practice recommendations
Bone Marrow Transplantation (2025)
-
BEAM/ATG or cyclophosphamide/ATG as conditioning regimen in autologous haemopoietic stem cell transplantation for multiple sclerosis: a retrospective analysis of the EBMT autoimmune diseases working party
Bone Marrow Transplantation (2025)