Abstract
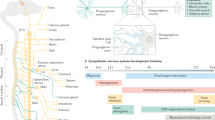
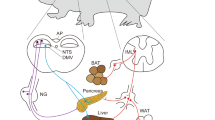
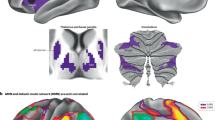
The autonomic nervous system (ANS) plays a pivotal role in regulating organ functions through descending brain-to-body signalling. The pathways involved are broadly categorized into two major branches: the sympathetic nervous system, which mediates ‘fight or flight’ responses, and the parasympathetic nervous system, which governs ‘rest and digest’ functions. Historically, the ANS was considered to mediate simple motor functions with limited neurochemical diversity. However, recent advances in neurotechnology have shown that brain-to-body communication is more complex and dynamic than previously appreciated. This review synthesizes current knowledge about the molecular, anatomical and functional diversity of autonomic motor neurons. Here we present a comparative analysis of the cellular architecture of the ANS and the suggested roles of distinct neuron populations. Additionally, we explore the emerging view that the ANS interacts with diverse systems involving metabolism, immunology and ageing, which extends its role beyond simple brain–organ modulation. Finally, we emphasize the need for cell-type-specific and longitudinal studies of the ANS to uncover novel mechanisms underlying body–brain interactions and to identify new translational opportunities for therapeutic interventions.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
We developed an online portal (available at https://ans-cell-atlas.com/) for an integrated view of published single-cell transcriptomics at the GEO database. All source code is available via GitHub at https://github.com/YTwTJ/Molecular-and-Functional-Diversity-of-the-Autonomic-Nervous-System. In Fig. 3, dot plots are generated from GEO datasets: SCG (GSE175421), stellate ganglion (GSE231924), CG-SMG (GSE278457), DMV (GSE172411) and nucleus ambiguus (GSE198709, GSE202760 and GSE211538). In Fig. 3, heat maps plot transcriptomic data available via the Human Protein Atlas at https://www.proteinatlas.org/humanproteome/tissue/data#hpa_tissues_rna.
References
Sammons, M. et al. Brain–body physiology: local, reflex, and central communication. Cell 187, 5877–5890 (2024).
Sternson, S. M. & Eiselt, A.-K. Three pillars for the neural control of appetite. Annu. Rev. Physiol. 79, 401–423 (2017).
Lin, E. E., Scott-Solomon, E. & Kuruvilla, R. Peripheral innervation in the regulation of glucose homeostasis. Trends Neurosci. 44, 189–202 (2021).
Wachsmuth, H. R., Weninger, S. N. & Duca, F. A. Role of the gut–brain axis in energy and glucose metabolism. Exp. Mol. Med. 54, 377–392 (2022).
Langhans, W., Watts, A. G. & Spector, A. C. The elusive cephalic phase insulin response: triggers, mechanisms, and functions. Physiol. Rev. 103, 1423–1485 (2023).
Ml, A. & Bb, L. Toward a wiring diagram understanding of appetite control. Neuron 95, 757–778 (2017).
Augustine, V., Lee, S. & Oka, Y. Neural control and modulation of thirst, sodium appetite, and hunger. Cell 180, 25–32 (2020).
Abraira, V. E. & Ginty, D. D. The sensory neurons of touch. Neuron 79, 618–639 (2013).
Travagli, R. A. & Anselmi, L. Vagal neurocircuitry and its influence on gastric motility. Nat. Rev. Gastroenterol. Hepatol. 13, 389–401 (2016).
Yu, C. D., Xu, Q. J. & Chang, R. B. Vagal sensory neurons and gut–brain signaling. Curr. Opin. Neurobiol. 62, 133–140 (2020).
Kim, M., Heo, G. & Kim, S.-Y. Neural signalling of gut mechanosensation in ingestive and digestive processes. Nat. Rev. Neurosci. 23, 135–156 (2022).
Prescott, S. L. & Liberles, S. D. Internal senses of the vagus nerve. Neuron 110, 579–599 (2022).
Barman, S. M. & Yates, B. J. Deciphering the neural control of sympathetic nerve activity: status report and directions for future research. Front. Neurosci. 11, 730 (2017).
Langley, J. N. On the regeneration of pre-ganglionic and of post-ganglionic visceral nerve fibres. J. Physiol. 22, 215–230 (1897).
Langley, J. N. The Autonomic Nervous System Part 1 (Heffer, 1921).
Gibbons, C. H. Basics of autonomic nervous system function. Handb. Clin. Neurol. 160, 407–418 (2019).
Guyenet, P. G. The sympathetic control of blood pressure. Nat. Rev. Neurosci. 7, 335–346 (2006).
Goldstein, D. S. Differential responses of components of the autonomic nervous system. Handb. Clin. Neurol. 117, 13–22 (2013).
Nakamura, K., Nakamura, Y. & Kataoka, N. A hypothalamomedullary network for physiological responses to environmental stresses. Nat. Rev. Neurosci. 23, 35–52 (2022).
Gibbins, I. Functional organization of autonomic neural pathways. Organogenesis 9, 169–175 (2013).
Jänig, W. & McLachlan, E. M. Characteristics of function-specific pathways in the sympathetic nervous system. Trends Neurosci. 15, 475–481 (1992).
Coverdell, T. C., Abbott, S. B. G. & Campbell, J. N. Molecular cell types as functional units of the efferent vagus nerve. Semin. Cell Dev. Biol. 156, 210–218 (2024).
Tao, J. et al. Highly selective brain-to-gut communication via genetically defined vagus neurons. Neuron 109, 2106–2115.e4 (2021). This study describes two genetically defined parasympathetic preganglionic neuron populations that form anatomical wiring with distinct enteric circuits.
Veerakumar, A., Yung, A. R., Liu, Y. & Krasnow, M. A. Molecularly defined circuits for cardiovascular and cardiopulmonary control. Nature 606, 739–746 (2022). This paper defines two distinct parasympathetic preganglionic neuron types that independently regulate cardiac or coordinated cardiopulmonary function through parallel efferent circuits.
Mapps, A. A. et al. Diversity of satellite glia in sympathetic and sensory ganglia. Cell Rep. 38, 110328 (2022). In this study, the authors reveal the molecular heterogeneity of satellite glia in sympathetic and sensory ganglia.
Sharma, S. et al. Tiered sympathetic control of cardiac function revealed by viral tracing and single cell transcriptome profiling. eLife 12, e86295 (2023). This study characterizes the morphological, electrophysiological and physiological properties of cardiac-innervating sympathetic postganglionic neurons.
Ziegler, K. A. et al. Immune-mediated denervation of the pineal gland underlies sleep disturbance in cardiac disease. Science 381, 285–290 (2023). In this study, the authors report that cardiac disease induces inflammation in the superior cervical ganglion, leading to sympathetic denervation of the pineal gland.
Wang, T., Teng, B., Yao, D. R., Gao, W. & Oka, Y. Organ-specific sympathetic innervation defines visceral functions. Nature 637, 895–902 (2025). This study identifies molecularly distinct sympathetic postganglionic neurons with organ-specific projections that independently control digestion and gastrointestinal transit.
Berthoud, H.-R. & Neuhuber, W. L. Vagal mechanisms as neuromodulatory targets for the treatment of metabolic disease. Ann. N. Y. Acad. Sci. 1454, 42–55 (2019).
Martinez-Sanchez, N. et al. The sympathetic nervous system in the 21st century: neuroimmune interactions in metabolic homeostasis and obesity. Neuron 110, 3597–3626 (2022).
Pongratz, G. & Straub, R. H. The sympathetic nervous response in inflammation. Arthritis Res. Ther. 16, 504 (2014).
Udit, S., Blake, K. & Chiu, I. M. Somatosensory and autonomic neuronal regulation of the immune response. Nat. Rev. Neurosci. 23, 157–171 (2022).
Chan, K. L., Poller, W. C., Swirski, F. K. & Russo, S. J. Central regulation of stress-evoked peripheral immune responses. Nat. Rev. Neurosci. 24, 591–604 (2023).
Martin, C. R., Osadchiy, V., Kalani, A. & Mayer, E. A. The brain–gut–microbiome axis. Cell. Mol. Gastroenterol. Hepatol. 6, 133–148 (2018).
Hodes, G. E., Kana, V., Menard, C., Merad, M. & Russo, S. J. Neuroimmune mechanisms of depression. Nat. Neurosci. 18, 1386–1393 (2015).
Hanoun, M., Maryanovich, M., Arnal-Estapé, A. & Frenette, P. S. Neural regulation of hematopoiesis, inflammation, and cancer. Neuron 86, 360–373 (2015).
Payne, S. C., Furness, J. B. & Stebbing, M. J. Bioelectric neuromodulation for gastrointestinal disorders: effectiveness and mechanisms. Nat. Rev. Gastroenterol. Hepatol. 16, 89–105 (2019).
Scott-Solomon, E., Boehm, E. & Kuruvilla, R. The sympathetic nervous system in development and disease. Nat. Rev. Neurosci. 22, 685–702 (2021).
Mather, M. Autonomic dysfunction in neurodegenerative disease. Nat. Rev. Neurosci. 26, 276–292 (2025).
LeBouef, T., Yaker, Z. & Whited, L. Physiology, autonomic nervous system. StatPearls https://www.ncbi.nlm.nih.gov/books/NBK538516/ (StatPearls Publishing, 2025).
Deuchars, S. A. & Lall, V. K. Sympathetic preganglionic neurons: properties and inputs. Compr. Physiol. 5, 829–869 (2015).
Llewellyn-Smith, I. J. in Central Regulation of Autonomic Functions (eds Llewellyn-Smith, I. J. & Verberne, A. J. M.) 98–119 (Oxford Univ. Press, 2011).
Jordan, D. in Central Regulation of Autonomic Functions (eds Llewellyn-Smith, I. J. & Verberne, A. J. M.) 120–139 (Oxford Univ. Press, 2011).
Langley, J. N. Sketch of the progress of discovery in the eighteenth century as regards the autonomic nervous system. J. Physiol. 50, 225–258 (1916).
Reuss, S. & Moore, R. Y. Neuropeptide Y-containing neurons in the rat superior cervical ganglion: projections to the pineal gland. J. Pineal Res. 6, 307–316 (1989).
Wojtkiewicz, J. et al. Immunohistochemical characterization of superior cervical ganglion neurons supplying porcine parotid salivary gland. Neurosci. Lett. 500, 57–62 (2011).
Zhu, Y. et al. Sympathetic neuropeptide Y protects from obesity by sustaining thermogenic fat. Nature 634, 243–250 (2024). This paper shows that sympathetic neuron-derived NPY sustains adipose thermogenesis and protects against obesity independently of food intake.
Uno, H. Sympathetic innervation of the sweat glands and piloarrector muscles of macaques and human beings. J. Invest. Dermatol. 69, 112–120 (1977).
van der Velden, V. H. & Hulsmann, A. R. Autonomic innervation of human airways: structure, function, and pathophysiology in asthma. Neuroimmunomodulation 6, 145–159 (1999).
Zeisel, A. et al. Molecular architecture of the mouse nervous system. Cell 174, 999–1014.e22 (2018).
Ernsberger, U., Reissmann, E., Mason, I. & Rohrer, H. The expression of dopamine beta-hydroxylase, tyrosine hydroxylase, and Phox2 transcription factors in sympathetic neurons: evidence for common regulation during noradrenergic induction and diverging regulation later in development. Mech. Dev. 92, 169–177 (2000).
Huesing, C. et al. Organization of sympathetic innervation of interscapular brown adipose tissue in the mouse. J. Comp. Neurol. 530, 1363–1378 (2022).
Goldstein, M., Fuxe, K. & Hökfelt, T. Characterization and tissue localization of catecholamine synthesizing enzymes. Pharmacol. Rev. 24, 293–309 (1972).
Bohn, M. C., Goldstein, M. & Black, I. B. Expression of phenylethanolamine N-methyltransferase in rat sympathetic ganglia and extra-adrenal chromaffin tissue. Dev. Biol. 89, 299–308 (1982).
Cahill, A. L., Eertmoed, A. L., Mangoura, D. & Perlman, R. L. Differential regulation of phenylethanolamine N-methyltransferase expression in two distinct subpopulations of bovine chromaffin cells. J. Neurochem. 67, 1217–1224 (1996).
Peter, D. et al. Differential expression of two vesicular monoamine transporters. J. Neurosci. 15, 6179–6188 (1995).
Schäfer, M. K., Schütz, B., Weihe, E. & Eiden, L. E. Target-independent cholinergic differentiation in the rat sympathetic nervous system. Proc. Natl Acad. Sci. USA 94, 4149–4154 (1997).
Sieber-Blum, M. & Ren, Z. Norepinephrine transporter expression and function in noradrenergic cell differentiation. Mol. Cell Biochem. 212, 61–70 (2000).
Li, Z., Caron, M. G., Blakely, R. D., Margolis, K. G. & Gershon, M. D. Dependence of serotonergic and other nonadrenergic enteric neurons on norepinephrine transporter expression. J. Neurosci. 30, 16730–16740 (2010).
Pfeil, U. et al. Expression of the high-affinity choline transporter, CHT1, in the rat trachea. Am. J. Respir. Cell Mol. Biol. 28, 473–477 (2003).
Hoover, D. B., Ganote, C. E., Ferguson, S. M., Blakely, R. D. & Parsons, R. L. Localization of cholinergic innervation in guinea pig heart by immunohistochemistry for high-affinity choline transporters. Cardiovasc. Res. 62, 112–121 (2004).
Okuda, T. & Haga, T. High-affinity choline transporter. Neurochem. Res. 28, 483–488 (2003).
Chien, H.-J. et al. Human pancreatic afferent and efferent nerves: mapping and 3-D illustration of exocrine, endocrine, and adipose innervation. Am. J. Physiol. Gastrointest. Liver Physiol. 317, G694–G706 (2019).
Keast, J. R. Visualization and immunohistochemical characterization of sympathetic and parasympathetic neurons in the male rat major pelvic ganglion. Neuroscience 66, 655–662 (1995).
Espinosa-Medina, I. et al. The sacral autonomic outflow is sympathetic. Science 354, 893–897 (2016).
Horn, J. P. The sacral autonomic outflow is parasympathetic: Langley got it right. Clin. Auton. Res. 28, 181–185 (2018).
Brumovsky, P. R. Dorsal root ganglion neurons and tyrosine hydroxylase — an intriguing association with implications for sensation and pain. Pain 157, 314–320 (2016).
Wang, Y. et al. The role of somatosensory innervation of adipose tissues. Nature 609, 569–574 (2022). This study demonstrates TH+ somatosensory innervation of the adipose tissue.
Lin, Y. S., Nosaka, S., Amakata, Y. & Maeda, T. Comparative study of the mammalian liver innervation: an immunohistochemical study of protein gene product 9.5, dopamine beta-hydroxylase and tyrosine hydroxylase. Comp. Biochem. Physiol. A Physiol. 110, 289–298 (1995).
Parker, T. L., Kesse, W. K., Mohamed, A. A. & Afework, M. The innervation of the mammalian adrenal gland. J. Anat. 183, 265–276 (1993).
McDougal, D. H. & Gamlin, P. D. Autonomic control of the eye. Compr. Physiol. 5, 439–473 (2015).
Li, Y. & Dahlström, A. Peripheral projections of NESP55 containing neurons in the rat sympathetic ganglia. Auton. Neurosci. 141, 1–9 (2008).
Jin, K. et al. Identification of lacrimal gland postganglionic innervation and its regulation of tear secretion. Am. J. Pathol. 190, 1068–1079 (2020).
Kummer, W., Fischer, A., Kurkowski, R. & Heym, C. The sensory and sympathetic innervation of guinea-pig lung and trachea as studied by retrograde neuronal tracing and double-labelling immunohistochemistry. Neuroscience 49, 715–737 (1992).
Rajendran, P. S. et al. Identification of peripheral neural circuits that regulate heart rate using optogenetic and viral vector strategies. Nat. Commun. 10, 1944 (2019).
Barrett, M. S., Hegarty, D. M., Habecker, B. A. & Aicher, S. A. Distinct morphology of cardiac- and brown adipose tissue-projecting neurons in the stellate ganglia of mice. Physiol. Rep. 10, e15334 (2022). This study shows that cardiac- and brown adipose tissue-projecting sympathetic postganglionic neurons are anatomically distinct and morphologically specialized.
Kuntz, A. & Jacobs, M. W. Components of periarterial extensions of celiac and mesenteric plexuses. Anat. Rec. 123, 509–520 (1955).
Quinson, N., Robbins, H. L., Clark, M. J. & Furness, J. B. Locations and innervation of cell bodies of sympathetic neurons projecting to the gastrointestinal tract in the rat. Arch. Histol. Cytol. 64, 281–294 (2001).
Liu, K. et al. Metabolic stress drives sympathetic neuropathy within the liver. Cell Metab. 33, 666–675.e4 (2021).
Ding, X. et al. Panicle-shaped sympathetic architecture in the spleen parenchyma modulates antibacterial innate immunity. Cell Rep. 27, 3799–3807.e3 (2019).
Smith-Edwards, K. M. et al. Sympathetic input to multiple cell types in mouse and human colon produces region-specific responses. Gastroenterology 160, 1208–1223.e4 (2021).
Mazur, U., Lepiarczyk, E., Janikiewicz, P. & Bossowska, A. Somatostatin immunoreactivity within the urinary bladder nerve fibers and paracervical ganglion urinary bladder projecting neurons in the female pig. J. Chem. Neuroanat. 117, 102007 (2021).
Osborn, J. W., Tyshynsky, R. & Vulchanova, L. Function of renal nerves in kidney physiology and pathophysiology. Annu. Rev. Physiol. 83, 429–450 (2021).
Torres, H. et al. Sympathetic innervation of the mouse kidney and liver arising from prevertebral ganglia. Am. J. Physiol. Regul. Integr. Comp. Physiol. 321, R328–R337 (2021). This work maps the spatial locations of kidney- and liver-innervating sympathetic postganglionic neurons.
François, M. et al. Sympathetic innervation of the interscapular brown adipose tissue in mouse. Ann. N. Y. Acad. Sci. 1454, 3–13 (2019).
Huesing, C. et al. Sympathetic innervation of inguinal white adipose tissue in the mouse. J. Comp. Neurol. 529, 1465–1485 (2021).
Vera, P. L., Haase, E. B. & Schramm, L. P. Origins of the sympathetic innervation of the cervical end of the uterus in the rat. Brain Res. 747, 140–143 (1997).
Houdeau, E., Rousseau, A., Meusnier, C., Prud’Homme, M. J. & Rousseau, J. P. Sympathetic innervation of the upper and lower regions of the uterus and cervix in the rat have different origins and routes. J. Comp. Neurol. 399, 403–412 (1998).
Tamamaki, N. & Nojyo, Y. Intracranial trajectories of sympathetic nerve fibers originating in the superior cervical ganglion in the rat: WGA-HRP anterograde labeling study. Brain Res. 437, 387–392 (1987).
Hirakawa, N., Morimoto, M. & Totoki, T. Sympathetic innervation of the young canine heart using antero- and retrograde axonal tracer methods. Brain Res. Bull. 31, 673–680 (1993).
Demer, J. L., Poukens, V., Miller, J. M. & Micevych, P. Innervation of extraocular pulley smooth muscle in monkeys and humans. Invest. Ophthalmol. Vis. Sci. 38, 1774–1785 (1997).
Beckers, H. J., Klooster, J., Vrensen, G. F. & Lamers, W. P. Facial parasympathetic innervation of the rat choroid, lacrimal glands and ciliary ganglion. An ultrastructural pterygopalatine tracing and immunohistochemical study. Ophthalmic Res. 25, 319–330 (1993).
van der Werf, F., Baljet, B., Prins, M. & Otto, J. A. Innervation of the lacrimal gland in the cynomolgous monkey: a retrograde tracing study. J. Anat. 188, 591–601 (1996).
Dartt, D. A. Neural regulation of lacrimal gland secretory processes: relevance in dry eye diseases. Prog. Retin. Eye Res. 28, 155–177 (2009).
Sheu, S.-H., Tapia, J. C., Tsuriel, S. & Lichtman, J. W. Similar synapse elimination motifs at successive relays in the same efferent pathway during development in mice. eLife 6, e23193 (2017).
Zubair, A. & Khan, Y. S. Neuroanatomy, otic ganglion. StatPearls https://www.ncbi.nlm.nih.gov/books/NBK554413/ (StatPearls Publishing, 2025).
Kalia, M. & Sullivan, J. M. Brainstem projections of sensory and motor components of the vagus nerve in the rat. J. Comp. Neurol. 211, 248–265 (1982).
Fox, E. A. & Powley, T. L. Longitudinal columnar organization within the dorsal motor nucleus represents separate branches of the abdominal vagus. Brain Res. 341, 269–282 (1985).
Bieger, D. & Hopkins, D. A. Viscerotopic representation of the upper alimentary tract in the medulla oblongata in the rat: the nucleus ambiguus. J. Comp. Neurol. 262, 546–562 (1987).
Dergacheva, O., Griffioen, K. J., Neff, R. A. & Mendelowitz, D. Respiratory modulation of premotor cardiac vagal neurons in the brainstem. Respir. Physiol. Neurobiol. 174, 102–110 (2010).
Browning, K. N., Coleman, F. H. & Travagli, R. A. Characterization of pancreas-projecting rat dorsal motor nucleus of vagus neurons. Am. J. Physiol. Gastrointest. Liver Physiol. 288, G950–G955 (2005).
Wang, L. & Taché, Y. The parasympathetic and sensory innervation of the proximal and distal colon in male mice. Front. Neuroanat. 18, 1422403 (2024).
Gourine, A. V. & Ackland, G. L. Cardiac vagus and exercise. Physiology 34, 71–80 (2019).
Moreira, T. S., Antunes, V. R., Falquetto, B. & Marina, N. Long-term stimulation of cardiac vagal preganglionic neurons reduces blood pressure in the spontaneously hypertensive rat. J. Hypertens. 36, 2444–2452 (2018).
Fonseca, R. C. et al. Vagus nerve regulates the phagocytic and secretory activity of resident macrophages in the liver. Brain Behav. Immun. 81, 444–454 (2019).
Machhada, A. et al. Optogenetic stimulation of vagal efferent activity preserves left ventricular function in experimental heart failure. JACC Basic Transl. Sci. 5, 799–810 (2020).
Dyavanapalli, J. Novel approaches to restore parasympathetic activity to the heart in cardiorespiratory diseases. Am. J. Physiol. Heart Circ. Physiol. 319, H1153–H1161 (2020).
Zasadny, F. M., Dyavanapalli, J., Dowling, N. M., Mendelowitz, D. & Kay, M. W. Cholinergic stimulation improves electrophysiological rate adaptation during pressure overload-induced heart failure in rats. Am. J. Physiol. Heart Circ. Physiol. 319, H1358–H1368 (2020).
Strain, M. M. et al. Dorsal motor vagal neurons can elicit bradycardia and reduce anxiety-like behavior. iScience 27, 109137 (2024).
Burnstock, G. Innervation of bladder and bowel. Ciba Found. Symp. 151, 18–26 (1990).
de Groat, W. C., Griffiths, D. & Yoshimura, N. Neural control of the lower urinary tract. Compr. Physiol. 5, 327–396 (2015).
Sharabi, A. F. & Carey, F. J. Anatomy, abdomen and pelvis, splanchnic nerves. StatPearls http://www.ncbi.nlm.nih.gov/books/NBK560504/ (StatPearls Publishing, 2025).
Kobori, N., Moore, A. N., Redell, J. B. & Dash, P. K. Caudal DMN neurons innervate the spleen and release CART peptide to regulate neuroimmune function. J. Neuroinflamm. 20, 158 (2023).
Anderson, C., McKinley, M., Martelli, D. & McAllen, R. Letter to the editor: parasympathetic innervation of the rodent spleen? Am. J. Physiol. Heart Circ. Physiol. 309, H2158 (2015).
Gautron, L. The parasympathetic innervation of the spleen: are we chasing a ghost? J. Anat. 240, 772–774 (2022).
Norvell, J. E. & Anderson, J. M. Assessment of possible parasympathetic innervation of the kidney. J. Auton. Nerv. Syst. 8, 291–294 (1983).
Cheng, X. et al. Anatomical evidence for parasympathetic innervation of the renal vasculature and pelvis. J. Am. Soc. Nephrol. 33, 2194–2210 (2022).
N’Guetta, P.-E. Y. et al. Comprehensive mapping of sensory and sympathetic innervation of the developing kidney. Cell Rep. 43, 114860 (2024).
Gibbins, I. L. Vasoconstrictor, vasodilator and pilomotor pathways in sympathetic ganglia of guinea pigs. Neuroscience 47, 657–672 (1992).
Gordan, R., Gwathmey, J. K. & Xie, L.-H. Autonomic and endocrine control of cardiovascular function. World J. Cardiol. 7, 204–214 (2015).
Gibbins, I. L. Vasomotor, pilomotor and secretomotor neurons distinguished by size and neuropeptide content in superior cervical ganglia of mice. J. Auton. Nerv. Syst. 34, 171–183 (1991).
Li, C. & Horn, J. P. Physiological classification of sympathetic neurons in the rat superior cervical ganglion. J. Neurophysiol. 95, 187–195 (2006).
Proctor, G. B. & Carpenter, G. H. Regulation of salivary gland function by autonomic nerves. Auton. Neurosci. 133, 3–18 (2007).
Izumi, H. Reflex parasympathetic vasodilatation in facial skin. Gen. Pharmacol. 26, 237–244 (1995).
Canning, B. J. Reflex regulation of airway smooth muscle tone. J. Appl. Physiol. 101, 971–985 (2006).
Olshansky, B., Sabbah, H. N., Hauptman, P. J. & Colucci, W. S. Parasympathetic nervous system and heart failure: pathophysiology and potential implications for therapy. Circulation 118, 863–871 (2008).
Ren, W., Hua, M., Cao, F. & Zeng, W. The sympathetic-immune milieu in metabolic health and diseases: insights from pancreas, liver, intestine, and adipose tissues. Adv. Sci. 11, e2306128 (2024).
Klein Wolterink, R. G. J., Wu, G. S., Chiu, I. M. & Veiga-Fernandes, H. Neuroimmune interactions in peripheral organs. Annu. Rev. Neurosci. 45, 339–360 (2022).
Shwartz, Y. et al. Cell types promoting goosebumps form a niche to regulate hair follicle stem cells. Cell 182, 578–593.e19 (2020).
Zhang, B. & Chen, T. Local and systemic mechanisms that control the hair follicle stem cell niche. Nat. Rev. Mol. Cell Biol. 25, 87–100 (2024).
Niu, X. et al. Mapping of extrinsic innervation of the gastrointestinal tract in the mouse embryo. J. Neurosci. 40, 6691–6708 (2020).
Guillot, J. et al. Sympathetic axonal sprouting induces changes in macrophage populations and protects against pancreatic cancer. Nat. Commun. 13, 1985 (2022).
Salamon, R. J. et al. Parasympathetic and sympathetic axons are bundled in the cardiac ventricles and undergo physiological reinnervation during heart regeneration. iScience 26, 107709 (2023).
Liu, S. et al. Somatotopic organization and intensity dependence in driving distinct NPY-expressing sympathetic pathways by electroacupuncture. Neuron 108, 436–450.e7 (2020).
Kumari, R. et al. Sympathetic NPY controls glucose homeostasis, cold tolerance, and cardiovascular functions in mice. Cell Rep. 43, 113674 (2024).
Lein, E. S. et al. Genome-wide atlas of gene expression in the adult mouse brain. Nature 445, 168–176 (2007).
Sternson, S. M., Atasoy, D., Betley, J. N., Henry, F. E. & Xu, S. An emerging technology framework for the neurobiology of appetite. Cell Metab. 23, 234–253 (2016).
Luo, L., Callaway, E. M. & Svoboda, K. Genetic dissection of neural circuits: a decade of progress. Neuron 98, 256–281 (2018).
Zeng, H. What is a cell type and how to define it? Cell 185, 2739–2755 (2022).
Miolan, J. P. & Niel, J. P. The mammalian sympathetic prevertebral ganglia: integrative properties and role in the nervous control of digestive tract motility. J. Auton. Nerv. Syst. 58, 125–138 (1996).
Kaestner, C. L., Smith, E. H., Peirce, S. G. & Hoover, D. B. Immunohistochemical analysis of the mouse celiac ganglion: an integrative relay station of the peripheral nervous system. J. Comp. Neurol. 527, 2742–2760 (2019).
Ernsberger, U., Deller, T. & Rohrer, H. The diversity of neuronal phenotypes in rodent and human autonomic ganglia. Cell Tissue Res. 382, 201–231 (2020).
Jobling, P. & Gibbins, I. L. Electrophysiological and morphological diversity of mouse sympathetic neurons. J. Neurophysiol. 82, 2747–2764 (1999).
Aubert, M. et al. Gene editing and elimination of latent herpes simplex virus in vivo. Nat. Commun. 11, 4148 (2020).
van Weperen, V. Y. H. et al. Single-cell transcriptomic profiling of satellite glial cells in stellate ganglia reveals developmental and functional axial dynamics. Glia 69, 1281–1291 (2021).
Davis, H., Paterson, D. J. & Herring, N. Post-ganglionic sympathetic neurons can directly sense raised extracellular Na+ via SCN7a/Nax. Front. Physiol. 13, 931094 (2022).
Sivori, M. et al. The pelvic organs receive no parasympathetic innervation. eLife 12, RP91576 (2024).
Kanda, H., Yamanaka, H., Dai, Y. & Noguchi, K. The neuronal and glial cell diversity in the celiac ganglion revealed by single-nucleus RNA sequencing. Sci. Rep. 15, 5510 (2025).
Li, E. et al. Control of lipolysis by a population of oxytocinergic sympathetic neurons. Nature 625, 175–180 (2024). This study identifies oxytocin-positive axons in the adipose tissue, serving as an endogenous regulator of adipose lipolysis and systemic metabolism.
Beaudet, M. M., Braas, K. M. & May, V. Pituitary adenylate cyclase activating polypeptide (PACAP) expression in sympathetic preganglionic projection neurons to the superior cervical ganglion. J. Neurobiol. 36, 325–336 (1998).
Harima, Y. et al. Parallel labeled-line organization of sympathetic outflow for selective organ regulation in mice. Nat. Commun. 15, 10478 (2024). This paper identifies molecularly distinct subtypes of spinal preganglionic neuron that form parallel anatomical wiring to either sympathetic prevertebral ganglia or the adrenal gland, selectively regulating gastrointestinal motility or glucose metabolism.
Berthoud, H. R., Carlson, N. R. & Powley, T. L. Topography of efferent vagal innervation of the rat gastrointestinal tract. Am. J. Physiol. 260, R200–R207 (1991).
Zsombok, A., Bhaskaran, M. D., Gao, H., Derbenev, A. V. & Smith, B. N. Functional plasticity of central TRPV1 receptors in brainstem dorsal vagal complex circuits of streptozotocin-treated hyperglycemic mice. J. Neurosci. 31, 14024–14031 (2011).
Derbenev, A. V., Stuart, T. C. & Smith, B. N. Cannabinoids suppress synaptic input to neurones of the rat dorsal motor nucleus of the vagus nerve. J. Physiol. 559, 923–938 (2004).
Hornung, E. et al. Neuromodulatory co-expression in cardiac vagal motor neurons of the dorsal motor nucleus of the vagus. iScience 27, 110549 (2024).
Coverdell, T. C., Abraham-Fan, R.-J., Wu, C., Abbott, S. B. G. & Campbell, J. N. Genetic encoding of an esophageal motor circuit. Cell Rep. 39, 110962 (2022). The authors show a molecularly distinct parasympathetic preganglionic neuron subtype that specifically innervates and controls oesophageal contraction.
Su, Y. et al. Brainstem Dbh+ neurons control allergen-induced airway hyperreactivity. Nature 631, 601–609 (2024).
Hibberd, T. J., Zagorodnyuk, V. P., Spencer, N. J. & Brookes, S. J. H. Identification and mechanosensitivity of viscerofugal neurons. Neuroscience 225, 118–129 (2012).
DePuy, S. D. et al. Glutamatergic neurotransmission between the C1 neurons and the parasympathetic preganglionic neurons of the dorsal motor nucleus of the vagus. J. Neurosci. 33, 1486–1497 (2013).
Kressel, A. M. et al. Identification of a brainstem locus that inhibits tumor necrosis factor. Proc. Natl Acad. Sci. USA 117, 29803–29810 (2020).
Muller, P. A. et al. Microbiota-modulated CART+ enteric neurons autonomously regulate blood glucose. Science 370, 314–321 (2020).
Zhang, T., Perkins, M. H., Chang, H., Han, W. & de Araujo, I. E. An inter-organ neural circuit for appetite suppression. Cell 185, 2478–2494.e28 (2022).
Bullmore, E. & Sporns, O. Complex brain networks: graph theoretical analysis of structural and functional systems. Nat. Rev. Neurosci. 10, 186–198 (2009).
Turrigiano, G. Homeostatic synaptic plasticity: local and global mechanisms for stabilizing neuronal function. Cold Spring Harb. Perspect. Biol. 4, a005736 (2012).
Bargmann, C. I. & Marder, E. From the connectome to brain function. Nat. Methods 10, 483–490 (2013).
LeCun, Y., Bengio, Y. & Hinton, G. Deep learning. Nature 521, 436–444 (2015).
Luo, L. Architectures of neuronal circuits. Science 373, eabg7285 (2021).
Zsombok, A., Desmoulins, L. D. & Derbenev, A. V. Sympathetic circuits regulating hepatic glucose metabolism: where we stand. Physiol. Rev. 104, 85–101 (2024).
Tomney, P. A., Hopkins, D. A. & Armour, J. A. Axonal branching of canine sympathetic postganglionic cardiopulmonary neurons. A retrograde fluorescent labeling study. Brain Res. Bull. 14, 443–452 (1985).
Seals, D. R. & Victor, R. G. Regulation of muscle sympathetic nerve activity during exercise in humans. Exerc. Sport. Sci. Rev. 19, 313–349 (1991).
DiBona, G. F. Neural control of the kidney: functionally specific renal sympathetic nerve fibers. Am. J. Physiol. Regul. Integr. Comp. Physiol. 279, R1517–R1524 (2000).
Morrison, S. F. Differential control of sympathetic outflow. Am. J. Physiol. Regul. Integr. Comp. Physiol. 281, R683–R698 (2001).
Muller, P. A. et al. Microbiota modulate sympathetic neurons via a gut-brain circuit. Nature 583, 441–446 (2020).
Asmus, S. E., Parsons, S. & Landis, S. C. Developmental changes in the transmitter properties of sympathetic neurons that innervate the periosteum. J. Neurosci. 20, 1495–1504 (2000).
Carmeliet, P. & Tessier-Lavigne, M. Common mechanisms of nerve and blood vessel wiring. Nature 436, 193–200 (2005).
Gallarda, B. W. et al. Segregation of axial motor and sensory pathways via heterotypic trans-axonal signaling. Science 320, 233–236 (2008).
Enomoto, H., Heuckeroth, R. O., Golden, J. P., Johnson, E. M. & Milbrandt, J. Development of cranial parasympathetic ganglia requires sequential actions of GDNF and neurturin. Development 127, 4877–4889 (2000).
Ekman, P., Levenson, R. W. & Friesen, W. V. Autonomic nervous system activity distinguishes among emotions. Science 221, 1208–1210 (1983).
Ju, S. H., Yun, H., Oh, Y., Choi, Y. & Sohn, J.-W. Melanocortin-4 receptors activate sympathetic preganglionic neurons and elevate blood pressure via TRPV1. Cell Rep. 41, 111579 (2022).
Steffens, A. B., Van der Gugten, J., Godeke, J., Luiten, P. G. & Strubbe, J. H. Meal-induced increases in parasympathetic and sympathetic activity elicit simultaneous rises in plasma insulin and free fatty acids. Physiol. Behav. 37, 119–122 (1986).
Panneton, W. M., Anch, A. M., Panneton, W. M. & Gan, Q. Parasympathetic preganglionic cardiac motoneurons labeled after voluntary diving. Front. Physiol. 5, 8 (2014).
Chen, H. et al. Postprandial parasympathetic signals promote lung type 2 immunity. Neuron https://doi.org/10.1016/j.neuron.2024.12.020 (2025).
Gonsalvez, D. G., Kerman, I. A., McAllen, R. M. & Anderson, C. R. Chemical coding for cardiovascular sympathetic preganglionic neurons in rats. J. Neurosci. 30, 11781–11791 (2010).
Wang, M., Wang, Q. & Whim, M. D. Fasting induces a form of autonomic synaptic plasticity that prevents hypoglycemia. Proc. Natl Acad. Sci. USA 113, E3029–E3038 (2016).
Jiang, H., Ding, X., Cao, Y., Wang, H. & Zeng, W. Dense intra-adipose sympathetic arborizations are essential for cold-induced beiging of mouse white adipose tissue. Cell Metab. 26, 686–692.e3 (2017).
Murano, I., Barbatelli, G., Giordano, A. & Cinti, S. Noradrenergic parenchymal nerve fiber branching after cold acclimatisation correlates with brown adipocyte density in mouse adipose organ. J. Anat. 214, 171–178 (2009).
Landry, M., Holmberg, K., Zhang, X. & Hökfelt, T. Effect of axotomy on expression of NPY, galanin, and NPY Y1 and Y2 receptors in dorsal root ganglia and the superior cervical ganglion studied with double-labeling in situ hybridization and immunohistochemistry. Exp. Neurol. 162, 361–384 (2000).
Tyrrell, S. & Landis, S. C. The appearance of NPY and VIP in sympathetic neuroblasts and subsequent alterations in their expression. J. Neurosci. 14, 4529–4547 (1994).
Hökfelt, T. et al. Coexistence of peptides with classical neurotransmitters. Experientia 43, 768–780 (1987).
Sann, H. & Pierau, F. K. Efferent functions of C-fiber nociceptors. Z. Rheumatol. 57, 8–13 (1998).
Andersson, K.-E. & Arner, A. Urinary bladder contraction and relaxation: physiology and pathophysiology. Physiol. Rev. 84, 935–986 (2004).
Ernsberger, U., Deller, T. & Rohrer, H. The sympathies of the body: functional organization and neuronal differentiation in the peripheral sympathetic nervous system. Cell Tissue Res. 386, 455–475 (2021).
Strosberg, A. D. Structure, function, and regulation of adrenergic receptors. Protein Sci. 2, 1198–1209 (1993).
Carlson, A. B. & Kraus, G. P. Physiology, cholinergic receptors. StatPearls http://www.ncbi.nlm.nih.gov/books/NBK526134/ (StatPearls Publishing, 2025).
Uhlén, M. et al. Proteomics. Tissue-based map of the human proteome. Science 347, 1260419 (2015).
Karlsson, M. et al. A single-cell type transcriptomics map of human tissues. Sci. Adv. 7, eabh2169 (2021).
Barrett, M. S. et al. Ischemia–reperfusion myocardial infarction induces remodeling of left cardiac-projecting stellate ganglia neurons. Am. J. Physiol. Heart Circ. Physiol. 326, H166–H179 (2024).
McEwen, B. S. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol. Rev. 87, 873–904 (2007).
de La Cruz, L., Bui, D., Moreno, C. M. & Vivas, O. Sympathetic motor neuron dysfunction is a missing link in age-associated sympathetic overactivity. eLife 12, RP91663 (2024).
Martelli, D., Yao, S. T., McKinley, M. J. & McAllen, R. M. Reflex control of inflammation by sympathetic nerves, not the vagus. J. Physiol. 592, 1677–1686 (2014).
Katayama, Y. et al. Signals from the sympathetic nervous system regulate hematopoietic stem cell egress from bone marrow. Cell 124, 407–421 (2006).
Morrison, S. J. & Scadden, D. T. The bone marrow niche for haematopoietic stem cells. Nature 505, 327–334 (2014).
Globig, A.-M. et al. The β1-adrenergic receptor links sympathetic nerves to T cell exhaustion. Nature 622, 383–392 (2023).
Yaniv, D., Mattson, B., Talbot, S., Gleber-Netto, F. O. & Amit, M. Targeting the peripheral neural-tumour microenvironment for cancer therapy. Nat. Rev. Drug Discov. 23, 780–796 (2024).
Jin, H., Li, M., Jeong, E., Castro-Martinez, F. & Zuker, C. S. A body–brain circuit that regulates body inflammatory responses. Nature 630, 695–703 (2024).
Pirzgalska, R. M. et al. Sympathetic neuron-associated macrophages contribute to obesity by importing and metabolizing norepinephrine. Nat. Med. 23, 1309–1318 (2017).
Wolf, Y. et al. Brown-adipose-tissue macrophages control tissue innervation and homeostatic energy expenditure. Nat. Immunol. 18, 665–674 (2017).
Cardoso, F. et al. Neuro-mesenchymal units control ILC2 and obesity via a brain–adipose circuit. Nature 597, 410–414 (2021).
Zhang, B. et al. Hyperactivation of sympathetic nerves drives depletion of melanocyte stem cells. Nature 577, 676–681 (2020).
Zhou, Y. et al. A brain-to-liver signal mediates the inhibition of liver regeneration under chronic stress in mice. Nat. Commun. 15, 10361 (2024).
Shivkumar, K. et al. Clinical neurocardiology defining the value of neuroscience-based cardiovascular therapeutics. J. Physiol. 594, 3911–3954 (2016).
Ajijola, O. A. et al. Bilateral cardiac sympathetic denervation for the management of electrical storm. J. Am. Coll. Cardiol. 59, 91–92 (2012).
Al-Khatib, S. M. et al. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: executive summary: a report of the American college of cardiology/American heart association task force on clinical practice guidelines and the heart rhythm society. Heart Rhythm. 15, e190–e252 (2018).
Ben-Menachem, E. Vagus-nerve stimulation for the treatment of epilepsy. Lancet Neurol. 1, 477–482 (2002).
Mauskop, A. Vagus nerve stimulation relieves chronic refractory migraine and cluster headaches. Cephalalgia 25, 82–86 (2005).
Turk, D. C. et al. Core outcome domains for chronic pain clinical trials: IMMPACT recommendations. Pain 106, 337–345 (2003).
Premchand, R. K. et al. Autonomic regulation therapy via left or right cervical vagus nerve stimulation in patients with chronic heart failure: results of the ANTHEM-HF trial. J. Card. Fail. 20, 808–816 (2014).
Hadaya, J. et al. Vagal nerve stimulation reduces ventricular arrhythmias and mitigates adverse neural cardiac remodeling post-myocardial infarction. JACC Basic Transl. Sci. 8, 1100–1118 (2023).
Berthoud, H.-R. The vagus nerve, food intake and obesity. Regul. Pept. 149, 15–25 (2008).
Matsubayashi, T. et al. Significant differences in sympathetic nerve fiber density among the facial skin nerves: a histologic study using human cadaveric specimens. Anat. Rec. 299, 1054–1059 (2016).
Donadio, V. et al. The autonomic innervation of hairy skin in humans: an in vivo confocal study. Sci. Rep. 9, 16982 (2019).
Kozłowska, A., Mikołajczyk, A. & Majewski, M. Detailed characterization of sympathetic chain ganglia (SChG) neurons supplying the skin of the porcine hindlimb. Int. J. Mol. Sci. 18, 1463 (2017).
Roberts, W. J. & Levitt, G. R. Histochemical evidence for sympathetic innervation of hair receptor afferents in cat skin. J. Comp. Neurol. 210, 204–209 (1982).
Chen, H. I. & Ta, C. The sympathetic efferent innervation of the cutaneous and muscle veins in cats. A comparative study using retrograde localization with horseradish peroxidase. J. Auton. Nerv. Syst. 46, 189–197 (1994).
Marfurt, C. F. & Ellis, L. C. Immunohistochemical localization of tyrosine hydroxylase in corneal nerves. J. Comp. Neurol. 336, 517–531 (1993).
Bergua, A., Kapsreiter, M., Neuhuber, W. L., Reitsamer, H. A. & Schrödl, F. Innervation pattern of the preocular human central retinal artery. Exp. Eye Res. 110, 142–147 (2013).
Marfurt, C. F., Kingsley, R. E. & Echtenkamp, S. E. Sensory and sympathetic innervation of the mammalian cornea. A retrograde tracing study. Invest. Ophthalmol. Vis. Sci. 30, 461–472 (1989).
Ehinger, B. Distribution of adrenergic nerves in the eye and some related structures in the cat. Acta Physiol. Scand. 66, 123–128 (1966).
Morgan, C., DeGroat, W. C. & Jannetta, P. J. Sympathetic innervation of the cornea from the superior cervical ganglion. An HRP study in the cat. J. Auton. Nerv. Syst. 20, 179–183 (1987).
Gibbins, I. L. & Morris, J. L. Co-existence of neuropeptides in sympathetic, cranial autonomic and sensory neurons innervating the iris of the guinea-pig. J. Auton. Nerv. Syst. 21, 67–82 (1987).
ten Tusscher, M. P., Klooster, J. & Vrensen, G. F. The innervation of the rabbit’s anterior eye segment: a retrograde tracing study. Exp. Eye Res. 46, 717–730 (1988).
Xue, Y. et al. The mouse autonomic nervous system modulates inflammation and epithelial renewal after corneal abrasion through the activation of distinct local macrophages. Mucosal Immunol. 11, 1496–1511 (2018).
Kuwayama, Y., Grimes, P. A., Ponte, B. & Stone, R. A. Autonomic neurons supplying the rat eye and the intraorbital distribution of vasoactive intestinal polypeptide (VIP)-like immunoreactivity. Exp. Eye Res. 44, 907–922 (1987).
Adeghate, E. A., Singh, J., Howarth, F. C. & Burrows, S. Control of porcine lacrimal gland secretion by non-cholinergic, non-adrenergic nerves: effects of electrical field stimulation, VIP and NPY. Brain Res. 758, 127–135 (1997).
Ding, C., Walcott, B. & Keyser, K. T. Sympathetic neural control of the mouse lacrimal gland. Invest. Ophthalmol. Vis. Sci. 44, 1513–1520 (2003).
Toshida, H. & Suto, C. Preganglionic parasympathetic denervation rabbit model for innervation studies. Cornea 37, S106–S112 (2018).
Freitag, P. & Engel, M. B. Autonomic innervation in rabbit salivary glands. Anat. Rec. 167, 87–105 (1970).
Teshima, T. H. N., Tucker, A. S. & Lourenço, S. V. Dual sympathetic input into developing salivary glands. J. Dent. Res. 98, 1122–1130 (2019).
Lahtivirta, S., Koistinaho, J. & Hervonen, A. A subpopulation of large neurons of the sympathetic superior cervical ganglion innervates the NGF-rich submandibular salivary gland in young adult and aged mice. J. Auton. Nerv. Syst. 50, 283–289 (1995).
Garrett, J. R. The autonomic innervation of rabbit salivary glands studied electron microscopically after 5-hydroxydopamine administration. Cell Tissue Res. 178, 551–562 (1977).
Melander, A. et al. Sympathetic innervation of the normal human thyroid. J. Clin. Endocrinol. Metab. 39, 713–718 (1974).
Melander, A., Sundler, F. & Westgren, U. Sympathetic innervation of the thyroid: variation with species and with age. Endocrinology 96, 102–106 (1975).
Amenta, F., Caporuscio, D., Ferrante, F., Porcelli, F. & Zomparelli, M. Cholinergic nerves in the thyroid gland. Cell Tissue Res. 195, 367–370 (1978).
Van Sande, J., Dumont, J. E., Melander, A. & Sundler, F. Presence and influence of cholinergic nerves in the human thyroid. J. Clin. Endocrinol. Metab. 51, 500–502 (1980).
Kalsbeek, A., Fliers, E., Franke, A. N., Wortel, J. & Buijs, R. M. Functional connections between the suprachiasmatic nucleus and the thyroid gland as revealed by lesioning and viral tracing techniques in the rat. Endocrinology 141, 3832–3841 (2000).
Bulc, M., Lewczuk, B., Prusik, M. & Całka, J. The foetal pig pineal gland is richly innervated by nerve fibres containing catecholamine-synthesizing enzymes, neuropeptide Y (NPY) and C-terminal flanking peptide of NPY, but it does not secrete melatonin. Histol. Histopathol. 28, 633–646 (2013).
Møller, M., Phansuwan-Pujito, P., Pramaulkijja, S., Kotchabhakdi, N. & Govitrapong, P. Innervation of the cat pineal gland by neuropeptide Y-immunoreactive nerve fibers: an experimental immunohistochemical study. Cell Tissue Res. 276, 545–550 (1994).
Romijn, H. J. Structure and innervation of the pineal gland of the rabbit, Oryctolagus cuniculus (L.). III. An electron microscopic investigation of the innervation. Cell Tissue Res. 157, 25–51 (1975).
Bowers, C. W., Dahm, L. M. & Zigmond, R. E. The number and distribution of sympathetic neurons that innervate the rat pineal gland. Neuroscience 13, 87–96 (1984).
Phansuwan-Pujito, P., Møller, M. & Govitrapong, P. Cholinergic innervation and function in the mammalian pineal gland. Microsc. Res. Tech. 46, 281–295 (1999).
Wang, W. et al. Age-related dopaminergic innervation augments T helper 2-type allergic inflammation in the postnatal lung. Immunity 51, 1102–1118.e7 (2019).
McGovern, A. E. & Mazzone, S. B. Characterization of the vagal motor neurons projecting to the guinea pig airways and esophagus. Front. Neurol. 1, 153 (2010).
Fontán, J. J., Diec, C. T. & Velloff, C. R. Bilateral distribution of vagal motor and sensory nerve fibers in the rat’s lungs and airways. Am. J. Physiol. Regul. Integr. Comp. Physiol. 279, R713–R728 (2000).
Pincus, A. B. et al. Multicolor labeling of airway neurons and analysis of parasympathetic heterogeneity. Sci. Rep. 12, 5006 (2022).
Oki, H., Inoue, S., Makishima, N., Takeyama, Y. & Shiokawa, A. Cardiac sympathetic innervation in patients with dilated cardiomyopathy-immunohistochemical study using anti-tyrosine hydroxylase antibody. Jpn. Circ. J. 58, 389–394 (1994).
Chuang, K.-S., Liu, W.-C., Liou, N.-H. & Liu, J.-C. Horseradish peroxidase localization of sympathetic postganglionic and parasympathetic preganglionic neurons innervating the monkey heart. Chin. J. Physiol. 47, 95–99 (2004).
Crick, S. J., Sheppard, M. N., Anderson, R. H., Polak, J. M. & Wharton, J. A quantitative study of nerve distribution in the conduction system of the guinea pig heart. J. Anat. 188, 403–416 (1996).
Crick, S. J., Anderson, R. H., Ho, S. Y. & Sheppard, M. N. Localisation and quantitation of autonomic innervation in the porcine heart II: endocardium, myocardium and epicardium. J. Anat. 195, 359–373 (1999).
Hopkins, D. A. & Armour, J. A. Localization of sympathetic postganglionic and parasympathetic preganglionic neurons which innervate different regions of the dog heart. J. Comp. Neurol. 229, 186–198 (1984).
Chang, C. M. et al. Nerve sprouting and sympathetic hyperinnervation in a canine model of atrial fibrillation produced by prolonged right atrial pacing. Circulation 103, 22–25 (2001).
Dickson, D. W. et al. Regional distribution of tyrosine hydroxylase and dopamine beta-hydroxylase activities in guinea pig heart. J. Auton. Nerv. Syst. 4, 319–326 (1981).
Kanazawa, H. et al. Heart failure causes cholinergic transdifferentiation of cardiac sympathetic nerves via gp130-signaling cytokines in rodents. J. Clin. Invest. 120, 408–421 (2010).
Pardini, B. J., Lund, D. D. & Schmid, P. G. Organization of the sympathetic postganglionic innervation of the rat heart. J. Auton. Nerv. Syst. 28, 193–201 (1989).
Jungen, C. et al. Disruption of cardiac cholinergic neurons enhances susceptibility to ventricular arrhythmias. Nat. Commun. 8, 14155 (2017).
Lizot, G. et al. Molecular and functional characterization of the mouse intrinsic cardiac nervous system. Heart Rhythm. 19, 1352–1362 (2022).
Batulevicius, D., Skripka, V., Pauziene, N. & Pauza, D. H. Topography of the porcine epicardiac nerve plexus as revealed by histochemistry for acetylcholinesterase. Auton. Neurosci. 138, 64–75 (2008).
Saburkina, I. et al. Morphological pattern of intrinsic nerve plexus distributed on the rabbit heart and interatrial septum. J. Anat. 224, 583–593 (2014).
Standish, A., Enquist, L. W., Escardo, J. A. & Schwaber, J. S. Central neuronal circuit innervating the rat heart defined by transneuronal transport of pseudorabies virus. J. Neurosci. 15, 1998–2012 (1995).
Mabe, A. M., Hoard, J. L., Duffourc, M. M. & Hoover, D. B. Localization of cholinergic innervation and neurturin receptors in adult mouse heart and expression of the neurturin gene. Cell Tissue Res. 326, 57–67 (2006).
De Matteis, R., Ricquier, D. & Cinti, S. TH-, NPY-, SP-, and CGRP-immunoreactive nerves in interscapular brown adipose tissue of adult rats acclimated at different temperatures: an immunohistochemical study. J. Neurocytol. 27, 877–886 (1998).
Burt, A. D. et al. Localization of adrenergic and neuropeptide tyrosine-containing nerves in the mammalian liver. Hepatology 9, 839–845 (1989).
Adori, C. et al. Disorganization and degeneration of liver sympathetic innervations in nonalcoholic fatty liver disease revealed by 3D imaging. Sci. Adv. 7, eabg5733 (2021).
Kwon, E. et al. Optogenetic stimulation of the liver-projecting melanocortinergic pathway promotes hepatic glucose production. Nat. Commun. 11, 6295 (2020).
Hwang, J. et al. The development of hepatic steatosis depends on the presence of liver-innervating parasympathetic cholinergic neurons in mice fed a high-fat diet. PLoS Biol. 22, e3002865 (2024).
Rodriguez-Diaz, R. et al. Innervation patterns of autonomic axons in the human endocrine pancreas. Cell Metab. 14, 45–54 (2011).
Krivova, Y. S., Proshchina, A. E., Otlyga, D. A., Leonova, O. G. & Saveliev, S. V. Prenatal development of sympathetic innervation of the human pancreas. Ann. Anat. 240, 151880 (2022).
Chiu, Y.-C., Hua, T.-E., Fu, Y.-Y., Pasricha, P. J. & Tang, S.-C. 3-D imaging and illustration of the perfusive mouse islet sympathetic innervation and its remodelling in injury. Diabetologia 55, 3252–3261 (2012).
Rinaman, L. & Miselis, R. R. The organization of vagal innervation of rat pancreas using cholera toxin-horseradish peroxidase conjugate. J. Auton. Nerv. Syst. 21, 109–125 (1987).
Cano, G., Sved, A. F., Rinaman, L., Rabin, B. S. & Card, J. P. Characterization of the central nervous system innervation of the rat spleen using viral transneuronal tracing. J. Comp. Neurol. 439, 1–18 (2001).
Felten, D. L., Ackerman, K. D., Wiegand, S. J. & Felten, S. Y. Noradrenergic sympathetic innervation of the spleen: I. Nerve fibers associate with lymphocytes and macrophages in specific compartments of the splenic white pulp. J. Neurosci. Res. 18, 28–36, 118–121 (1987).
Bellinger, D. et al. Age-related changes in noradrenergic sympathetic innervation of the rat spleen is strain dependent. Brain Behav. Immun. 16, 247–261 (2002).
Murray, K. et al. Neuroanatomy of the spleen: mapping the relationship between sympathetic neurons and lymphocytes. PLoS ONE 12, e0182416 (2017).
Walter, G. C., Phillips, R. J., McAdams, J. L. & Powley, T. L. Individual sympathetic postganglionic neurons coinnervate myenteric ganglia and smooth muscle layers in the gastrointestinal tract of the rat. J. Comp. Neurol. 524, 2577–2603 (2016).
Shapiro, R. E. & Miselis, R. R. The central organization of the vagus nerve innervating the stomach of the rat. J. Comp. Neurol. 238, 473–488 (1985).
Hayakawa, T., Kuwahara, S., Maeda, S., Tanaka, K. & Seki, M. Direct synaptic contacts on the myenteric ganglia of the rat stomach from the dorsal motor nucleus of the vagus. J. Comp. Neurol. 498, 352–362 (2006).
Gao, H. et al. Morphological and electrophysiological features of motor neurons and putative interneurons in the dorsal vagal complex of rats and mice. Brain Res. 1291, 40–52 (2009).
Parker, D. R. et al. Sympathetic pathways target cholinergic neurons in the human colonic myenteric plexus. Front. Neurosci. 16, 863662 (2022).
Holets, V. & Elde, R. Sympathoadrenal preganglionic neurons: their distribution and relationship to chemically-coded fibers in the kitten intermediolateral cell column. J. Auton. Nerv. Syst. 7, 149–163 (1983).
Vera, P. L., Hurwitz, B. E. & Schneiderman, N. Sympathoadrenal preganglionic neurons in the adult rabbit send their dendrites into the contralateral hemicord. J. Auton. Nerv. Syst. 30, 193–198 (1990).
Lever, J. D. Nerve fibres in the adrenal cortex of the rat. Nature 171, 882–883 (1953).
Sakakura, K. et al. Anatomic assessment of sympathetic peri-arterial renal nerves in man. J. Am. Coll. Cardiol. 64, 635–643 (2014).
Barbe, M. F. et al. Clarification of the innervation of the bladder, external urethral sphincter and clitoris: a neuronal tracing study in female mongrel hound dogs. Anat. Rec. 301, 1426–1441 (2018).
McConnell, J., Benson, G. S. & Wood, J. G. Autonomic innervation of the urogenital system: adrenergic and cholinergic elements. Brain Res. Bull. 9, 679–694 (1982).
Pinsard, M. et al. Anatomic and functional mapping of human uterine innervation. Fertil. Steril. 117, 1279–1288 (2022).
Dail, W. G. & Evan, A. P. Ultrastructure of adrenergic terminals and SIF cells in the superior cervical ganglion of the rabbit. Brain Res. 148, 469–477 (1978).
Luebke, J. I. & Wright, L. L. Characterization of superior cervical ganglion neurons that project to the submandibular glands, the eyes, and the pineal gland in rats. Brain Res. 589, 1–14 (1992).
Fioretto, E. T. et al. Macro- and microstructure of the superior cervical ganglion in dogs, cats and horses during maturation. Cell Tissues Organs 186, 129–140 (2007).
Toscano, C. P., de Melo, M. P., Matera, J. M., Loesch, A. & Ribeiro, A. A. C. M. The developing and restructuring superior cervical ganglion of guinea pigs (Cavia porcellus var. albina). Int. J. Dev. Neurosci. 27, 329–336 (2009).
Mitsuoka, K., Kikutani, T. & Sato, I. Morphological relationship between the superior cervical ganglion and cervical nerves in Japanese cadaver donors. Brain Behav. 7, e00619 (2017).
Boljanović, J. et al. Arterial supply and morphological characteristics of sympathetic neurons in the human superior cervical ganglion. Front. Neuroanat. 18, 1372180 (2024).
Erulkar, S. D. & Woodward, J. K. Intracellular recording from mammalian superior cervical ganglion in situ. J. Physiol. 199, 189–203 (1968).
Purves, D. & Wigston, D. J. Neural units in the superior cervical ganglion of the guinea pig. J. Physiol. 334, 169–178 (1983).
Ivanov, A. & Purves, D. Ongoing electrical activity of superior cervical ganglion cells in mammals of different size. J. Comp. Neurol. 284, 398–404 (1989).
Ivanov, A. Y. A. & Skok, V. I. Neuronal mechanisms responsible for ongoing activity of rabbit superior cervical ganglion neurons. J. Auton. Nerv. Syst. 41, 61–66 (1992).
Cowen, T., Haven, A. J., Milner, P., Lincoln, J. & Burnstock, G. Increase in neuropeptide Y, but not noradrenaline, in the superior cervical ganglion of rabbits chronically exposed to cold. J. Auton. Nerv. Syst. 24, 175–178 (1988).
Baffi, J. et al. Neuropeptides in the human superior cervical ganglion. Brain Res. 570, 272–278 (1992).
Klimaschewski, L., Kummer, W. & Heym, C. Localization, regulation and functions of neurotransmitters and neuromodulators in cervical sympathetic ganglia. Microsc. Res. Tech. 35, 44–68 (1996).
Masliukov, P. M., Konovalov, V. V., Emanuilov, A. I. & Nozdrachev, A. D. Development of neuropeptide Y-containing neurons in sympathetic ganglia of rats. Neuropeptides 46, 345–352 (2012).
Kokubun, S., Sato, T., Yajima, T. & Ichikawa, H. Distribution of postganglionic neurons which contain dopamine β-hydroxylase, tyrosine hydroxylase, neuropeptide Y and vasoactive intestinal polypeptide in the human middle cervical ganglion. Tissue Cell 58, 42–50 (2019).
Sapio, M. R. et al. Comparative analysis of dorsal root, nodose and sympathetic ganglia for the development of new analgesics. Front. Neurosci. 14, 615362 (2020).
Bosnjak, Z. J. & Kampine, J. P. Electrophysiological and morphological characterization of neurons in stellate ganglion of cats. Am. J. Physiol. 248, R288–R292 (1985).
Mo, N., Wallis, D. I. & Watson, A. Properties of putative cardiac and non-cardiac neurones in the rat stellate ganglion. J. Auton. Nerv. Syst. 47, 7–22 (1994).
Kwon, O. J. et al. Morphological spectra of adult human stellate ganglia: implications for thoracic sympathetic denervation. Anat. Rec. 301, 1244–1250 (2018).
McKinnon, M. L. et al. Dramatically amplified thoracic sympathetic postganglionic excitability and integrative capacity revealed with whole-cell patch-clamp recordings. eNeuro 6, ENEURO.0433–18.2019 (2019).
Blackman, J. G. & Purves, R. D. Intracellular recordings from ganglia of the thoracic sympathetic chain of the guinea-pig. J. Physiol. 203, 173–198 (1969).
Gilbert, R., Ryan, J. S., Horackova, M., Smith, F. M. & Kelly, M. E. Actions of substance P on membrane potential and ionic currents in guinea pig stellate ganglion neurons. Am. J. Physiol. 274, C892–C903 (1998).
Nozdrachev, A. D., Fateev, M. M., Jiménez, B. & Morales, M. A. Circuits and projections of cat stellate ganglion. Arch. Med. Res. 34, 106–115 (2003).
Lindh, B., Staines, W., Hökfelt, T., Terenius, L. & Salvaterra, P. M. Immunohistochemical demonstration of choline acetyltransferase-immunoreactive preganglionic nerve fibers in guinea pig autonomic ganglia. Proc. Natl Acad. Sci. USA 83, 5316–5320 (1986).
Darvesh, S., Nance, D. M., Hopkins, D. A. & Armour, J. A. Distribution of neuropeptide-like immunoreactivity in intact and chronically decentralized middle cervical and stellate ganglia of dogs. J. Auton. Nerv. Syst. 21, 167–180 (1987).
Hill, E. L. & Elde, R. Vasoactive intestinal peptide distribution and colocalization with dopamine-beta-hydroxylase in sympathetic chain ganglia of pig. J. Auton. Nerv. Syst. 27, 229–239 (1989).
Häppölä, O., Lakomy, M., Majewski, M., Wasowicz, K. & Yanaihara, N. Distribution of neuropeptides in the porcine stellate ganglion. Cell Tissue Res. 274, 181–187 (1993).
Ernsberger, U. & Rohrer, H. Development of the cholinergic neurotransmitter phenotype in postganglionic sympathetic neurons. Cell Tissue Res. 297, 339–361 (1999).
Gola, M. & Niel, J. P. Electrical and integrative properties of rabbit sympathetic neurones re-evaluated by patch clamping non-dissociated cells. J. Physiol. 460, 327–349 (1993).
Lundberg, J. M. et al. Organizational principles in the peripheral sympathetic nervous system: subdivision by coexisting peptides (somatostatin-, avian pancreatic polypeptide-, and vasoactive intestinal polypeptide-like immunoreactive materials). Proc. Natl Acad. Sci. USA 79, 1303–1307 (1982).
Macrae, I. M., Furness, J. B. & Costa, M. Distribution of subgroups of noradrenaline neurons in the coeliac ganglion of the guinea-pig. Cell Tissue Res. 244, 173–180 (1986).
Lindh, B. et al. Topography of NPY-, somatostatin-, and VIP-immunoreactive, neuronal subpopulations in the guinea pig celiac-superior mesenteric ganglion and their projection to the pylorus. J. Neurosci. 6, 2371–2383 (1986).
Lakomy, M., Häppölä, O., Majewski, M. & Wasowicz, K. Neuropeptides in the porcine coeliac-superior mesenteric ganglion. Folia Histochem. Cytobiol. 31, 181–191 (1993).
Fox, E. A. & Powley, T. L. Morphology of identified preganglionic neurons in the dorsal motor nucleus of the vagus. J. Comp. Neurol. 322, 79–98 (1992).
Huang, X. F., Törk, I. & Paxinos, G. Dorsal motor nucleus of the vagus nerve: a cyto- and chemoarchitectonic study in the human. J. Comp. Neurol. 330, 158–182 (1993).
Fogel, R., Zhang, X. & Renehan, W. E. Relationships between the morphology and function of gastric and intestinal distention-sensitive neurons in the dorsal motor nucleus of the vagus. J. Comp. Neurol. 364, 78–91 (1996).
Jarvinen, M. K. & Powley, T. L. Dorsal motor nucleus of the vagus neurons: a multivariate taxonomy. J. Comp. Neurol. 403, 359–377 (1999).
Ford, T. W., Bennett, J. A., Kidd, C. & McWilliam, P. N. Neurones in the dorsal motor vagal nucleus of the cat with non-myelinated axons projecting to the heart and lungs. Exp. Physiol. 75, 459–473 (1990).
Rogers, R. C., Hermann, G. E. & Travagli, R. A. Brainstem pathways responsible for oesophageal control of gastric motility and tone in the rat. J. Physiol. 514, 369–383 (1999).
Mussa, B. M., Sartor, D. M. & Verberne, A. J. M. Dorsal vagal preganglionic neurons: differential responses to CCK1 and 5-HT3 receptor stimulation. Auton. Neurosci. 156, 36–43 (2010).
de Souza, J. G. V. et al. Electrophysiological properties and morphology of cardiac and pulmonary motoneurons within the dorsal motor nucleus of the vagus of rats. Neuroscience 551, 153–165 (2024).
Armstrong, D. M., Ross, C. A., Pickel, V. M., Joh, T. H. & Reis, D. J. Distribution of dopamine-, noradrenaline-, and adrenaline-containing cell bodies in the rat medulla oblongata: demonstrated by the immunocytochemical localization of catecholamine biosynthetic enzymes. J. Comp. Neurol. 212, 173–187 (1982).
Raggenbass, M., Dubois-Dauphin, M., Charpak, S. & Dreifuss, J. J. Neurons in the dorsal motor nucleus of the vagus nerve are excited by oxytocin in the rat but not in the guinea pig. Proc. Natl Acad. Sci. USA 84, 3926–3930 (1987).
Armstrong, D. M., Manley, L., Haycock, J. W. & Hersh, L. B. Co-localization of choline acetyltransferase and tyrosine hydroxylase within neurons of the dorsal motor nucleus of the vagus. J. Chem. Neuroanat. 3, 133–140 (1990).
Kitahama, K. et al. Dopaminergic neurons in the cat dorsal motor nucleus of the vagus, demonstrated by dopamine, AADC and TH immunohistochemistry. Neurosci. Lett. 146, 5–9 (1992).
Zheng, Z. L., Rogers, R. C. & Travagli, R. A. Selective gastric projections of nitric oxide synthase-containing vagal brainstem neurons. Neuroscience 90, 685–694 (1999).
Gańko, M., Rychlik, A. & Całka, J. Immunohistochemical characterization of neurons and neuronal processes in the dorsal vagal nucleus of the pig. Pol. J. Vet. Sci. 16, 9–16 (2013).
Tsumori, T., Oka, T., Yokota, S., Niu, J.-G. & Yasui, Y. Intrapancreatic ganglia neurons receive projection fibers from melanocortin-4 receptor-expressing neurons in the dorsal motor nucleus of the vagus nerve of the mouse. Brain Res. 1537, 132–142 (2013).
Fogarty, M. J. Dendritic morphology of motor neurons and interneurons within the compact, semicompact, and loose formations of the rat nucleus ambiguus. Front. Cell Neurosci. 18, 1409974 (2024).
McAllen, R. M. & Spyer, K. M. Two types of vagal preganglionic motoneurones projecting to the heart and lungs. J. Physiol. 282, 353–364 (1978).
Delgado-García, J. M., López-Barneo, J., Serra, R. & González-Barón, S. Electrophysiological and functional identification of different neuronal types within the nucleus ambiguus in the cat. Brain Res. 277, 231–240 (1983).
Mendelowitz, D. Firing properties of identified parasympathetic cardiac neurons in nucleus ambiguus. Am. J. Physiol. 271, H2609–H2614 (1996).
Chen, Y. et al. Inspiratory-activated and inspiratory-inhibited airway vagal preganglionic neurons in the ventrolateral medulla of neonatal rat are different in intrinsic electrophysiological properties. Respir. Physiol. Neurobiol. 180, 323–330 (2012).
Abe, C. et al. C1 neurons mediate a stress-induced anti-inflammatory reflex in mice. Nat. Neurosci. 20, 700–707 (2017).
Souza, G. M. P. R., Stornetta, R. L., Stornetta, D. S., Guyenet, P. G. & Abbott, S. B. G. Adrenergic C1 neurons monitor arterial blood pressure and determine the sympathetic response to hemorrhage. Cell Rep. 38, 110480 (2022).
Gu, X. et al. Neurons in the caudal ventrolateral medulla mediate descending pain control. Nat. Neurosci. 26, 594–605 (2023).
Murray, K., Barboza, M., Rude, K. M., Brust-Mascher, I. & Reardon, C. Functional circuitry of neuro-immune communication in the mesenteric lymph node and spleen. Brain Behav. Immun. 82, 214–223 (2019).
Wengrowski, A. M. et al. Optogenetic release of norepinephrine from cardiac sympathetic neurons alters mechanical and electrical function. Cardiovasc. Res. 105, 143–150 (2015).
Lyons, C. E. et al. Optogenetic-induced sympathetic neuromodulation of brown adipose tissue thermogenesis. FASEB J. 34, 2765–2773 (2020).
Schiller, M. et al. Optogenetic activation of local colonic sympathetic innervations attenuates colitis by limiting immune cell extravasation. Immunity 54, 1022–1036.e8 (2021).
Pereira, M. M. A. et al. A brain-sparing diphtheria toxin for chemical genetic ablation of peripheral cell lineages. Nat. Commun. 8, 14967 (2017).
Acknowledgements
We thank the members of the Oka lab for helpful discussion and comments. Y.O. is supported by Startup funds from the President and Provost of California Institute of Technology, the Biology and Biological Engineering Division of California Institute of Technology, the New York Stem Cell Foundation, the Alfred P. Sloan Foundation, the Edward Mallinckrodt Foundation, and Heritage Medical Research Institute.
Author information
Authors and Affiliations
Contributions
T.W. researched data for the article. T.W. and A.T. developed transcriptomic data visualization portal. O.A.A. wrote the translational implications section. T.W. and Y.O. conceived and wrote the article.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Neuroscience thanks B. Davis and the other, anonymous, reviewers for their contribution to the peer review of this manuscript.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, T., Tufenkjian, A., Ajijola, O.A. et al. Molecular and functional diversity of the autonomic nervous system. Nat. Rev. Neurosci. 26, 607–622 (2025). https://doi.org/10.1038/s41583-025-00941-2
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41583-025-00941-2