Abstract
The HIV-1 envelope glycoprotein (Env) trimer mediates cell entry and is conformationally dynamic1,2,3,4,5,6,7,8. Imaging by single-molecule fluorescence resonance energy transfer (smFRET) has revealed that, on the surface of intact virions, mature pre-fusion Env transitions from a pre-triggered conformation (state 1) through a default intermediate conformation (state 2) to a conformation in which it is bound to three CD4 receptor molecules (state 3)8,9,10. It is currently unclear how these states relate to known structures. Breakthroughs in the structural characterization of the HIV-1 Env trimer have previously been achieved by generating soluble and proteolytically cleaved trimers of gp140 Env that are stabilized by a disulfide bond, an isoleucine-to-proline substitution at residue 559 and a truncation at residue 664 (SOSIP.664 trimers)5,11,12,13,14,15,16,17,18. Cryo-electron microscopy studies have been performed with C-terminally truncated Env of the HIV-1JR-FL strain in complex with the antibody PGT15119. Both approaches have revealed similar structures for Env. Although these structures have been presumed to represent the pre-triggered state 1 of HIV-1 Env, this hypothesis has never directly been tested. Here we use smFRET to compare the conformational states of Env trimers used for structural studies with native Env on intact virus. We find that the constructs upon which extant high-resolution structures are based predominantly occupy downstream conformations that represent states 2 and 3. Therefore, the structure of the pre-triggered state-1 conformation of viral Env that has been identified by smFRET and that is preferentially stabilized by many broadly neutralizing antibodies—and thus of interest for the design of immunogens—remains unknown.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding authors upon reasonable request.
Code availability
The full source code of SPARTAN34, which was used for all analysis of smFRET data, is available at http://www.scottcblanchardlab.com/software.
References
Harrison, S. C. Viral membrane fusion. Nat. Struct. Mol. Biol. 15, 690–698 (2008).
Wyatt, R. & Sodroski, J. The HIV-1 envelope glycoproteins: fusogens, antigens, and immunogens. Science 280, 1884–1888 (1998).
Kwong, P. D. et al. Structure of an HIV gp120 envelope glycoprotein in complex with the CD4 receptor and a neutralizing human antibody. Nature 393, 648–659 (1998).
Liu, J., Bartesaghi, A., Borgnia, M. J., Sapiro, G. & Subramaniam, S. Molecular architecture of native HIV-1 gp120 trimers. Nature 455, 109–113 (2008).
Ozorowski, G. et al. Open and closed structures reveal allostery and pliability in the HIV-1 envelope spike. Nature 547, 360–363 (2017).
Haynes, B. F. & Burton, D. R. Developing an HIV vaccine. Science 355, 1129–1130 (2017).
Sanders, R. W. & Moore, J. P. Native-like Env trimers as a platform for HIV-1 vaccine design. Immunol. Rev. 275, 161–182 (2017).
Munro, J. B. et al. Conformational dynamics of single HIV-1 envelope trimers on the surface of native virions. Science 346, 759–763 (2014).
Herschhorn, A. et al. Release of gp120 restraints leads to an entry-competent intermediate state of the HIV-1 envelopeglycoproteins. m Bio 7, e01598-e16 (2016).
Ma, X. et al. HIV-1 Env trimer opens through an asymmetric intermediate in which individual protomers adopt distinct conformations. eLife 7, e34271 (2018).
Sanders, R. W. et al. A next-generation cleaved, soluble HIV-1 Env trimer, BG505 SOSIP.664gp140, expresses multiple epitopes for broadly neutralizing but not non-neutralizing antibodies. PLoS Pathog. 9, e1003618 (2013).
Julien, J. P. et al. Crystal structure of a soluble cleaved HIV-1 envelope trimer. Science 342, 1477–1483 (2013).
Lyumkis, D. et al. Cryo-EM structure of a fully glycosylated soluble cleaved HIV-1 envelope trimer. Science 342, 1484–1490 (2013).
Pancera, M. et al. Structure and immune recognition of trimeric pre-fusion HIV-1 Env. Nature 514, 455–461 (2014).
Wang, H. et al. Cryo-EM structure of a CD4-bound open HIV-1 envelope trimer reveals structural rearrangements of the gp120 V1V2 loop. Proc. Natl Acad. Sci. USA 113, E7151–E7158 (2016).
Bartesaghi, A., Merk, A., Borgnia, M. J., Milne, J. L. & Subramaniam, S. Prefusion structure of trimeric HIV-1 envelope glycoprotein determined by cryo-electron microscopy. Nat. Struct. Mol. Biol. 20, 1352–1357 (2013).
Gristick, H. B. et al. Natively glycosylated HIV-1 Env structure reveals new mode for antibody recognition of the CD4-binding site. Nat. Struct. Mol. Biol. 23, 906–915 (2016).
Kwon, Y. D. et al. Crystal structure, conformational fixation and entry-related interactions of mature ligand-free HIV-1 Env. Nat. Struct. Mol. Biol. 22, 522–531 (2015).
Lee, J. H., Ozorowski, G. & Ward, A. B. Cryo-EM structure of a native, fully glycosylated, cleaved HIV-1 envelope trimer. Science 351, 1043–1048 (2016).
Chuang, G. Y. et al. Structure-based design of a soluble prefusion-closed HIV-1 Env trimer with reduced CD4 affinity and improved immunogenicity. J. Virol. 91, e02268-e16 (2017).
Sakin, V. et al. A versatile tool for live-cell imaging and super-resolution nanoscopy studies of HIV-1 Env distribution and mobility. Cell Chem. Biol. 24, 635–645 (2017).
Sen, J., Jacobs, A. & Caffrey, M. Role of the HIV gp120 conserved domain 5 in processing and viral entry. Biochemistry 47, 7788–7795 (2008).
Alsahafi, N., Debbeche, O., Sodroski, J. & Finzi, A. Effects of the I559P gp41 change on the conformation and function of the human immunodeficiency virus (HIV-1) membrane envelope glycoprotein trimer. PLoS ONE 10, e0122111 (2015); correction 10, e0129405 (2015).
Pancera, M. et al. Crystal structures of trimeric HIV envelope with entry inhibitors BMS-378806 and BMS-626529. Nat. Chem. Biol. 13, 1115–1122 (2017).
Caskey, M. et al. Viraemia suppressed in HIV-1-infected humans by broadly neutralizing antibody 3BNC117. Nature 522, 487–491 (2015).
Caskey, M. et al. Antibody 10-1074 suppresses viremia in HIV-1-infected individuals. Nat. Med. 23, 185–191 (2017).
Nishimura, Y. et al. Early antibody therapy can induce long-lasting immunity to SHIV. Nature 543, 559–563 (2017).
Sok, D. et al. Rapid elicitation of broadly neutralizing antibodies to HIV by immunization in cows. Nature 548, 108–111 (2017).
Gardner, M. R. et al. AAV-expressed eCD4-Ig provides durable protection from multiple SHIV challenges. Nature 519, 87–91 (2015).
Castillo-Menendez, L. R., Nguyen, H. T. & Sodroski, J. Conformational differences between functional human immunodeficiency virus envelope glycoprotein trimers and stabilized soluble trimers. J. Virol. 93, e01709–e01718 (2019).
Tang, G. et al. EMAN2: an extensible image processing suite for electron microscopy. J. Struct. Biol. 157, 38–46 (2007).
Lin, C. W. & Ting, A. Y. Transglutaminase-catalyzed site-specific conjugation of small-molecule probes to proteins in vitro and on the surface of living cells. J. Am. Chem. Soc. 128, 4542–4543 (2006).
Yin, J., Lin, A. J., Golan, D. E. & Walsh, C. T. Site-specific protein labeling by Sfp phosphopantetheinyl transferase. Nat. Protoc. 1, 280–285 (2006).
Juette, M. F. et al. Single-molecule imaging of non-equilibrium molecular ensembles on the millisecond timescale. Nat. Methods 13, 341–344 (2016).
Dave, R., Terry, D. S., Munro, J. B. & Blanchard, S. C. Mitigating unwanted photophysical processes for improved single-molecule fluorescence imaging. Biophys. J. 96, 2371–2381 (2009).
Aitken, C. E., Marshall, R. A. & Puglisi, J. D. An oxygen scavenging system for improvement of dye stability in single-molecule fluorescence experiments. Biophys. J. 94, 1826–1835 (2008).
McKinney, S. A., Joo, C. & Ha, T. Analysis of single-molecule FRET trajectories using hidden Markov modeling. Biophys. J. 91, 1941–1951 (2006).
Zhong, P. et al. Cell-to-cell transmission can overcome multiple donor and target cell barriers imposed on cell-free HIV. PLoS ONE 8, e53138 (2013).
Poss, M. & Overbaugh, J. Variants from the diverse virus population identified at seroconversion of a clade A human immunodeficiency virus type 1-infected woman have distinct biological properties. J. Virol. 73, 5255–5264 (1999).
Acknowledgements
We thank A. B. Ward, R. W. Sanders and P. J. Bjorkman for discussions, R. Blakemore for assistance with molecular modelling, D. Burton and M. Feinberg for reagents including PGT121, PGT122, PGT145, PGT151 and NC-Cow1, NC-Cow8, NC-Cow9 and NC-Cow10 antibodies, and the AIDS Research and the Reference Reagent Program (Division of AIDS, NIAID, NIH) for the antibodies 3BNC117, 10-1074, PG9 and PG16. This work was supported by NIH grants RO1 GM116654 and UM1 AI100645 to W.M., RO1 GM098859 to S.C.B., RO1 AI124982 and RO1 AI100645 to J.G.S., K22 AI116262 to J.B.M., CRC Tier 2 RCHS0235 and a CIHR foundation grant 352417 to A.F., PO1 GM056550 to W.M., J.G.S., S.C.B, A.B.S. and C.A., by a Brown Coxe Fellowship to M.L., a fellowship from the China Scholarship Council-Yale World Scholars to X.M., by the International AIDS Vaccine Initiative’s (IAVI’s) Neutralizing Antibody Consortium to P.D.K. and by the Intramural Research Program of the Vaccine Research Center (NIAID, NIH) to P.D.K. and A.B.M., and by the SFB1129 and the Emmy-Noether programme (project number 317530061) of the German Research Foundation to E.A.L. and I.N.-S., respectively.
Reviewer information
Nature thanks David Millar, Alexandra Trkola and the other anonymous reviewer(s) for their contribution to the peer review of this work.
Author information
Authors and Affiliations
Contributions
M.L., X.M., L.R.C.-M., N.A., J.G., J.B.M., A.F., P.D.K., S.C.B., J.G.S. and W.M. conceived these experiments. M.L., X.M., U.E., N.R., K.W., J.R.G., and B.P.C. performed mutagenesis, and M.L. and X.M. generated fluorescently labelled viruses. M.L., X.M. and D.S.T. performed labelling and smFRET imaging. M.L., X.M., S.C.B. and W.M. analysed the data. L.R.C.-M., J.G., M.C., N.A., A.F., D.P., B.Z., T.Z. and A.B.M. performed protein expression, purification and antibody-binding experiments. L.R.C.-M. performed negative-stain electron microscopy. J.B.M. and C.A. performed photophysical measurements and molecular dynamics simulations. A.S., J.A., A.B.S.III, I.N.-S., E.A.L., M.R.G. and M.F. provided reagents. M.L., P.D.K, S.C.B., J.G.S. and W.M. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
S.C.B. holds an equity interest in Lumidyne Technologies. M.F. is a co-founder of Emmune, a company developing eCD4-Ig for clinical use. E.A.L. holds the patent WO 2012/104422 A1. S.C.B., J.B.M. and W.M. hold the patent US 959385324 B2.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
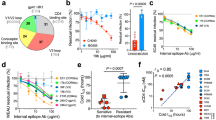
Extended Data Fig. 1 Tagged BG505 sgp140 SOSIP.664 proteins largely retain their known immunogenic features and preferentially sample state-2-like conformations.
a, Schematics for wild-type (WT) BG505 Env and BG505 sgp140 SOSIP.664 with D7324 affinity tag; V1–Q3 peptide in green, V4–A1 peptide in red. b, Validation of tagged BG505 sgp140 SOSIP.664. Top, antigenic profile of 100% untagged (WT), 100% double-tagged V1V4 (V1–Q3 V4–A1), and 20:1 of untagged to double-tagged BG505 sgp140 SOSIP.664. Binding by the indicated VRC01, 17b, PG9, 19b, PGT151 and 902090 antibodies was assessed from two independent ELISA assays in hexaplets and displayed as percentage of 2G12 binding (mean ± s.d.). The epitope for the antibody 902090 was more exposed in the 100%-tagged BG505 sgp140 SOSIP.664 than in the untagged BG505 sgp140 SOSIP.664, although this was not the case for the 1:20 tagged:wild-type trimers used for our smFRET analyses. The insertion of the Q3 tag into all three V1 regions of Env may exert local effects on the V2 β-barrel that contains the 902090 epitope (residues 171–177). Bottom, reference-free negative-staining electron microscopy two-dimensional class averages with representative trimeric density map of the BG505 sgp140 SOSIP.664 (wild type:V1V4-tagged at a 20:1 ratio) used for smFRET imaging. A Fourier shell correlation is also provided. c, Antigenic characteristics of BG505 sgp140 DS-SOSIP.664 (left) and 100% V1V4-tagged BG505 DS-SOSIP.Mut4 (right), determined by MSD. Antibodies are labelled. CD4bs, CD4 binding site; CD4i, CD4-induced; V1V2, V1V2-directed; V3, V3 glycan site-directed; gp120/gp41, interface between gp120 and gp41. Antigenic profiles of BG505 DS-SOSIP.664 (left) and 100% V1V4-tagged DS-SOSIP.Mut4 (right) after V3-negative selection were assessed by a panel of CD4-induced antibodies (17 and 48b, with and without sCD4), CD4 binding site antibodies (VRC01, VRC03, b12 and weakly neutralizing F105), V1V2-directed antibodies (PGT145 and VRC26.25), V3 glycan site-directed antibodies (2G12, PGT121, PGT128) and weakly neutralizing V3-directed antibodies (447-52D, 3074 and 2557, with and without soluble CD4), gp41–gp120 interface antibodies (PGT151, 35O22, 8ANC195 and VRC34.01) and the negative-control antibody CR9114 (an influenza virus antibody that does not recognize HIV-1 Env). ECL, electrochemiluminescence. d, The indicated BG505 sgp140 SOSIP.664 variants exhibit predominantly state-2-like conformations. FRET histograms for V1V4-tagged BG505 sgp140 SOSIP.664 with molecules after V3-negative selection (left), and for the stabilized BG505 sgp140 SOSIP.664 variant DS-SOSIP.Mut420 (right) (see Methods). Histograms represent mean ± s.e.m., determined from three independent populations of smFRET traces.
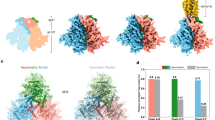
Extended Data Fig. 2 Binding of PGT151 stabilizes a state-2-like conformational state of HIV-1 Env.
a, Structure of HIV-1JR-FL Env(ΔCT) in complex with PGT15119. Two PGT151 antigen-binding fragments are distant from the positions of the gp120 variable loops (V1 and V4) that carry the fluorophores. b, Population FRET histograms of unliganded HIV-1JR-FL Env(ΔCT), and HIV-1JR-FL Env(ΔCT) in the presence of 10 μg ml−1 sCD4D1D2–Igαtp. c–f, Addition of PGT151 at neutralizing concentrations (10 μg ml−1) shifts the conformational landscapes for enzymatically labelled HIV-1JR-FL (c, e) and HIV-1BG505 (d, f) from the unliganded preference towards state 1 (red solid lines) to a preference for state 2 (blue solid lines). g, The addition of PGT151 to BG505 sgp140 SOSIP.664 did not alter the dominance of the state-2-like conformation exhibited in the absence of PGT151. h, Schematic of use of amber-suppressor tRNAs to introduce unnatural amino acids that can be clicked with fluorophores (h, top; see Methods), and schematic comparison between the Q3 and A1 double tag used for enzymatically labelling and click-labelling of V1 and V4 of HIV-1JR-FL (h, middle) and HIV-1BG505 Env (h, bottom). To introduce the unnatural amino acid TCO*, Asn136 in the V1 loop of HIV-1JR-FL or Ser401 in the V4 loop of HIV-1BG505 was genetically altered to an amber (TAG) stop codon. i–l, Experiment as in c–f, characterizing the conformational landscape upon binding of PGT151 to click-labelled HIV-1JR-FL V1–Asn136TAG V4–A1 (i, k), and HIV-1BG505 V1–Q3 V4–Ser401TAG (j, l). Neutralization data (mean ± s.d.) are averaged from three independent experiments in triplicates (c, d, i, j). FRET population histograms represent mean ± s.e.m., determined from three independent populations of smFRET traces.
Extended Data Fig. 3 SOS and I559P effects on infectivity and conformational plasticity of sgp140 SOSIP.664.
a, SOS and/or I559P (IP) changes introduced into native HIV-1BG505 Q23 Env do not influence Env processing or virus incorporation. Env expression, processing and virus incorporation for HIV-1BG505 Q23 carrying SOS, I559P and SOS and I559P (SOS&IP) changes were tested by centrifugation of viruses from cell culture supernatants, followed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis in the presence of dithiothreitol, and western blotting using the antiserum to HIV-1 gp120 (NIH AIDS reagent no. 288) and HIV-1 p24 monoclonal antibody (NIH AIDS reagent no. 3537). Experiments were repeated twice. b, The structure-stabilizing modifications A501C and T605C (SOS) and I559P used in the design of BG505 sgp140 SOSIP.664 abort HIV-1 infectivity. Infectivity of HIV-1BG505 Q23 SOS and I559P was measured by a Gaussia Luciferase assay, and normalized to that of wild-type HIV-1BG505 Q23. c, FRET histogram of HIV-1JR-FL Env carrying SOS, confirming similar data for HIV-1BG505 Env that the SOS change is largely responsible for the state 2 stabilization of Env on virus. d, e, FRET histograms of HIV-1BG505 Env in the absence (unliganded, d) or the presence of sCD4D1D2–Igαtp (e). f, g, Experiments as in d, e for BG505 sgp140 SOSIP.664. h–k, FRET histograms of HIV-1BG505 Env and BG505 sgp140 SOSIP.664 in the presence of the entry inhibitors BMS-378806 (h, i) and BMS-626529 (j, k). l–m, Neutralization of HIV-1BG505 by sCD4D1D2–Igαtp (l), BMS-378806 (m) and BMS-626529 (n). Red arrows indicate concentrations used in smFRET experiments. Histograms correspond to those in the main figures: in d (Fig. 1e), e (Fig. 2b, top), f (Fig. 1f), g (Fig. 2c, top), j (Fig. 2b, bottom) and k (Fig. 2c, bottom). Infectivity and neutralization curves represent mean ± s.d. from three replicates in triplicates. FRET population histograms represent mean ± s.e.m., determined from three independent populations of smFRET traces.
Extended Data Fig. 4 Conformational remodelling of HIV-1BG505 and BG505 sgp140 SOSIP.664 by sCD4D1D2–Igαtp and BMS-626529.
a, b, Examples of fluorescence traces of BG505 sgp140 SOSIP.664 in the presence of 10 μg ml−1 sCD4D1D2–Igαtp (a), and 100 μM BMS-626529 (b). Arrows indicate single-step photobleaching events that define the background of our smFRET assay. c–e, Transition density plots of HIV-1BG505 in the absence (c) or presence (d) of sCD4D1D2–Igαtp, or in the presence of BMS-626529 (e). Transition density plots that indicate the relative frequency of state-to-state transitions were generated from individual traces (180 traces in Fig. 1e, 147 traces in Fig. 2b (top), and 116 traces in Fig. 2b (bottom)). n, number of total transitions observed. f–h, Transition density plots of BG505 sgp140 SOSIP.664 under the same experimental conditions as those shown in c–e.
Extended Data Fig. 5 Many bNAbs neutralize and exhibit preference for the state 1 conformation of HIV-1.
a–c, Neutralization of native HIV-1BG505 by bNAbs that recognize different Env epitopes: V3 glycan site-directed bNAbs 10-1074, PGT121 and PGT122 (a); CD4 binding site bNAbs 3BNC117, VRC01 and VRC03 (b); and V1V2 glycan bNAbs PG9, PG16 and PGT145 (c). Only bNAbs that potently neutralize HIV-1BG505 or HIV-1JR-FL and allowed smFRET imaging at an antibody concentration 5 times above the 95% inhibitory concentration were analysed further (Fig. 3b, d). Neutralization data (mean ± s.d.) were averaged from three independent experiments in triplicate. d, FRET histogram that shows that HIV-1BG505 Env remains in state 1 in the presence of PG9 (50 μg ml−1). FRET population histograms represent mean ± s.e.m., determined from three independent populations of smFRET traces.
Extended Data Fig. 6 Conformational preferences of non-neutralizing antibodies for HIV-1 Env on virus.
a, b, FRET histograms and overlaid landscapes of HIV-1NL4-3 in the presence of 100 μg ml−1 17b (a) and 100 μg ml−1 F105 (b) acquired after 0 min, 30 min and 60 min of incubation. c, d, FRET histograms and overlaid landscapes of HIV-1BG505 in the presence of 17b (c) and F105 (d), acquired after 0 min, 30 min and 60 min of incubation. Non-neutralizing antibodies have preference for the state 3 conformation of Env. Note that in contrast to the tier 1 HIV-1 isolate NL4-3, the tier 2 isolate BG505 does not respond to 17b. FRET population histograms represent mean ± s.e.m., determined from three independent groups of smFRET traces.
Extended Data Fig. 7 Antibodies isolated from cows immunized using BG505 sgp140 SOSIP.664 immunogens exhibit a preference for state 2.
a, FRET histogram of HIV-1BG505(T332N). b, Neutralization curves of HIV-1BG505 by NC-Cow1, NC-Cow8, NC-Cow9 and NC-Cow10 antibodies. Data are presented as mean ± s.d. determined from three independent experiments in triplicate. c–e, FRET histograms of native HIV-1BG505 in the presence of 10 μg ml−1 NC-Cow1 (c), NC-Cow8 (d) and NC-Cow10 (e). f, The FRET histogram of HIV-1BG505 that carries the T332N substitution in Env is overlaid with that of wild-type HIV-1BG505. The T332N substitution in HIV-1BG505 Env does not detectably change the conformation of the Env. g, h, Cow antibodies (NC-Cow1, NC-Cow8, NC-Cow9 and NC-Cow10) shift the conformational landscape of native Env on the virus from state 1 towards that of BG505 sgp140 SOSIP.664 (state 2). FRET population histograms (a, c–e) represent mean ± s.e.m., from three independent populations of smFRET traces. i, j, Ligand preferences for states 1 and 2 probed by antibody staining of cell-expressed HIV-1JR-FL Env(ΔCT). Increasing amounts of the first ligand were pre-bound to cells for 1 h. The cells were washed, incubated with the second dye-labelled probe for 30 min, and the binding was quantified by flow cytometry. The ratio of measured mean fluorescence intensity (MFI) was normalized to that seen in the absence of pre-bound ligand (Methods). Matched combinations (state 1 and state 1 or state 2 and state 2) and non-matched combinations (state 1 and state 2 or state 2 and state 1) at the highest concentration of pre-bound first ligand were compared, and statistical significance was evaluated using a paired Student’s two-sided t-test. *P < 0.05. Note that the strong interference between 3BNC117 and PGT151 is due to a steric clash between the two antibodies, and was included as a control.
Extended Data Fig. 8 Validating the behaviour of dyes used for smFRET.
a, The 50-ns molecular dynamics simulations of fluorophore tumbling on the BG505 sgp140 SOSIP.664 trimer (4ZMJ) shows that dyes in V1 and V4 are far from the viral membrane. Molecular dynamics simulation was performed to account for movements of loops, enzymatic labelling tags, linkers and dyes to describe the possible dye tumbling space within 50 ns. The sampled space was docked into the approximately 20 Å structure of the HIV-1 virus Env spike determined by cryo-electron tomography4. A 50-ns molecular dynamics simulation is not temporally comparable to the time resolution of single-molecule imaging at 40 ms, or the timescale of observed conformational changes of Env (milliseconds to seconds). b–d, Conformational properties of the HIV-1BG505 Env remain highly similar when the dyes are flipped. b, Reference FRET histograms of HIV-1BG505 that carries Cy3B in V1 and Cy5 in V4, in unliganded form (from Fig. 1e), in the presence of PGT151 (from Fig. 1h) or in the presence of sCD4D1D2–Igαtp (from Fig. 2b). c, FRET histograms of HIV-1BG505 Env that carries Cy5 in V1 and Cy3B in V4 (see Methods), in the absence and in the presence of 10 μg ml−1 PGT151 or 10 μg ml−1 sCD4D1D2–Igαtp, respectively. d, Overlaid conformational landscapes of HIV-1BG505 Env labelled as in c with flipped dyes (green), compared to HIV-1BG505 Env labelled as in b (red). FRET population histograms represent mean ± s.e.m., determined from three independent populations of smFRET traces.
Extended Data Fig. 9 Suppressed HIV-1JR-FL that carries amber positions in gp120 and gp41 enables smFRET imaging of Env from two distinct perspectives.
a, Schematic of tagged sites in HIV-1JR-FL Env that were used for enzymatic labelling and amber stop codon (TAG)-suppressed incorporation of unnatural amino acids for click labelling. HIV-1JR-FL V1–Q3 V4–A1 carries the Q3 peptide in the V1 loop and the A1 peptide in the V4 loop. HIV-1 JR-FL V1–Asn136TAG V4–A1 carries a TAG at position Asn136 in V1 and the A1 peptide in V4. HIV-1JR-FL V4–A1 α6–Arg542TAG carries the A1 tag in gp120 V4 and a TAG at Arg542 in the α6 helix of gp41. b, c, Neutralization of HIV-1JR-FL wild type, 100%-peptide-tagged V1–Q3 V4–A1, 100%-amber-suppressed V1–Asn136TAG V4–A1 and V4–A1 α6–Arg542TAG by sCD4D1D2–Igαtp (b), and eCD4-Ig(Q40A, mim2) (c). Neutralization curves (b, c) represent mean ± s.d. from three replicates in triplicates.
Supplementary information
Supplementary Table 1
This table shows the fluorescence lifetime of free and conjugated Cy3 and Cy5 used in smFRET imaging.
Rights and permissions
About this article
Cite this article
Lu, M., Ma, X., Castillo-Menendez, L.R. et al. Associating HIV-1 envelope glycoprotein structures with states on the virus observed by smFRET. Nature 568, 415–419 (2019). https://doi.org/10.1038/s41586-019-1101-y
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41586-019-1101-y
This article is cited by
-
Conformational trajectory of the HIV-1 fusion peptide during CD4-induced envelope opening
Nature Communications (2025)
-
The membrane-proximal external region of human immunodeficiency virus (HIV-1) envelope glycoprotein trimers in A18-lipid nanodiscs
Communications Biology (2025)
-
The KT Jeang retrovirology prize 2024: Walther Mothes
Retrovirology (2024)
-
Simulation-driven design of stabilized SARS-CoV-2 spike S2 immunogens
Nature Communications (2024)
-
HIV-1 Env trimers asymmetrically engage CD4 receptors in membranes
Nature (2023)

John P Moore
Lu et al. report that recombinant HIV-1 envelope glycoprotein (Env) immunogens, BG505 SOSIP.664 trimers, differ from the native (virion-associated) form of the Env trimer, as judged by an smFRET assay of conformational flexibility1. Specifically, smFRET reports that probe-modified
BG505 SOSIP.664 trimers occupy three conformational states but predominantly state 2, whereas the Env proteins on BG505 HIV-1 virions are mostly in state 1. Both forms of Env occasionally adopt a “fully open” state 3 conformation that resembles the CD4 receptor-bound structure1. The authors argue that because recombinant SOSIP.664 trimers are not in what they define as the state 1 conformation, they may be sub-optimal immunogens for inducing broadly neutralizing antibodies (bNAbs)1.
It is inevitable that SOSIP.664 trimers differ from virion Env. The recombinant trimers are engineered to lack the transmembrane and cytoplasmic domains that are present in the native trimer, and also to contain stabilizing changes2,3. These modifications are collectively necessary to produce an antigenically relevant immunogen in practical amounts3. As a result, my colleagues and I consistently refer to SOSIP.664 trimers as “native-like”, and have never claimed that they are identical to the truly native Env structure on infectious viruses2-4. The question arises, however, as to whether the various conclusions drawn by Lu et al. based on smFRET data are both appropriate and relevant to immunogen design1.
Eight months ago, the Bjorkman group published a study of SOSIP
trimers involving an alternative spectroscopy technique to assess protein
conformational transitions: double electron-electron resonance (DEER) spectroscopy5. The use of this method, together with knowledge of the smFRET data previously published and/or presented at multiple
scientific conferences over a more than 2-year period, led to the following
report: There is “a single pre-dominant state for the closed BG505 SOSIP apex, rather than the three states interpreted from the smFRET studies of unliganded virion-bound Env (Munro et al., 2014). This DEER result was
consistent with closed SOSIP (Ward and Wilson, 2017) and non-SOSIP (Lee et al., 2016) Env structures, as well as the closed, unliganded Env
conformation on virions (Liu et al., 2008), and revealed no evidence for distinct conformational states with respect to the V1V2 – V4 distance in the absence of sCD4 as suggested by smFRET (Ma et al., 2018; Munro et
al., 2014).” The authors also concluded that the single distance measured between DEER probes in Env locations near the smFRET probes must correspond to smFRET state 2 (i.e., highest FRET, smallest inter-probe distance) because “…steric constraints should prevent shorter distances separating V1 and V4” 5. In summary, the DEER experiments showed that BG505 SOSIP.664 trimers were in a single conformational state with respect to the trimer apex and the distance between the probes in the V1 and V4 loops5. Moreover, all of the distances measured using DEER were consistent with the x-ray crystallography and cryo-EM structures of various SOSIP trimers whereas smFRET reports that the same trimers transition between three different states1,5,6. Given how long ago the DEER spectroscopy paper was published (8/21/18) and its obvious relevance, it is hard to understand why Lu et al. ignored it in their paper (accepted 3/8/19)
when they acknowledge holding “discussions” with its senior author (P.J.
Bjorkman)1.
Why might the two spectroscopy techniques lead to different conclusions? The smFRET method involves the indirect capture of probe-modified SOSIP.664 trimers onto a solid phase in imaging chambers; when
added, antibody probes remain in contact with the trimers for prolonged periods1. The extent to which the V3 region at the SOSIP.664 trimer apex is accessible to non-neutralizing antibodies is quite assay-dependent,
with capture ELISA, SPR, NS-EM and ITC techniques yielding different answers2,3,6,7,8. Whether and how the trimers are immobilized, and the period of exposure to antibody probes, may influence how different assays report on the exposure of V3 and, by extension, the flexibility of the trimer apex. DEER spectroscopy, a solution-phase technique, reports that the V3 region of SOSIP.664 trimers is rigid5. That conclusion is consistent with data
derived by, e.g., NS-EM but not by capture ELISA or smFRET1,2,8. The multiple conformations of SOSIP.664 trimers detected by smFRET but not by DEER may, therefore, reflect subtle and perhaps unappreciated assay-dependent variables. Alternative approaches to how SOSIP trimers are used for smFRET research, including perhaps the provision of a membrane lipid environment may be worth pursuing (a membrane is present when smFRET
is used to study virion Env1).
Lu et al. cite chemical cross-linking studies on cell membrane-associated Env as supporting their conclusion that “SOSIP.664 proteins are in a conformation that is distinct from the native Env” 1,9. However, Env proteins on the surfaces of cells or virus-like particles are highly heterogeneous10-12.
As smFRET now reports that the native Env trimer on infectious virions is predominantly in state 1, either the smFRET data are misleading or cell membrane-associated Env is not an appropriate surrogate for the infectious virus. In either case, it seems imprudent to assert that the cross-linking and smFRET studies are mutually supportive1,9.
I concur with Lu et al.’s suggestion for more research on what forms of Env are best able to induce bNAbs1. Since the BG505 SOSIP.664 trimer was
described in 2013, several groups have identified dozens of structure-guided
design modifications that improve both the stability and antigenicity of
native-like soluble trimers,3.6,13-15. Further incremental improvements should continue to emerge. The smFRET data and interpretations have been presented at multiple meetings over the past 2-years. If this biophysical technique does eventually illustrate how to design and deliver better immunogens, then so much the better.
We should also consider that the immune system responds to adjuvanted, solution-phase proteins over a period measured in hours or days. The smFRET and DEER methods measure conformational transitions over a time scale of micro- to milli-seconds1,5. Any conformation that a HIV-1 Env trimer can adopt on that time scale will certainly be sampled by the immune system under the in vivo conditions relevant to vaccine delivery. The SOSIP.664 trimer and its later generation derivatives consistently present bNAb epitopes from every known cluster except the absent MPER, with high affinity, as shown by multiple techniques3,4,6,16. However, while the absence of a bNAb epitope(s) from an immunogen is clearly problematic, the presence of one does not mandate that it will induce similar antibodies; antigenicity is not the same as immunogenicity. The key difficulty in HIV-1 vaccine design is not nowadays the presentation of bNAb epitopes to the immune system, it is finding ways to persuade the host to respond to those epitopes. Virion Env, the form favored by Lu et al., induces bNAbs in only a minority of HIV-1 infected people after many years of constant antigenic stimulus accompanied by neutralization escape and multiple cycles of somatic
hyper-mutation14-17. It is highly unlikely that any purified HIV-1 Env protein, whether a SOSIP trimer or virion-derived, could mimic those processes to induce bNAbs by itself. Among other knowledge gaps, we must better understand how such highly glycosylated and atypical immunogens interact with antigen-presenting cells, T cells and B cells17-19. Various groups are already pursuing persuasive pathways to bNAb induction that harness both structural biology and immunology insights while using SOSIP trimers as a design platform3,14,15,17,18.
The clear discrepancies between the smFRET and DEER endpoints must be satisfactorily resolved so as to avoid adopting artefactual approaches to immunogen design. Of the two, only the DEER data are consistent with the multiple high-resolution structures of Env trimers, SOSIP and otherwise, observed by X-ray crystallography and electron microscopy. Cryo-EM is capable of detecting multiple conformations of proteins, even minority ones, but the many cryo-EM structures of closed SOSIP trimers have always revealed only a single, predominant conformation6,14,15.
John P. Moore, Weill Cornell Medical College, New York
1. Lu, S. et al. Associating HIV-1 envelope glycoprotein structures with states on the virus observed by smFRET. Nature 25, 578-587 (2019).
2. Sanders, R.W. et al. A next-generation cleaved, soluble HIV-1 Env trimer, BG505 SOSIP.664 gp140, expresses multiple epitopes for broadly neutralizing but not non-neutralizing antibodies. PLoS Pathog. 9, e1003618 (2013).
3. Sanders, R.W. & Moore, J. P. Native-like Env trimers as a platform for HIV-1 vaccine design. Immunol. Rev. 275, 161-182 (2017).
4. Crispin, M., Ward, A.B. & Wilson, I.A. Structure and immune recognition of the HIV glycan shield. Annu. Rev. Biophys. [Epub ahead of print] (2018).
5. Stadtmueller, B.M. et al. DEER spectroscopy measurements reveal multiple conformations of HIV-1 SOSIP envelopes that show similarities with envelopes on native virions. Immunity 49, 235-246 (2018).
6. Ward, A.B., & Wilson, I.A. The HIV-1 envelope glycoprotein structure: nailing down a moving target. Immunol. Rev. 275, 21-32 (2017).
7. Yasmeen, A. et al. Differential binding of neutralizing and non-neutralizing antibodies to native-like soluble HIV-1 Env trimers, uncleaved Env proteins, and monomeric subunits. Retrovirology 11, 41 (2014).
8. Pugach, P. et al. A native-like SOSIP.664 trimer based on a HIV-1 subtype B env gene. J. Virol. 89, 3380-3395. (2015).
9. Castillo-Menendez, L.R., Nguyen, H.T. & Sodroski, J. Conformational differences between functional human immunodeficiency virus
envelope glycoprotein trimers and stabilized soluble trimers. J. Virol. 93,
e01709-18 (2019).
10. Herrera, C. et al. Nonneutralizing antibodies to the CD4-binding site on the gp120 subunit of human immunodeficiency virus type 1 do not interfere with the activity of a neutralizing antibody against the same site. J. Virol. 77, 1084-1091 (2003).
11. Pancera, M. & Wyatt, R. Selective recognition of oligomeric HIV-1 primary isolate envelope glycoproteins by potently neutralizing ligands requires efficient precursor cleavage. Virology 332, 145-156 (2005).
12. Crooks, E.T., Tong, T., Osawam K. & Binley, J.M. Enzyme digests eliminate nonfunctional Env from HIV-1 particle surfaces, leaving native Env trimers intact and viral infectivity unaffected. J. Virol. 85, 5825-5839 (2011).
13. Torrents de la Peña, A. & Sanders, R.W. Stabilizing HIV-1
envelope glycoprotein trimers to induce neutralizing antibodies. Retrovirology 15, 63 (2018).
14. Kwong, P.D. & Mascola, J.R. HIV-1 vaccines based on antibody identification, B cell ontogeny, and epitope structure. Immunity 48, 855-871 (2018).
15. Andrabi, R., Bhiman, J.N. & Burton, D.R. Strategies for a multi-stage neutralizing antibody-based HIV vaccine. Curr. Opin. Immunol. 53,143-151 (2018).
16. Kadelka, C. et al. Distinct, IgG1-driven antibody response landscapes demarcate individuals with broadly HIV-1 neutralizing activity. J. Exp. Med. 215, 1589-1608 (2018).
17. McGuire, A.T. Targeting broadly neutralizing antibody precursors: a
naïve approach to vaccine design. Curr. Opin. HIV AIDS [Epub ahead of print] (2019).
18. Tokatlian, T. et al. Innate immune recognition of glycans targets HIV nanoparticle immunogens to germinal centers. Science 363, 649-654 (2019).
19. Heß, R. et al. Glycosylation of HIV Env impacts IgG subtype responses to vaccination. Viruses 11, E153 (2019).
Conflicts statement: John P. Moore is listed as a co-inventor of the BG505 SOSIP.664 trimer on a patent filed in 2013 by the International AIDS
Vaccine Initiative, a non-profit organization, but has had no financial benefit
from the patent.
Author contribution: John P. Moore wrote this article but benefited from the insights of several colleague
Walther Mothes Replied to John P Moore
We thank Dr. Moore for his thoughtful comments.
Understanding the nature and distribution of conformational states sampled by functional human immunodeficiency virus (HIV-1) envelope glycoproteins (Env) on the surface of viruses can guide the design of therapeutic and prophylactic interventions. Single-molecule fluorescence resonance energy transfer (smFRET) gave us technical access to the conformational states of Env on the surface of HIV-1 virions under natural conditions. This advance revealed that the predominant, pretriggered Env on the virus adopts a conformational State 1 that is distinct from currently available high-resolution Env trimer structures (1). Our observation that many broadly neutralizing antibodies and some small-molecule entry inhibitors recognize this pretriggered state provides a compelling reason to define this Env conformation. We have kept to a minimum speculation about what our discovery means for the design of HIV-1 Env immunogens. Clinical trials will provide ample opportunity to test experimentally how well antibodies elicited using current State-2 Env immunogens recognize native Env on viruses.
The conclusion from our smFRET studies that currently available high-resolution Env trimer structures predominantly exhibit a State 2-like conformation is supported by recent DEER investigations performed by the group of Pamela Bjorkman (2). Although, unlike smFRET, DEER cannot be used to study the conformational dynamics of the Env trimer or Env conformations on the surface of HIV-1 virions, Stadtmueller et al. did evaluate the conformation of soluble gp140 SOSIP.664 trimers. Both methods (smFRET and DEER) agree that the soluble gp140 SOSIP.664 trimers reside in a State 2 conformation and open into State 3 upon binding CD4. It is indeed remarkable that these two distinct biophysical approaches lead to such similar conclusions despite the many technical disparities of the two spectroscopic methods. As Stadtmueller et al. was published during the late stages of our manuscript review process, we overlooked the opportunity to reference this work. We will add a correction to our report to address this shortcoming.
With respect to smFRET and DEER measurements of soluble gp140 SOSIP.664 trimers, there are many reasons why smFRET may detect conformational heterogeneities at the Env trimer apex and a greater number of discrete conformations in both the unliganded and CD4-bound forms. Sample preparations, labeling and measurements are very different. The observations of different dynamic features by both methods suggest that further efforts, potentially leveraging the power of smFRET, DEER and Molecular Dynamics approaches, will be needed to delineate the full repertoire of HIV-1 Env conformational states leading to virus entry.
Going forward, we should not lose sight of the opportunities provided by our results. If, as smFRET suggests, an additional conformational state of Env predominates on the surface of primary HIV-1 virions, we should be able to visualize it using other structural methods. We envision using smFRET to explore why the State-1 conformation of Env is stabilized on the surface of viruses, and to use that knowledge to enrich State 1 for structural and immunologic studies.
Walther Mothes, Joe Sodroski and Scott Blanchard
1. Lu M, Ma X, Castillo-Menendez LR, Gorman J, Alsahafi N, Ermel U, Terry DS, Chambers M, Peng D, Zhang B, Zhou T, Reichard N, Wang K, Grover JR, Carman BP, Gardner MR, Nikic-Spiegel I, Sugawara A, Arthos J, Lemke EA, Smith AB, 3rd, Farzan M, Abrams C, Munro JB, McDermott AB, Finzi A, Kwong PD, Blanchard SC, Sodroski JG, Mothes W. 2019. Associating HIV-1 envelope glycoprotein structures with states on the virus observed by smFRET. Nature 568:415-419.
2. Stadtmueller BM, Bridges MD, Dam KM, Lerch MT, Huey-Tubman KE, Hubbell WL, Bjorkman PJ. 2018. DEER Spectroscopy Measurements Reveal Multiple Conformations of HIV-1 SOSIP Envelopes that Show Similarities with Envelopes on Native Virions. Immunity 49:235-246 e234.
John P Moore Replied to Walther Mothes
The senior authors of the paper by Lu et al. consider it
“remarkable that these two distinct biophysical approaches (i.e., smFRET and DEER) lead to such similar conclusions”. That opinion is not shared by spectroscopy experts I have consulted, so I encourage Nature readers to study both papers and draw their own conclusions. The smFRET assay reports that SOSIP trimers adopt three distinct conformations (States 1, 2, 3), whereas DEER shows there is only one (State 2) (refs 1,2). Religious wars have been fought over the differences between three deities and one.
The different output of the two assays is not a trivial matter that should be “overlooked” in the way the DEER paper was during the 7-month period between the publications of the two papers. Given the disparate outcomes, and the substantial likelihood that immobilization artefacts affect the smFRET system used by Lu et al., the wider spectroscopy community should consider assessing the relative merits of smFRET and DEER for the present purpose. In a similar controversy over the cryo-EM structure of a HIV-1 Env trimer (3,4), the opinions of otherwise uninvolved leaders in the cryo-EM field were decisive in determining where the truth lay (5,6,7).
Lu et al. seek to “enrich (virion-associated) State 1 for structural and immunologic studies”, a task some of the co-authors have been engaged in for many years now without any reported success. Virologists and immunologists familiar with HIV-1 infection and vaccine design have long been aware that the virus has evolved multiple mechanisms to minimize the induction of neutralizing antibodies (NAbs), particularly ones with the necessary breadth (bNAbs). It seems rather optimistic to assume that using virion-associated Env as a fixed-composition immunogen would be sufficient to induce bNAbs. In my earlier comment, I note
that “State-2 Env immunogens”, for example the DS-SOSIP (8) or SOSIP.664 (9) trimers now in clinical trials, are highly unlikely to do so either. However, I also refer to their use in more sophisticated immunogen design programs that
may offer a greater chance of a successful outcome.
John P. Moore, Weill Cornell
Medical College, New York
1. Lu, S. et al. Associating HIV-1 envelope glycoprotein structures with states on the virus observed by smFRET.
Nature 25, 578-587 (2019).
2. Stadtmueller, B.M. et al. DEER spectroscopy measurements reveal multiple conformations of HIV-1 SOSIP
envelopes that show similarities with envelopes on native virions. Immunity 49, 235-246 (2018).
3. Mao Y, Wang L, Gu C, Herschhorn A, Désormeaux A, Finzi A, Xiang SH, Sodroski JG. Molecular architecture of the uncleaved HIV-1 envelope glycoprotein trimer. Proc Natl Acad Sci U S A 110, 12438-12443 (2013).
4. Mao Y, Wang L, Gu C, Herschhorn A, Xiang SH, Haim H, Yang X, Sodroski J. Subunit organization of the
membrane-bound HIV-1 envelope glycoprotein trimer. Nat Struct Mol Biol 19, 893-899 (2012).
5. Subramaniam S. Structure of trimeric HIV-1 envelope glycoproteins. Proc Natl Acad Sci U S A 110, E4172-4174 (2013).
6. Van Heel M. Finding trimeric HIV-1 envelope
glycoproteins in random noise. Proc Natl Acad Sci U S A 110, E4175-4177 (2013).
7. Henderson R. Avoiding the pitfalls of single particle cryo-electron microscopy: Einstein from noise. Proc Natl Acad Sci U S A 110, 18037-18041 (2013).
8. Chuang GY, Geng H, Pancera M, Xu K, Cheng C, Acharya P, Chambers M, Druz A, Tsybovsky Y, Wanninger TG, Yang Y,
Doria-Rose NA, Georgiev IS, Gorman J, Joyce MG, O'Dell S, Zhou T, McDermott AB, Mascola JR, Kwong PD. Structure-based design of a soluble prefusion-closed HIV-1 Env trimer with reduced CD4 affinity and improved immunogenicity. J Virol 91, e02268-16 (2017).
9. Dey AK, Cupo A, Ozorowski G, Sharma VK, Behrens AJ, Go EP, Ketas TJ, Yasmeen A, Klasse PJ, Sayeed E, Desaire
H, Crispin M, Wilson IA, Sanders RW, Hassell T, Ward AB, Moore JP. cGMP production and analysis of BG505 SOSIP.664, an extensively glycosylated, trimeric HIV-1 envelope glycoprotein vaccine candidate. Biotechnol Bioeng 115, 885-899 (2018).