Abstract
Gas exchange and ion regulation at gills have key roles in the evolution of vertebrates1,2,3,4. Gills are hypothesized to have first acquired these important homeostatic functions from the skin in stem vertebrates, facilitating the evolution of larger, more-active modes of life2,3,5. However, this hypothesis lacks functional support in relevant taxa. Here we characterize the function of gills and skin in a vertebrate (lamprey ammocoete; Entosphenus tridentatus), a cephalochordate (amphioxus; Branchiostoma floridae) and a hemichordate (acorn worm; Saccoglossus kowalevskii) with the presumed burrowing, filter-feeding traits of vertebrate ancestors6,7,8,9. We provide functional support for a vertebrate origin of gas exchange at the gills with increasing body size and activity, as direct measurements in vivo reveal that gills are the dominant site of gas exchange only in ammocoetes, and only with increasing body size or challenges to oxygen supply and demand. Conversely, gills of all three taxa are implicated in ion regulation. Ammocoete gills are responsible for all ion flux at all body sizes, whereas molecular markers for ion regulation are higher in the gills than in the skin of amphioxus and acorn worms. This suggests that ion regulation at gills has an earlier origin than gas exchange that is unrelated to vertebrate size and activity—perhaps at the very inception of pharyngeal pores in stem deuterostomes.
Similar content being viewed by others
Main
Pharyngeal gill arches and their derivatives have key roles in vertebrate evolution1,2,3,4,10. In early vertebrates and fishes, many of these roles relate to gills functioning as the primary site of gas exchange and ion regulation1,2,3,4. However, when and how vertebrate gills acquired these homeostatic functions is unclear.
Gas exchange and ion regulation are at present hypothesized to first recruit gills in stem vertebrates2,3,5. In this classic scenario, vertebrate ancestors used gills to filter-feed and were constrained to a small, worm-like existence by relying on the skin to breathe and to regulate the acid–base ion balance. Shifting these homeostatic functions to the gills relaxed these constraints, and facilitated the evolution of larger, armoured and more-active modes of life.
Support for this point of view is compelling but incomplete. Fossil and developmental studies support a stem vertebrate origin for structures that enhance gas exchange at gills8,11,12,13,14,15,16. However, these structures might simply enhance an already dominant capacity at gills, and there are no equivalent data for ionoregulatory structures. Work in larval teleosts provides some functional support, as gas exchange and ion regulation shift from the skin to the gills with increasing body size, dermal thickness and activity during embryonic development17,18. However, these data are potentially confounded by rapid developmental changes in morphology and behaviour, and/or derived aspects of teleost morphology and life history.
Here we characterize gill and skin function in a vertebrate (lamprey ammocoete; E. tridentatus), a cephalochordate (amphioxus; B. floridae) and a hemichordate (acorn worm; S. kowalevskii) that have small, worm-shaped bodies and the burrowing, filter-feeding traits of vertebrate ancestors2,6,7,9,19. Our results support a vertebrate origin for gas exchange at gills with increasing body size and activity, but an earlier chordate or deuterostome origin for ion regulation at gills. These findings are a functional complement to existing fossil and developmental data with regard to when and how vertebrate gills acquired their primary role in gas exchange, but they question the origins of ion homeostasis in vertebrates.
Gas exchange and ion regulation in ammocoetes
We first sought to test the effects of body size, dermal thickness and activity on the recruitment of gills for gas exchange and ion regulation. Ammocoetes, or at least a prolonged filter-feeding life stage, are likely to be a derived feature of modern lampreys20. However, we chose them as our model system because they closely resemble the presumed morphology and life history of vertebrate ancestors in being small, worm-shaped burrowers with filter-feeding gills21. Even if these traits were acquired secondarily through convergent evolution, they are likely to constrain the function of gills and skin for gas exchange and ion regulation in similar ways, and the absence of these traits in larval teleosts is a major limitation of previous work5. Post-embryonic ammocoetes are also a better test than larval teleosts for how dermal thickness (t) and body surface-area-to-volume ratio (SA:V) affect gill function, as they capture a tenfold drop in the diffusive capacity of the skin (SA t−1 g−1) with little change to morphology or behaviour in this slow-growing, years-long life stage21 (Extended Data Fig. 1).
We used a divided chamber to directly measure contributions by skin and gills to different gas and ion fluxes simultaneously in vivo. Measurements were taken at rest (normoxia; 10 °C) and during acute challenges to oxygen supply (hypoxia; 20 °C) and demand (normoxia; 26 °C) to simulate activity and promote maximum gill recruitment. We predicted that gas exchange and ion regulation would shift from the skin to the gills with increasing body size and challenges to oxygen supply and demand.
Gas exchange at gills appears to be driven by increasing body size and activity in post-embryonic ammocoetes (Fig. 1). Oxygen uptake occurred primarily across the skin of our smallest ammocoetes in all conditions, and gill recruitment increased progressively with body size, temperature and hypoxia. Results for ammonia (Fig. 1) and a representative sample of CO2 excretion (Extended Data Fig. 2a) mirror those for oxygen uptake at rest, and were thus not measured during thermal or hypoxic challenges. Total flux rates for gases (Extended Data Fig. 3a,b) are consistent with previous measurements in free-swimming ammocoetes22, which suggests that our experimental preparation did not affect gas exchange in any unexpected way. This includes a close match between post-exercise rates of oxygen uptake and those measured during our acute thermal challenge, supporting this treatment as a proxy for maximum activity. To our knowledge, we are the first to measure CO2 excretion in ammocoetes in water, but the respiratory exchange ratio of around 0.7 is within the expected range.
Percentage of total body oxygen uptake (blue; n = 40), ammonia excretion (orange; n = 36) and sodium uptake (pink; n = 18) occurring at the gills in normoxia at 10 °C. Grey and white symbols indicate the percentage of total body oxygen uptake occurring at the gills in thermal (normoxia at 26 °C; n = 26) and hypoxic (4 kPa partial pressure of oxygen (PO2) at 20 °C; n = 22) challenges, respectively. Data are presented as individuals and expressed as a function of body mass (g). Linear regression lines with 95% confidence intervals are fitted to each flux for each treatment. A multiple linear regression analysis of oxygen uptake with ANOVA finds a significant effect of body mass (P < 0.0001) and treatment (P < 0.0001), but no interaction (P = 0.2356, F = 1.471).
Conversely, ion regulation at the gills appears to be unrelated to body size and activity in post-embryonic ammocoetes (Fig. 1). All ion uptake in ammocoetes occurred at the gills, and there was no evidence for a shift from the skin. This is especially notable because our smallest ammocoetes had a body SA:V, dermal thickness and rate of sodium uptake that were similar to those of newly hatched salmonids, which acquire 80% of sodium at the skin17,23 (Extended Data Figs. 1 and 3c). A representative sample for calcium uptake in our smallest ammocoetes was also entirely at the gills (Extended Data Fig. 2a), and although the relative levels of calcium uptake by skin and by gills in larval teleosts are unknown, our total uptake rates of around 3 nmol g−1 h−1 (Extended Data Fig. 2c) are consistent with rainbow trout acclimated to similar levels of environmental calcium24. The uncoupling of sodium uptake from ammonia excretion differs from observations in freshwater teleosts25. However, the reliance of ammocoetes on the skin for a large share of ammonia excretion is consistent with reports in Petromyzon marinus26. To our knowledge, these are the first direct measurements of ion uptake in lampreys at any life stage.
Pre-vertebrate origins of gill function
The complete absence of ion flux at the skin of even our smallest ammocoetes suggests that the evolutionary origins of ion regulation at vertebrate gills might be unrelated to body size, dermal thickness or activity, and/or that they might precede the origin of vertebrates. However, these results are from a single taxon, and despite resembling vertebrate ancestors in many ways, ammocoetes have specialized and derived traits that may confound our results. Specifically, their freshwater residency and osmoregulatory physiology might increase gill recruitment for ion regulation in a way that deviates from the ancestral condition1.
A pre-vertebrate origin for gas exchange at gills is also possible. Gills were not the primary site of gas exchange in our smallest ammocoetes, but they did contribute to a substantial extent, especially in hypoxia (around 45–50%). Hypoxia is common in the presumed burrowing lifestyle of vertebrate ancestors and could have selected for gas exchange at gills before the origin of vertebrates and their transition to larger, more-active modes of life. However, vertebrate-specific pharyngeal adaptations that enhance gill ventilation, perfusion and diffusion may underlie the observed contributions (muscular pharyngeal pump, pillar cells and so on)8.
To test for pre-vertebrate origins of gas exchange and ion regulation at gills, we next characterized gill function in the hemichordate S. kowalevskii and cephalochordate B. floridae. We inferred a common ancestry for gill function shared between these representatives and ammocoetes. The relatively simple gill bars of S. kowalevskii and B. floridae differ from the more-elaborate gill arches and lamellae of ammocoetes, but comparisons between these gill structures are strengthened by a shared developmental origin from homologous outpockets of pharyngeal endoderm7,19. As with ammocoetes, S. kowalevskii and B. floridae resemble vertebrate ancestors in being small, worm-like burrowers with filter-feeding gills. However, they are marine osmoconformers that lack the vertebrate pharyngeal adaptations that enhance gill ventilation, perfusion and diffusion, thus addressing many of the caveats associated with ammocoetes.
Gas exchange in acorn worms
The acorn worm S. kowalevskii was too fragile for the divided chamber that was used to measure gill recruitment for gas exchange in ammocoetes. We instead used the regenerative capacity of S. kowalevskii27 to separate worms into viable halves with and without gills for respirometry. We predicted that if S. kowalevskii gills were a primary site of gas exchange, then whole worms and anterior worm halves with gills would have a greater capacity for gas exchange than posterior worm halves without gills. As with ammocoetes, measurements were also made during acute thermal and hypoxic challenges to ensure maximum recruitment of gills. However, only oxygen uptake and ammonia excretion are reported, as we lack the resolution to measure CO2 excretion in seawater.
Our results indicate that the gills of S. kowalevskii are not a primary site of gas exchange. Worm halves without gills had rates of oxygen uptake and ammonia excretion that were at least as high as whole worms and worm halves with gills in all treatments (Fig. 2). Rates are similar to those of other aquatic ectotherms22, and temperature effects (ratio between rates over a span of 10 °C (Q10)) lower than 2 for oxygen uptake and higher than 3 for ammonia excretion support 20 °C as a suitable thermal challenge near the upper limit of our winter-acclimated worms28.
a,b, Oxygen uptake (a) and ammonia excretion (b) in whole worms (black), posterior halves (white) and anterior halves (grey) in normoxia at 10 °C and 20 °C and in hypoxia at 20 °C. Posterior halves lack the pharynx and gills. Data are presented as mean ± s.d. with individuals superimposed (n = 8 for all groups, except n = 6 for ammonia excretion at 10 °C). Letters indicate significant differences within treatments (one-way ANOVA with Tukey’s post-hoc test; P < 0.05). Ammonia excretion not measured in hypoxia (see Methods). Exact P-values: P = 0.8256 (a, 10 °C), P = 0.8732 (a, 20 °C), P = 0.0253 (a, hypoxia), P = 0.6115 (b, 10 °C) and P = 0.0826 (b, 20 °C).
Notably, posterior worm halves without gills had slightly higher rates of oxygen uptake in hypoxia than did whole worms and anterior halves with gills (Fig. 2a). The reasons for this are unknown, but worm halves without gills might simply have a shorter diffusion distance, as the posterior body wall is generally thinner29. Alternatively, whole worms and anterior halves with gills might reduce their oxygen demand in hypoxia relative to posterior halves without gills. Many organisms suppress their activity and metabolic rate in hypoxia30, which requires sensory and processing capacities that posterior worm halves may lack. Sensory perception and processing in hemichordates are largely unstudied, but concentrated cell bodies of neurons in the proboscis and collar of anterior worm halves are speculated to have such a role31. Regardless of why oxygen uptake is higher in halves without gills in hypoxia, the results still indicate that gills are not a primary site for gas exchange.
We were unable to measure gill recruitment for gas exchange in the cephalochordate B. floridae. The morphology of this organism precludes the methods that were used to isolate gill function in ammocoetes and acorn worms. However, morphometric work by others estimates that cephalochordate gills have less than 5% of the total body diffusive capacity32. Diffusive capacity is an excellent predictor of the contributions of gills and skin to gas exchange at rest in larval fishes, coming within 2% of direct measurements made in divided chambers23,33. Functional data are preferred to account for the effects of convection during challenges to oxygen supply and demand, but cephalochordate gills are still unlikely to be a primary site of gas exchange with such a small diffusive potential—especially without the vertebrate pharyngeal adaptations that enhance gill perfusion and ventilation. For example, ammocoetes challenged with temperature and hypoxia only increase the contributions of gills to gas exchange by approximately twofold from rest, despite a closed circulatory system and robust, muscular ventilation (Fig. 1). Thus, even if cephalochordate gills had vertebrate-like convection, contributions to gas exchange would probably still be less than 10%.
Ion regulation in acorn worms and amphioxus
The high ion content of seawater hindered our use of radioisotopes to directly measure ion flux at the gills and skin of amphioxus and acorn worms as we did for ammocoetes. We instead measured common markers for specialized cells called ionocytes that drive trans-epithelial ion regulation in vertebrates25. Markers include (1) activity or expression of key ion transporters such as Na+,K+-ATPase (NKA) and V-type H+-ATPase (VHA), which power most ion-transport pathways in ionocytes25; (2) expression of forkhead box protein I (foxI), which drives ionocyte specification and differentiation in vertebrates34,35,36; and (3) colocalization of foxI with key ion transporters in discrete epithelial cells.
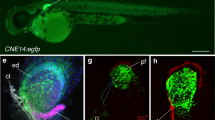
The gills of B. floridae showed a strong signal for vertebrate-like ionocytes across all markers. The activities of NKA and VHA in the gills were tenfold higher than those in the skin (Fig. 3a), matching the pattern in ammocoetes (Extended Data Fig. 4), and consistent with levels in other vertebrate gills37,38. Expression of a putative sodium–proton exchanger (NHE), carbonic anhydrase and foxI was also four-, four- and tenfold higher in the gills than in the skin, respectively (Fig. 3b and Extended Data Fig. 5). But, perhaps most compelling, the expression of foxI and NKA was restricted to a discrete epithelial domain in each pharyngeal gill bar (Fig. 4a–f, outlined section), colocalizing as observed in the NKA-rich ionocytes of zebrafish34. Furthermore, these foxI+NKA+ cells were also immunopositive for a Na-K-Cl cotransporter (NKCC; Fig. 4d–f), which is another hallmark of gill ionocytes in marine teleosts and lampreys25.
a–d, ATPase activity (a,c) and gene expression (b,d) for ionocyte markers in amphioxus (a,b) and acorn worms (c,d). White and grey symbols represent skin and gills, respectively. Gene expression relative to geometric mean of EF1A and 18S. Data are presented as mean ± s.d. with individuals superimposed (n = 10). Asterisks indicate significant differences between skin and gills for a single marker with two-tailed Student’s t-test. In all figures, *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001 and ****P ≤ 0.0001. Exact P-values: P < 0.0001 (a, NKA and VHA), P = 0.3228 (b, AE), P = 0.0003 (b, NHE), P = 0.0412 (b, CA), P < 0.0001 (b, foxI), P = 0.0009 (c, NKA), P = 0.3646 (c, VHA), P = 0.7728 (d, AE), P = 0.1655 (d, NHE), P = 0.3281 (d, CA) and P < 0.0001 (d, foxI). AE, anion exchanger; CA, carbonic anhydrase.
a–i, Masson’s trichrome staining (a,g), mRNA in situ hybridization by chain reaction for foxI (b,c,h,i), and immunostaining for NKA and NKCC (d–f) in the pharyngeal gill bars of amphioxus (a–f) and acorn worms (g–i). Putative ionocytes are in outlined sections. The dashed box in g is the area shown in h,i. Experiments were repeated independently three times with similar results. Scale bars, 50 μm.
These findings are consistent with immunohistochemical evidence for the presence of ion transporters in the gills of B. floridae39, and with work in Branchiostoma japonicum, in which NKA expression is sevenfold higher in the gills than in the skin40. However, we believe this to be the first discovery in cephalochordates of a distinct ionocyte cell type on the basis of specific co-expression of multiple molecular markers including foxI.
The gills of S. kowalevskii are also implicated in ion regulation, but the results are less clear. As in amphioxus, foxI expression was eightfold higher in the gills than in the skin (Fig. 3d), and in situ hybridization revealed that this expression was similarly restricted to a discrete epithelial domain on each pharyngeal gill bar (Fig. 4g–i). However, we were unable to detect specific expression of NKA, VHA or NKCC by immunofluorescence. This might be an issue with antibody reactivity, but could also be linked to broadly distributed ion-transport machinery. Indeed, ATPase activities and gene expression for most other markers in S. kowalevskii were similar across the gills, skin and other tissues (Extended Data Figs. 4a,d and 6 and Extended Data Tables 1 and 2).
In light of these results, S. kowalevskii gills might have the majority of ionocytes for epithelial ion transport as indicated by foxI expression, but all tissues might have a high capacity for intracellular ion regulation. A high capacity for intracellular pH regulation by tissues without ionocytes is common among CO2-tolerant fishes, in which extracellular pH regulation by ionocytes at gills often becomes limited or insufficient for the protection of other tissues during severe acid–base disturbances41. Indeed, acorn worms may require such a capacity in their burrows during intertidal fluctuations to CO2 and salinity30, or during regeneration when tissue fragments must maintain ion homeostasis independently. Alternatively, the foxI+ cells in S. kowalevskii might be latent homologues42 with a shared specification pathway that was subsequently coopted for, or gave rise to, ionocytes in chordates.
Origins of gill function
Together, our results support a vertebrate origin for gas exchange at gills with increasing body size and activity, but an earlier chordate or deuterostome origin for ion regulation (Extended Data Fig. 7). Of our study taxa, only ammocoete gills are recruited for gas exchange, and only secondarily with increasing body size or challenges to oxygen supply and demand. By contrast, the gills of all three taxa are implicated in ion regulation, with especially strong support in ammocoetes and amphioxus.
For gas exchange, this work is a key functional complement to fossil and developmental studies that support the role of gill recruitment in the stem vertebrate transition to larger, armoured and more-active modes of life8,11,13,14,15,16. Furthermore, it suggests that hypoxia associated with the burrowing behaviour of vertebrate ancestors is another potentially important selective pressure driving this recruitment. However, this work challenges our understanding of when and how gills acquired their primary role in ion regulation. Our findings suggest that ion regulation did not shift from the skin to the gills with gas exchange in stem vertebrates, as previously hypothesized5, and that it is unrelated to body size, dermal thickness and activity. Instead, gills are likely to have acquired a function in ion regulation before the origin of vertebrates, either in stem chordates or near the inception of pharyngeal pores in stem deuterostomes.
A pre-vertebrate origin for ion regulation at gills also questions our understanding of its ancestral function. Maintaining the extracellular acid–base balance is widely accepted as the ancestral function of ion regulation at vertebrate gills1, and this might be so for deuterostomes in general as well. However, there is little support for robust extracellular acid–base regulation in non-vertebrate deuterostomes43, and they may lack the required circulatory adaptations29. Early deuterostomes might have instead relied on intracellular regulation by tissues for ion homeostasis, and we find support for this capacity in S. kowalevskii.
Alternatively, ion regulation at early deuterostome gills might have been linked to feeding. As in our study species, pharyngeal gill bars of early deuterostomes are believed to be filter-feeding structures with a mucociliary epithelium that captured food from the inhalant water flow2,3,7,8,9,44. Filter-feeding may rely on epithelial ion regulation at gills to drive active nutrient uptake, as seen in crustaceans45, and/or to ensure proper secretion of mucins for particle entrapment and transport46. A function in mucin secretion is of particular note because the ionocytes that have this role in the mucociliary epithelia of mammalian lungs and amphibian skin are likely to be homologous with those of teleost fish gills that maintain the extracellular ion balance. Although embryonic origins have yet to be determined in all cases, ionocyte homology across these vertebrate taxa is supported by foxI orthologues controlling cell specification and differentiation34,35,36. foxI also serves as an important marker for ionocytes in adult tissue, as its expression persists in mature cells. The foxI+ cells that we observe in adult amphioxus and acorn worm gills are thus consistent with the presence of homologous, vertebrate-like ionocytes in these tissues too—especially in amphioxus, in which they co-express other ionocyte markers such as NKA and NKCC. Furthermore, the areas that correspond to these foxI+ domains have previously been described as distinct regions of non-ciliated secretory cells with mucin vesicles47,48 that, in amphioxus, form the primary source of mucin in the pharyngeal gill bars49 (Extended Data Fig. 8).
We propose that ancestral ionocytes served to facilitate mucin secretion for filter-feeding near the origin of pharyngeal gill pores in stem deuterostomes. These cells and their machinery perhaps then served as an exaptation to maintain the extracellular ion and acid–base balance with the evolution of an improved circulatory system in vertebrates. Regulation of extracellular ion homeostasis by the skin of larval teleosts might therefore be a derived embryonic trait that is expressed before the completion of gill development, rather than a relic of the ancestral condition as previously hypothesized5.
Methods
Animal collection and husbandry
Ammocoetes (E. tridentatus) were collected by dip-net from the Salmon River near Langley, British Columbia (49° 07' 39.2" N, 122° 34' 55.5" W) and transported to the University of British Columbia’s Vancouver campus for experimentation between 2014 and 2018. Animals were held at 10 °C on a 12-h–12-h photoperiod in recirculating aquaria (96 l) filled with dechlorinated Vancouver city tap water (Na+, 0.06 mM; Cl−, 0.05 mM; Ca2+, 0.03 mM; Mg2+, 0.007 mM; K+, 0.004 mM; alkalinity in mg CaCO3 l−1, 3.3; pH 6.5)50 and 5 cm of burrowing substrate. Ammocoetes were fed baker’s yeast twice weekly, and water changes were made every five days.
Acorn worms (S. kowalevskii) were purchased from the Marine Biological Laboratory in Woods Hole, USA, and shipped to the University of British Columbia’s Point Grey campus in Vancouver, Canada. Worms were held in static, 40-l seawater tanks (10 worms per tank; 34 parts per thousand (ppt) artificial seawater made from Instant Ocean salt mix) at 10 °C on a 12-h–12-h light–dark photoperiod. Tanks were supplied with sandy substrate, and 30% water changes were performed twice weekly. Acorn worms used in experiments had a mean wet mass of 0.105 ± 0.009 g (n = 60).
Amphioxus (B. floridae) were purchased from Gulf Specimen Marine Laboratories in Panacea, Florida, USA, and also shipped to the University of British Columbia’s Point Grey campus in Vancouver, Canada. B. floridae were held in identical conditions to S. kowalevskii, but maintained at 20 °C. Amphioxus used in experiments had a mean wet mass of 0.049 ± 0.005 g (n = 20).
All animals from all species were held for at least 1–2 weeks under their respective conditions and fasted 24–48 h before experimentation. All animal care and experimentation conformed to the guidelines set by the Canadian Council on Animal Care (CCAC) and was approved by the University of British Columbia’s Animal Care Committee (ACC) under the animal use protocol A19-0284.
Ammocoete in vivo protocols
Divided flux chambers
Ammocoetes were placed in divided flux chambers similar to those used previously33. To place ammocoetes in chambers, animals were lightly anaesthetized (0.05 g l−1 MS-222; tricaine methanesulfonate, Syndel) and passed through small holes in latex dental dams (Hygenic Corporation). Latex dams were fitted immediately posterior to branchial pores to separate the head and gills from the body. Once fitted, anterior and posterior chamber halves were placed on opposing sides of the latex dam to encompass anterior and posterior portions of the animal. Each acrylic chamber half was fitted with a stir bar, overflow tube and two needle ports for oxygen probes and water sampling. Chamber dimensions varied to accommodate the large range of sizes of the animals, and were such that body mass approximated 5–20% of chamber volume. Once in the flux chambers, animals were flushed with fresh water free of anaesthetic and subjected to one of three experimental protocols as described below. At protocol completion, animals were euthanized with a lethal dose of MS-222, cut into anterior and posterior halves at the point of chamber division and weighed, and a subset of animals were analysed for surface area as described below. All experiments were performed during daylight hours (08:00–19:00) under identical photoperiods.
Normoxia at 10 °C and 20 °C
Ammocoetes in open flux chambers were acclimated for 2 h in an aerated water bath at 10 °C or 20 °C. Chambers were sealed following acclimation, and water PO2 was recorded from anterior and posterior chamber halves simultaneously with needle-type optodes (Presens). Chamber halves were flushed when oxygen saturation fell below 80%, and PO2 measurements were repeated for three cycles. During each cycle at 20 °C, 20-μl samples of water were also taken at regular intervals from each chamber half through needle ports with a gas tight syringe (Hamilton). Water samples were immediately analysed for total CO2 as described below. After PO2 measurements and CO2 sampling, chambers were opened to the air, and a radioisotope (22Na or 45Ca) was added to either anterior or posterior halves. Water samples of 750 μl were taken from both chamber halves at 5 min, 2 h and 4 h after isotope addition. Five hundred microlitres of each sample was immediately frozen at −20 °C for later measurement of total ammonia, and the remaining sample was analysed for ion content and radioactivity. Surface area and wet mass for ammocoete halves were calculated following measurements for radioactivity as described below.
Normoxia at 26 °C
Animals in open flux chambers were subjected to an acute temperature ramp from 10 °C to 26 °C over 2 h in an aerated water bath. An upper temperature of 26 °C was used because it yielded the maximum ventilation rate. Chambers were sealed at 26 °C, and water PO2 was recorded from both chamber halves as in normoxia at 10 °C. Ventilation frequency was recorded for 1 min during each cycle for all animals.
Hypoxia at 20 °C
Ammocoetes in open flux chambers were subjected to acute hypoxia by bubbling nitrogen into the surrounding water bath (20 °C). The water bath PO2 was reduced to around 4 kPa (about 20% saturation) over 20 min, and animals were left to acclimate for 1 h before chamber halves were sealed. Water PO2 was recorded by optodes in both chamber halves simultaneously once sealed, and the ventilation frequency was recorded for 1 min. Trials were terminated when chamber oxygen fell below 3% saturation.
Acorn worm in vivo protocols
Normoxia at 10 °C
Eight whole worms were transferred to acrylic respirometers (4 ml) in an aerated water bath at 10 °C. Each respirometer was fitted with a stir bar, overflow tube and needle-type oxygen probe (Presens). After a 1-h acclimation period in respirometers open to the water bath, respirometers were sealed and the PO2 was recorded until it fell to around 80% saturation. Respirometers were then manually flushed, and the cycle was repeated twice more. After the third cycle, a razor blade was used to cut animals in half with a transverse section immediately posterior to the hindmost gill pores. Animal halves were left to recover in 10 ml aerated chambers at 10 °C for at least 12 h after fragmentation. After recovery, animal halves were returned to 4-ml respirometers and subjected to the same measurements of PO2 as whole animals. At trial completion, animal halves were euthanized with a lethal dose of MS-222 and weighed. For measurements of ammonia excretion, a separate group of six whole worms was placed in 10-ml aerated chambers. From the aerated chambers, 1-ml water samples were taken every 30 min for 4 h and frozen at −20 °C for later measurement of total ammonia. After the final water sample, animals were cut in half as above and left to recover overnight. Animal halves were then subjected to the same measurements as whole worms, and euthanized and weighed at trial completion.
Normoxia at 20 °C
Acorn worms were subjected to the same protocol as outlined for normoxia at 10 °C, but all measurements were performed at 20 °C and oxygen uptake and ammonia excretion were measured simultaneously in the same animals. Eight whole worms were warmed to 20 °C from 10 °C overnight in a water bath at 2.5 °C h−1 before experimentation. The temperature of 20 °C was chosen as an ecologically relevant thermal challenge that would substantially increase oxygen demand from 10 °C, but not result in mortality over 24–36 h. This temperature was chosen on the basis of previous work with summer-acclimated worms51 that recommended that rearing temperatures should not exceed 24 °C.
Hypoxia at 20 °C
Eight whole worms at 10 °C were warmed to 20 °C as in the previous protocol. Worms were placed in submerged respirometers open to the water bath. The water bath PO2 was then reduced to around 4 kPa (about 20% saturation) with a nitrogen sparge. After a 1-h acclimation at this reduced PO2, respirometers were sealed and the PO2 was recorded until it dropped to around 1% saturation. Whole worms were then euthanized with a lethal dose of MS-222 and weighed. A different, second set of eight whole animals at 10 °C was partitioned into anterior and posterior halves as in the previous protocols. Worm halves recovered for 12 h, and were then subjected to the same temperature ramp, experimental protocol and measurements as whole animals in hypoxia. This second, naive set of worms was used for fragmentation to avoid any potential confounding effects that pre-exposure of whole animals to hypoxia might have on gas exchange. Ammonia excretion was not measured in hypoxia because (1) PO2 below around 20% saturation could not be maintained in open respirometers; and (2) ammonia production during closed respirometry trials was below assay sensitivity.
Gas and ion flux analyses
O2 uptake
Mean slopes were taken from the linear portions of three O2 cycles for each chamber (acorn worms) or chamber half (ammocoetes) recorded with OXY-4V2_11TX data acquisition software (Presens). Mean slopes were then adjusted with background respiration values from blank trials to yield a ∆PO2 h−1 (mm Hg O2 h−1). From these mean rates, chamber volumes (ml), animal or fragment wet mass (g) and O2 solubility values52 (μmol O2 ml−1 mm Hg−1) were used to calculate oxygen uptake rates for whole and fragmented acorn worms (\(\dot{M}\)O2, in μmol O2 g−1 h−1). For ammocoetes, oxygen uptake rates were calculated in the same way for each chamber half, and then summed to yield whole-body \(\mathop{M}\limits^{.}\)O2.
Ammonia excretion
Water samples were analysed for total ammonia (NH3 and NH4+; hereafter written as NH3/4+) colorometrically53 using a SpectraMax 190 microplate reader (Molecular Devices). The difference in ammonia concentration between initial and final water samples was used to calculate the rate of change in the concentration of ammonia (nmol NH3/4+ ml−1 h−1) for a chamber (acorn worms) or chamber half (ammocoetes). From this rate, chamber volumes and animal or fragment wet mass (g) were used to calculate ammonia excretion rates for whole or fragmented acorn worms (\(\mathop{M}\limits^{.}\)NH3/4+). For ammocoetes, ammonia excretion rates were calculated in the same way for each chamber half, and then summed to yield whole-body \(\mathop{M}\limits^{.}\)NH3/4+.
CO2 excretion
Water samples (20 μl) were analysed for total CO2 as before54. Total CO2 was expressed as a concentration (μmol ml−1) and all values for a given trial were plotted as a function of time (h). The slope of this line equals the rate of change in CO2 concentration for a chamber half (μmol CO2 ml−1 h−1). From this rate, chamber half volumes (ml) and animal wet mass (g) were used to calculate CO2 excretion rates for each chamber half, and then summed to yield whole-body \(\mathop{M}\limits^{.}\)CO2 (μmol CO2 g−1 h−1).
Na+ uptake
At trial completion, ammocoetes were rinsed three times with 5 mM cold NaCl to displace surface-bound 22Na and once with deionized water. Whole ammocoetes and water samples were measured for radioactivity in counts per minute (cpm) with a gamma counter (PerkinElmer). Trials were discarded if activity above background was found in water samples from the chamber half that did not receive isotope injection. Water Na+ concentration was determined by flame atomic absorption spectrometry (Varian). Sodium uptake rates were calculated as in a previous study55 and reported as μmol Na+ g−1 h−1. Owing to the absence of sodium uptake from posterior chamber halves, uptake rates were only calculated from trials where isotope was added to anterior chamber halves.
Ca2+ uptake
Calcium uptake rates were measured in a subset of our smallest ammocoetes (0.08 ± 0.003 g, n = 15) to determine whether the pattern observed for Na+ uptake could be applied to ion regulation more generally. Analysis was identical to that for Na+ uptake with the following exceptions. At trial onset, 100 μl of 45Ca working solution was injected to either chamber half to yield around 0.4 μCi per ml of chamber water. At trial completion, ammocoetes were rinsed twice in a 10 mM cold CaCl2 solution, once in 50 mM EDTA and once in deionized water. Whole ammocoetes were digested overnight at 40 °C in a 5:1 volume:mass ratio of 1 M nitric acid. Digest aliquots and water samples were then diluted five times in Ultima Gold AB liquid scintillation cocktail (PerkinElmer) and measured for radioactivity with a liquid scintillation counter (LSC-2000; Beckman Coulter). A quench curve was constructed with varying amounts of tissue digest so that counting efficiency could be corrected to be the same as that of water samples. Calcium uptake was reported as nmol Ca2+ g−1 h−1 and calculated as described previously56. Owing to the absence of calcium uptake from posterior chamber halves, uptake rates were only calculated from trials in which isotope was added to anterior chambers (n = 8).
Per cent flux at ammocoete gills
Flux rates for anterior and posterior chamber halves were adjusted with epidermal surface area to represent flux at gills and skin, respectively17,57. After surface area correction, the per cent flux at gills was then calculated by dividing anterior chamber flux rates by total flux rates.
Ammocoete epidermal surface area, thickness and cutaneous diffusion capacity
At trial completion, a subset of 15 euthanized ammocoetes was fixed in 70% ethanol for 48 h after mass and length measurements. The epidermis was removed from each half, flattened under coverslips and photographed with a dissecting scope. Epidermal surface area (cm2) for each half was measured in photos with ImageJ (NIH), and the percentage of the total body surface area present in anterior chamber halves was plotted as a function of body mass (g). These data were fitted with an allometric curve (y = 32.058x−0.075, R2 = 0.81) that was used to calculate anterior surface area for all ammocoetes.
For epidermal thickness, a subset of ammocoetes was euthanized, weighed, measured for length, fixed and prepared for sectioning as described previously58. For each individual, 5-μm transverse sections were made at midpoints of the pharynx, body and anus. Epidermal thickness was measured at all three midpoints under a microscope with ImageJ (NIH) and used to calculate a mean thickness (μm) for each individual. Mean epidermal thicknesses for all ammocoetes were plotted as a function of body mass and fitted with an allometric curve (R2 = 0.92; Extended Data Fig. 1b).
To calculate cutaneous diffusion capacity, mass-specific surface area (cm2 g−1) of ammocoetes sampled during experimentation was plotted as a function of wet mass (g) and fitted with an allometric curve (R2 = 0.99; Extended Data Fig. 1a). The equation of this line was divided by that for epidermal thickness to yield a model estimating cutaneous diffusion capacity (cm2 μm−1 g−1; Extended Data Fig. 1c).
Tissue sampling for ionocyte markers
Animals were pinned to silicone-coated Petri dishes in seawater for dissection. Tissue samples were carefully excised with the aid of a dissecting microscope, transferred to RNase-free bullet tubes, flash-frozen in liquid N2 and stored at −80 °C. For B. floridae, tissue samples were taken for gills (all pharyngeal gill bars), skin (outer dermis excluding the atrial lining), hepatic caecum and muscle (dorsal section immediately posterior to atriopore). For S. kowalevskii, tissue samples were taken for gills (all pharyngeal gill bars), skin (outer dermis), intestine (entire gut tube posterior to hindmost gill pores) and muscle (entire proboscis). Animals of a given species were all sampled within a 4-h period, and each dissection was under 10 min. A total of 20 animals per species were sampled (10 for ATPase measurements and 10 for gene expression). NKA activity was also measured in gill, skin, intestine and muscle of E. tridentatus (n = 10).
ATPase activity
NKA and VHA activities were measured using modified versions of the protocols outlined previously38,59. NKA and VHA activities are reported as μmol ADP per mg protein per hour. Additional detail is provided in the Supplementary Information.
Gene expression
Target sequences (Extended Data Table 1) were identified in B. floridae and S. kowalevskii with reciprocal best BLAST hits for characterized proteins in Danio rerio and/or Homo sapiens60. Species-specific primers for B. floridae and S. kowalevskii were designed for corresponding transcripts from RefSeq mRNA databases (NCBI txid7739 and txid10224, respectively) with PrimerQuest (Integrated DNA Technologies) for use with quantitative PCR with reverse transcription (qRT–PCR; primer sequences listed in Extended Data Table 2). Tissue homogenization, RNA extraction, cDNA synthesis and qRT–PCR were performed as described previously61. Gene expression values are normalized to skin and reported relative to the geometric mean of 18S (accession numbers M97571.1 and L28054.1 for B. floridae and S. kowalevskii, respectively) and EF1A (accession numbers XM_002604676.1 and NM_001184809.1 for B. floridae and S. kowalevskii, respectively). Additional detail is provided in the Supplementary Information, including sequence alignments assessing ionocyte marker suitability (Supplementary Figs. 1–8 and Supplementary Table 1) and upstream scans for putative promoter binding sites for foxI (Supplementary Table 2). Sequence accession numbers used for gene expression are also listed in the figure source data files.
Microscopy
Whole S. kowalevskii and B. floridae were euthanized as above and fixed in 4% paraformaldehyde in DEPC-treated phosphate-buffered saline (PBS) overnight at 4 °C. Fixed samples were then rinsed in PBS and dehydrated into 100% methanol for storage at −20 °C. Pharyngeal regions were dissected from dehydrated samples before paraffin embedding and sectioning.
Paraffin embedding, sectioning (7 μm thickness) and immunofluorescence were performed according to a previous report62. Antibodies were diluted as follows: rabbit anti-NKA (1:200), mouse anti-NKCC (1:100; T4, Developmental Studies Hybridoma Bank), goat anti-rabbit 647 (1:500; Thermo Fisher Scientific) and goat anti-mouse 488 (1:500; Thermo Fisher Scientific). Rabbit anti-NKA is NAK121 (ref. 63) and was provided by J. Wilson. Negative controls were performed in tissue sections for each species by running the staining protocol without each primary antibody to confirm the absence of non-specific signal. Additional protocol detail is provided in the Supplementary Information.
mRNA in situ hybridization by chain reaction was performed on paraffin sections as described previously64, with modifications as per another previous report65. Hairpins and custom probe sets were purchased from Molecular Instruments for foxI transcripts in S. kowalevksii (lot PRK271, amplifier B1) and B. floridae (lot PRK274, amplifier B1).
Masson’s trichrome and Alcian blue stainings were performed as described before66. All sections were imaged on a Zeiss Axioscope A1 with Colibri 7 light source (ZEISS). Images were captured in ZEN (v.2.3; ZEISS), and edited for Fig. 4 with Adobe Creative Suite. All experiments were repeated at least three times independently with similar results.
Statistics and reproducibility
Data were analysed with Prism 9 for macOS (v.9.3.1; GraphPad Software). Ammocoete gill contributions to gas exchange and ion regulation were subjected to a multiple linear regression analysis with ANOVA using treatment and body mass as factors. Gas exchange in whole and fragmented acorn worms was analysed with a one-way ANOVA and Tukey’s post-hoc test. Activity and expression of ionocyte markers in amphioxus and acorn worms were analysed with a two-tailed Student’s t-test (Fig. 3), one-way ANOVA with Tukey’s post-hoc test (Extended Data Figs. 4a,b,d, 5 and 6b,d) or Kruskal–Wallis test with Dunn’s post-hoc test (Extended Data Figs. 4c,e and 6a,c). All data were analysed with t-tests or ANOVA passed tests of normality and equal variance, but some did require log or root transformation. Kruskal–Wallis tests were only used when normality and equal variance were not achieved by simple data transformation.
Sample sizes were determined from previous studies that found meaningful biological effects with similar methods17,57. Experiments were not replicated, so that the number of animals euthanized could be kept as low as reasonably allowable. For in vivo measurements of gas and ion flux, animals were randomly assigned to one of three experimental treatments (normoxia, high temperature or hypoxia). However, blinding was not possible as water temperature and oxygen levels required close monitoring and manipulation. All remaining in vitro data collection and analyses were blind to treatments.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
All data generated and analysed by this study are publicly accessible (https://doi.org/10.5281/zenodo.7022487). NCBI accession numbers for species and sequences used in gene expression are as follows: txid7739 (M97571.1, XM_002605269.1, XM_002594257.1, XM_002604676.1, XM_002608239.1 and XM_002597600.1) and txid10224 (L28054.1, XM_002736626.2, XM_006814054.1, NM_001184809.1, XM_002734648.1 and XM_002741628.2). NCBI accession numbers for species and sequences used in protein alignments are as follows: txid7739 (XP_002605315.1, XP_002594302.1, XP_002608285.1 and XP_002597646.1), txid10224 (XP_002736672.2, XP_006814117.1, XP_002734694.1 and XP_002741674.2), txid7955 (NP_001161738.1, NP_001032314.1, NP_001289693.1, NP_001032198.1, XP_696967.3, XP_009296364.1, NP_001315073.1, NP_001075158.1, NP_954685.2, NP_001107879.2, NP_001159683.1, NP_001104671.1, XP_009295179.1, NP_957107.1, NP_001017571.2, XP_694982.3, NP_571142.1, NP_571577.1, NP_859424.1, NP_957278.1, NP_944598.2, NP_944599.2, NP_944600.1, NP_001070174.2, NP_956196.1, NP_001030156.1, XP_021335149.1, XP_021334721.1, NP_001106952.1, NP_001107567.1, XP_00929331.1, NP_001106943.1, NP_001091726.2, NP_001025248.2 and NP_001008586.1) and txid9606 (NP_000333.1, NP_001186621.1, NP_005061.3, NP_001076002.1, NP_001209.1, NP_940986.1, NP_036245.1, NP_000058.1, NP_000708.1, NP_001354154.1, NP_001257431.1, NP_001014435.1, NP_001308767.1, NP_001207.2, NP_005240.3, NP_003914.1, NP_005241.1, NP_036320.2, NP_658982.1, NP_997309.2, NP_001129121.1, NP_001445.2, NP_001032242.1, NP_001091954.1, NP_597812.1, NP_001171486.1, NP_003038, NP_003039, NP_004165, NP_001310902.1, NP_001171122.1, NP_001244220.1 and NP_001247420.1). Relevant accession numbers are also provided in the figure source data files. A putative DNA-binding domain was identified in NP_005241.1 using the PROSITE database (https://prosite.expasy.org). Source data are provided with this paper.
Change history
28 October 2022
In the PDF version of this article initially published, Extended Data Figure 5 was an incorrect image and has now been replaced in the PDF version of the article. The HTML version was unaffected.
References
Evans, D. Gill Na+/H+ and Cl−/HCO3− exchange systems evolved before the vertebrates entered fresh water. J. Exp. Biol. 113, 465–469 (1984).
Gans, C. & Northcutt, R. G. Neural crest and the origin of vertebrates: a new head. Science 220, 268–273 (1983).
Northcutt, R. G. The new head hypothesis revisited. J. Exp. Zoolog. B 304, 274–297 (2005).
Halstead, L. B. & Lawson, J. D. The vertebrate invasion of fresh water. Philos. Trans. R. Soc. B 309, 243–258 (1985).
Brauner, C. J. & Rombough, P. J. Ontogeny and paleophysiology of the gill: new insights from larval and air-breathing fish. Respir. Physiol. Neurobiol. 184, 293–300 (2012).
Purnell, M. A. Feeding in extinct jawless heterostracan fishes and testing scenarios of early vertebrate evolution. Proc. R. Soc. B 269, 83–88 (2002).
Simakov, O. et al. Hemichordate genomes and deuterostome origins. Nature 527, 459–465 (2015).
Green, S. A., Simoes-Costa, M. & Bronner, M. E. Evolution of vertebrates as viewed from the crest. Nature 520, 474–482 (2015).
Lowe, C. J., Clarke, D. N., Medeiros, D. M., Rokhsar, D. S. & Gerhart, J. The deuterostome context of chordate origins. Nature 520, 456–465 (2015).
Ronco, F. et al. Drivers and dynamics of a massive adaptive radiation in cichlid fishes. Nature 589, 76–81 (2021).
Gillis, J. A. & Tidswell, O. R. A. The origin of vertebrate gills. Curr. Biol. 27, 729–732 (2017).
Green, S. A. & Bronner, M. E. The lamprey: a jawless vertebrate model system for examining origin of the neural crest and other vertebrate traits. Differentiation 87, 44–51 (2014).
Mongera, A. et al. Genetic lineage labeling in zebrafish uncovers novel neural crest contributions to the head, including gill pillar cells. Development 140, 916–925 (2013).
Morris, S. C. & Caron, J.-B. A primitive fish from the Cambrian of North America. Nature 512, 419–422 (2014).
Shu, D.-G. et al. Lower Cambrian vertebrates from south China. Nature 402, 42–46 (1999).
Xian-guang, H., Aldridge, R. J., Siveter, D. J., Siveter, D. J. & Xiang-hong, F. New evidence on the anatomy and phylogeny of the earliest vertebrates. Proc. R. Soc. B 269, 1865–1869 (2002).
Fu, C., Wilson, J. M., Rombough, P. J. & Brauner, C. J. Ions first: Na+ uptake shifts from the skin to the gills before O2 uptake in developing rainbow trout, Oncorhynchus mykiss. Proc. R. Soc. B 277, 1553–1560 (2010).
Rombough, P. The functional ontogeny of the teleost gill: which comes first, gas or ion exchange? Comp. Biochem. Physiol. A 148, 732–742 (2007).
Gillis, J. A., Fritzenwanker, J. H. & Lowe, C. J. A stem-deuterostome origin of the vertebrate pharyngeal transcriptional network. Proc. R. Soc. B 279, 237–246 (2012).
Miyashita, T., Gess, R. W., Tietjen, K. & Coates, M. I. Non-ammocoete larvae of Palaeozoic stem lampreys. Nature 591, 408–412 (2021).
Dawson, H. A., Quintella, B. R., Almeida, P. R., Treble, A. J. & Jolley, J. C. in Lampreys: Biology, Conservation and Control (ed. Docker, M. F.) 75–137 (Springer, 2015).
Wilkie, M. P., Bradshaw, P. G., Joanis, V., Claude, J. F. & Swindell, S. L. Rapid metabolic recovery following vigorous exercise in burrow‐dwelling larval sea lampreys (Petromyzon marinus). Physiol. Biochem. Zool. 74, 261–272 (2001).
Wells, P. & Pinder, A. The respiratory development of Atlantic salmon. I. Morphometry of gills, yolk sac and body surface. J. Exp. Biol. 199, 2725–2736 (1996).
Perry, S. F. & Wood, C. M. Kinetics of branchial calcium uptake in the rainbow trout: effects of acclimation to various external calcium levels. J. Exp. Biol. 116, 411–433 (1985).
Hwang, P.-P. & Lin, L. Y. in The Physiology of Fishes 4th edn (eds Evans, D. H., Claiborne, J. B. & Currie, S.) 205–234 (CRC Press, 2013).
Blair, S. D., Wilkie, M. P. & Edwards, S. L. Rh glycoprotein immunoreactivity in the skin and its role in extrabranchial ammonia excretion by the sea lamprey (Petromyzon marinus) in freshwater. Can. J. Zool. 95, 95–105 (2017).
Tweedell, K. S. Regeneration of the enteropneust, Saccoglossus kowalevskii. Biol. Bull. 120, 118–127 (1961).
Schulte, P. M. The effects of temperature on aerobic metabolism: towards a mechanistic understanding of the responses of ectotherms to a changing environment. J. Exp. Biol. 218, 1856–1866 (2015).
Barrington, E. J. The Biology of Hemichordata and Protochordata (Oliver and Boyd, 1965).
Richards, J. G. Physiological, behavioral and biochemical adaptations of intertidal fishes to hypoxia. J. Exp. Biol. 214, 191–199 (2011).
Miyamoto, N. & Wada, H. in Oxford Research Encyclopedia of Neuroscience https://doi.org/10.1093/acrefore/9780190264086.013.204 (Oxford Univ. Press, 2018).
Schmitz, A., Gemmel, M. & Perry, S. F. Morphometric partitioning of respiratory surfaces in amphioxus (Branchiostoma lanceolatum Pallas). J. Exp. Biol. 203, 3381–3390 (2000).
Wells, P. & Pinder, A. The respiratory development of Atlantic salmon. II. Partitioning of oxygen uptake among gills, yolk sac and body surfaces. J. Exp. Biol. 199, 2737–2744 (1996).
Hsiao, C.-D. et al. A positive regulatory loop between foxi3a and foxi3b is essential for specification and differentiation of zebrafish epidermal ionocytes. PLoS ONE 2, e302 (2007).
Montoro, D. T. et al. A revised airway epithelial hierarchy includes CFTR-expressing ionocytes. Nature 560, 319–324 (2018).
Quigley, I. K., Stubbs, J. L. & Kintner, C. Specification of ion transport cells in the Xenopus larval skin. Development 138, 705–714 (2011).
Richards, J. G., Semple, J. W., Bystriansky, J. S. & Schulte, P. M. Na+/K+-ATPase α-isoform switching in gills of rainbow trout (Oncorhynchus mykiss) during salinity transfer. J. Exp. Biol. 206, 4475–4486 (2003).
Tresguerres, M., Katoh, F., Fenton, H., Jasinska, E. & Goss, G. G. Regulation of branchial V-H+-ATPase, Na+/K+-ATPase and NHE2 in response to acid and base infusions in the Pacific spiny dogfish (Squalus acanthias). J. Exp. Biol. 208, 345–354 (2005).
Cuoghi, I., Lazzaretti, C., Mandrioli, M., Mola, L. & Pederzoli, A. Immunohistochemical analysis of the distribution of molecules involved in ionic and pH regulation in the lancelet Branchiostoma floridae (Hubbs, 1922). Acta Histochem. 120, 33–40 (2018).
Li, M., Jiang, C., Zhang, Y. & Zhang, S. Activities of amphioxus GH-like protein in osmoregulation: insight into origin of vertebrate GH family. Int. J. Endocrinol. 2017, 9538685 (2017).
Sackville, M. A. et al. Water pH limits extracellular but not intracellular pH compensation in the CO2-tolerant freshwater fish Pangasianodon hypophthalmus. J. Exp. Biol. 221, jeb190413 (2018).
Stone, J. R. & Hall, B. K. Latent homologues for the neural crest as an evolutionary novelty. Evol. Dev. 6, 123–129 (2004).
Stumpp, M. & Hu, M. Y. in Acid–Base Balance and Nitrogen Excretion in Invertebrates (eds Weihrauch, D. & O’Donnell, M.) 261–273 (Springer, 2017).
Gonzalez, P. & Cameron, C. B. The gill slits and pre-oral ciliary organ of Protoglossus (Hemichordata: Enteropneusta) are filter-feeding structures. Biol. J. Linn. Soc. 98, 898–906 (2009).
Blewett, T. A. & Goss, G. G. A novel pathway of nutrient absorption in crustaceans: branchial amino acid uptake in the green shore crab (Carcinus maenas). Proc. R. Soc. B 284, 20171298 (2017).
Quinton, P. M. Role of epithelial HCO3− transport in mucin secretion: lessons from cystic fibrosis. Am. J. Physiol. Cell Physiol. 299, C1222–C1233 (2010).
Pardos, F. & Benito, J. Estudio histológico de lar faringe de Glossobalanus minutus (Enteropneusta, Ptychoderidae). Bol. R. Soc. Espanõla Hist. Nat. 80, 101–118 (1982).
Ruppert, E. E. in Microscopic Anatomy of Invertebrates Vol. 15 (eds Harrison, F. W. & Ruppert, E. E.) 349–504 (John Wiley & Sons, 1997).
Mallatt, J. The suspension feeding mechanism of the larval lamprey Petromyzon marinus. J. Zool. 194, 103–142 (1981).
Water Quality Control Annual Report http://www.metrovancouver.org/services/parks/ParksPublications/2018WaterQualityMonitoringReport.pdf (Metro Vancouver, 2018).
Lowe, C. J., Tagawa, K., Humphreys, T., Kirschner, M. & Gerhart, J. in Methods in Cell Biology Vol. 74 (eds Ettensohn, C. A., Wray, G. A. & Wessel, G. M.) 171–194 (Elsevier, 2004).
Boutilier, R. G., Heming, T. A. & Iwama, G. K. in Fish Physiology Vol. 10 (eds Hoar, W.S. & Randall, D. J.) 403–430 (Elsevier, 1984).
Verdouw, H., Van Echteld, C. J. A. & Dekkers, E. M. J. Ammonia determination based on indophenol formation with sodium salicylate. Water Res. 12, 399–402 (1978).
Lee, D. J., Gutbrod, M., Ferreras, F. M. & Matthews, P. G. D. Changes in hemolymph total CO2 content during the water-to-air respiratory transition of amphibiotic dragonflies. J. Exp. Biol. 221, jeb181438 (2018).
Brauner, C. J. & Wood, C. M. Ionoregulatory development and the effect of chronic silver exposure on growth, survival, and sublethal indicators of toxicity in early life stages of rainbow trout (Oncorhynchus mykiss). J. Comp. Physiol. B 172, 153–162 (2002).
Zimmer, A. M., Brix, K. V. & Wood, C. M. Mechanisms of Ca2+ uptake in freshwater and seawater-acclimated killifish, Fundulus heteroclitus, and their response to acute salinity transfer. J. Comp. Physiol. B 189, 47–60 (2019).
Zimmer, A. M., Wright, P. A. & Wood, C. M. What is the primary function of the early teleost gill? Evidence for Na+/NH4+ exchange in developing rainbow trout (Oncorhynchus mykiss). Proc. R. Soc. B 281, 20141422 (2014).
Sackville, M., Wilson, J. M., Farrell, A. P. & Brauner, C. J. Water balance trumps ion balance for early marine survival of juvenile pink salmon (Oncorhynchus gorbuscha). J. Comp. Physiol. B 182, 781–792 (2012).
McCormick, S. D. Methods for nonlethal gill biopsy and measurement of Na+, K+-ATPase activity. Can. J. Fish. Aquat. Sci. 50, 656–658 (1993).
Ward, N. & Moreno-Hagelsieb, G. Quickly finding orthologs as reciprocal best hits with BLAT, LAST, and UBLAST: how much do we miss? PLoS ONE 9, e101850 (2014).
Gibbons, T. C., Metzger, D. C. H., Healy, T. M. & Schulte, P. M. Gene expression plasticity in response to salinity acclimation in threespine stickleback ecotypes from different salinity habitats. Mol. Ecol. 26, 2711–2725 (2017).
Hirschberger, C. & Gillis, J. A. The pseudobranch of jawed vertebrates is a mandibular arch-derived gill. Development 149, dev200184 (2022).
Uchida, K., Kaneko, T., Miyazaki, H., Hasegawa, S. & Hirano, T. Excellent salinity tolerance of Mozambique tilapia (Oreochromis mossambicus): elevated chloride cell activity in the branchial and opercular epithelia of the fish adapted to concentrated seawater. Zoolog. Sci. 17, 149–160 (2000).
Choi, H. M. T. et al. Third-generation in situ hybridization chain reaction: multiplexed, quantitative, sensitive, versatile, robust. Development 145, dev165753 (2018).
Criswell, K. E. & Gillis, J. A. Resegmentation is an ancestral feature of the gnathostome vertebral skeleton. eLife 9, e51696 (2020).
Witten, P. E. & Hall, B. K. Seasonal changes in the lower jaw skeleton in male Atlantic salmon (Salmo salar L.): remodelling and regression of the kype after spawning. J. Anat. 203, 435–450 (2003).
Acknowledgements
We thank P. J. Rombough for discussion that helped inspire this work. This study was funded in part by Natural Sciences and Engineering Council of Canada Discovery Grants to C.J.B. (2018-04172) and C.B.C. (1283784), and a Royal Society University Research Fellowship (UF130182, URF\R\191007) and Royal Society Research Fellows Enhancement Award (RGF/EA/180087) to J.A.G. M.A.S. was supported by an NSERC CGS-D scholarship.
Author information
Authors and Affiliations
Contributions
M.A.S., C.J.B. and C.B.C. conceived the study. M.A.S. performed all experiments and data analyses and wrote the manuscript. J.A.G. oversaw all microscopy. All authors provided manuscript edits and comments and approved the final version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks Dorit Hockman, Christopher Lowe and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Cutaneous diffusive capacity of ammocoetes (E. tridentatus).
Mass-specific surface area (a; n = 15) and epidermal thickness (b; n = 16) measured in different subsets of ammocoetes. Diffusive capacity of the skin (c) calculated from allometric curves fitted to epidermal thickness (a) and mass-specific surface area (b). All data expressed as a function of wet mass (g).
Extended Data Fig. 2 Gill contributions to CO2 and calcium flux in ammocoetes (E. tridentatus).
Gill contributions (a) and whole-body flux rates (b, c) for CO2 excretion (white), oxygen uptake (grey) and calcium uptake (green) in normoxia at 20 °C. Individual data points plotted as a function of wet mass (g), n = 8 for all groups except n = 7 for gill contributions to calcium uptake
Extended Data Fig. 3 Whole-body rates of gas and ion flux in ammocoetes (E. tridentatus).
Rates of oxygen uptake (a; n = 40), ammonia excretion (b; n = 36) and sodium uptake (c; n = 21) in normoxia at 10 °C, 26 °C (grey; oxygen only, n = 26) or hypoxia at 20 °C (white; oxygen only, n = 22). Individual data points plotted as a function of wet mass (g)
Extended Data Fig. 4 ATPase activities in acorn worms (S. kowalevskii), amphioxus (B. floridae) and ammocoetes (E. tridentatus).
Na+/K+-ATPase (a–c; NKA) and V-H+-ATPase (d,e; VHA) activities in multiple tissues of S. kowalevskii (a,d), B. floridae (b,e) and E. tridentatus (c). Data presented as means±sd with individuals superimposed (n = 10 for all tissues except n = 9 for proboscis and hepatic caecum). One-way ANOVA with Tukey’s test (a,b,d) or Kruskal-Wallis with Dunn’s test (c,e), P < 0.05. Letters indicate significant differences between tissues. P < 0.0001 (a–c,e), P = 0.6203 (d).
Extended Data Fig. 5 Gene expression for ionocyte markers in amphioxus (B. floridae).
Anion exchanger (a; AE, P < 0.0001), sodium–proton exchanger (b; NHE, P < 0.0001), carbonic anhydrase (c; CA, P = 0.0004) and forkhead box protein I (d; FoxI, P < 0.0001) expression in skin, gill, hepatic caecum and muscle of B. floridae. Expression is relative to geometric mean of EF1A and 18S. Data presented as means±sd with individuals superimposed (n = 10). One-way ANOVA with Tukey’s test, P < 0.05. Letters indicate significant differences between tissues
Extended Data Fig. 6 Gene expression for ionocyte markers in acorn worms (S. kowalevskii).
Anion exchanger (a; AE, P = 0.0163), sodium-proton exchanger (b; NHE, P < 0.0001), carbonic anhydrase (c; CA, P = 0.0431) and forkhead box protein I (d; FoxI, P < 0.0001) expression in skin, gill, intestine and proboscis of S. kowalevskii. Expression is relative to geometric mean of EF1A and 18S. Data presented as means±sd with individuals superimposed (n = 10). Letters indicate significant differences between tissues. One-way ANOVA with Tukey’s test (b,d) or Kruskal-Wallis with Dunn’s test (a,c), P < 0.05
Extended Data Fig. 7 Origins of gill function.
Our findings support a novel stem chordate or deuterostome origin for ion regulation at gills (pink), perhaps near the inception of pharyngeal gill pores and their role in filter-feeding (black). A vertebrate origin for gas exchange at gills (blue) with increasing body size and activity is also supported by this work, and consistent with fossil and developmental studies (references in text). Data not collected for clades in grey.
Extended Data Fig. 8 Acidic mucins in the foxI+ domains of the pharyngeal gill bars in B. floridae.
Positive staining for Alcian blue shows acidic mucins in the foxI+ domains of the pharyngeal gill bars in B. floridae (outlined section). Counterstained with Nuclear Fast Red, scale bar = 50 μm. This experiment was repeated independently three times with similar results.
Supplementary information
Supplementary Information
This file contains Supplementary Methods; Supplementary Data Tables 1-2; Supplementary Figures 1–8 and Supplementary References.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sackville, M.A., Cameron, C.B., Gillis, J.A. et al. Ion regulation at gills precedes gas exchange and the origin of vertebrates. Nature 610, 699–703 (2022). https://doi.org/10.1038/s41586-022-05331-7
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41586-022-05331-7
This article is cited by
-
Chromosome-scale genome assembly and gene annotation of the hydrothermal vent annelid Alvinella pompejana yield insight into animal evolution in extreme environments
BMC Biology (2025)
-
Ammonia excretion by the fish gill: discoveries and ideas that shaped our current understanding
Journal of Comparative Physiology B (2024)
-
The physiological significance of plasma-accessible carbonic anhydrase in the respiratory systems of fishes
Journal of Comparative Physiology B (2024)
-
Structure and function of the larval teleost fish gill
Journal of Comparative Physiology B (2024)
-
Causes and consequences of gas bubble trauma on fish gill function
Journal of Comparative Physiology B (2024)