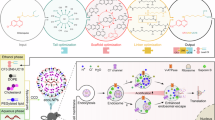
Abstract
Pre-eclampsia is a placental disorder that affects 3–5% of all pregnancies and is a leading cause of maternal and fetal morbidity worldwide1,2. With no drug available to slow disease progression, engineering ionizable lipid nanoparticles (LNPs) for extrahepatic messenger RNA (mRNA) delivery to the placenta is an attractive therapeutic option for pre-eclampsia. Here we use high-throughput screening to evaluate a library of 98 LNP formulations in vivo and identify a placenta-tropic LNP (LNP 55) that mediates more than 100-fold greater mRNA delivery to the placenta in pregnant mice than a formulation based on the Food and Drug Administration-approved Onpattro LNP (DLin-MC3-DMA)3. We propose an endogenous targeting mechanism based on β2-glycoprotein I adsorption that enables LNP delivery to the placenta. In both inflammation- and hypoxia-induced models of pre-eclampsia, a single administration of LNP 55 encapsulating vascular endothelial growth factor (VEGF) mRNA resolves maternal hypertension until the end of gestation. In addition, with our VEGF mRNA LNP 55 therapeutic, we demonstrate improvements in fetal health and partially restore placental vasculature, the local and systemic immune landscape and serum levels of soluble Fms-like tyrosine kinase-1, a clinical biomarker of pre-eclampsia1. Together, these results demonstrate the potential of this mRNA LNP platform for treating placental disorders such as pre-eclampsia.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Demultiplexed next-generation sequencing data from b-DNA LNP screening are available at https://upenn.box.com/v/VEGF-LNPs-pre-eclampsia. Source data are provided with this paper.
Change history
03 February 2025
A Correction to this paper has been published: https://doi.org/10.1038/s41586-025-08605-y
References
Chappell, L. C., Cluver, C. A., Kingdom, J. & Tong, S. Pre-eclampsia. Lancet 398, 341–354 (2021).
Swingle, K. L., Ricciardi, A. S., Peranteau, W. H. & Mitchell, M. J. Delivery technologies for women’s health applications. Nat. Rev. Bioeng. 1, 408–425 (2023).
Adams, D. et al. Patisiran, an RNAi therapeutic, for hereditary transthyretin amyloidosis. New Engl. J. Med. 379, 11–21 (2018).
Geisler, H. C., Safford, H. C. & Mitchell, M. J. Rational design of nanomedicine for placental disorders: birthing a new era in women’s reproductive health. Small 20, 2300852 (2023).
Gilbert, J. S. et al. Recombinant vascular endothelial growth factor 121 infusion lowers blood pressure and improves renal function in rats with placentalischemia-induced hypertension. Hypertension 55, 380–385 (2010).
Mateus, J. et al. Endothelial growth factor therapy improves preeclampsia-like manifestations in a murine model induced by overexpression of sVEGFR-1. Am. J. Physiol. Heart Circ. Physiol. 301, H1781–H1787 (2011).
Li, Z. et al. Recombinant vascular endothelial growth factor 121 attenuates hypertension and improves kidney damage in a rat model of preeclampsia. Hypertension 50, 686–692 (2007).
David, A. L. et al. Local delivery of VEGF adenovirus to the uterine artery increases vasorelaxation and uterine blood flow in the pregnant sheep. Gene Ther. 15, 1344–1350 (2008).
David, A. L. Maternal uterine artery VEGF gene therapy for treatment of intrauterine growth restriction. Placenta 59, S44–S50 (2017).
Mehta, V. et al. Long-term increase in uterine blood flow is achieved by local overexpression of VEGF-A 165 in the uterine arteries of pregnant sheep. Gene Ther. 19, 925–935 (2012).
Carr, D. J. et al. Uteroplacental adenovirus vascular endothelial growth factor gene therapy increases fetal growth velocity in growth-restricted sheep pregnancies. Hum. Gene Ther. 25, 375–384 (2014).
Woods, A. K. et al. Adenoviral delivery of VEGF121 early in pregnancy prevents spontaneous development of preeclampsia in BPH/5 mice. Hypertension 57, 94–102 (2011).
Haase, N. et al. RNA interference therapeutics targeting angiotensinogen ameliorate preeclamptic phenotype in rodent models. J. Clin. Invest. 130, 2928–2942 (2020).
Turanov, A. A. et al. RNAi modulation of placental sFLT1 for the treatment of preeclampsia. Nat. Biotechnol. 36, 1164–1173 (2018).
Yin, H. et al. Non-viral vectors for gene-based therapy. Nat. Rev. Genet. 15, 541–555 (2014).
Swingle, K. L., Hamilton, A. G. & Mitchell, M. J. Lipid nanoparticle-mediated delivery of mRNA therapeutics and vaccines. Trends Mol. Med. 27, 616–617 (2021).
Polack Fernando, P. et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. New Engl. J. Med. 383, 2603–2615 (2020).
Baden Lindsey, R. et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. New Engl. J. Med. 384, 403–416 (2021).
Sato, Y., Kinami, Y., Hashiba, K. & Harashima, H. Different kinetics for the hepatic uptake of lipid nanoparticles between the apolipoprotein E/low density lipoprotein receptor and the N-acetyl-d-galactosamine/asialoglycoprotein receptor pathway. J. Control. Release 322, 217–226 (2020).
Cheng, Q. et al. Selective organ targeting (SORT) nanoparticles for tissue-specific mRNA delivery and CRISPR–Cas gene editing. Nat. Nanotechnol. 15, 313–320 (2020).
Dilliard, S. A., Cheng, Q. & Siegwart, D. J. On the mechanism of tissue-specific mRNA delivery by selective organ targeting nanoparticles. Proc. Natl Acad. Sci. USA 118, e2109256118 (2021).
LoPresti, S. T., Arral, M. L., Chaudhary, N. & Whitehead, K. A. The replacement of helper lipids with charged alternatives in lipid nanoparticles facilitates targeted mRNA delivery to the spleen and lungs. J. Control. Release 345, 819–831 (2022).
Swingle, K. L. et al. Ionizable lipid nanoparticles for in vivo mRNA delivery to the placenta during pregnancy. J. Am. Chem. Soc. https://doi.org/10.1021/jacs.2c12893 (2023).
Young, R. E. et al. Systematic development of ionizable lipid nanoparticles for placental mRNA delivery using a design of experiments approach. Bioact. Mater. 34, 125–137 (2024).
Chaudhary, N. et al. Lipid nanoparticle structure and delivery route during pregnancy dictate mRNA potency, immunogenicity, and maternal and fetal outcomes. Proc. Natl Acad. Sci. USA 121, e2307810121 (2024).
Safford, H. C. et al. Orthogonal design of experiments for engineering of lipid nanoparticles for mRNA delivery to the placenta. Small 20, e2303568 (2024).
Geisler, H. C. et al. EGFR-targeted ionizable lipid nanoparticles enhance in vivo mRNA delivery to the placenta. J. Control. Release 371, 455–469 (2024).
Paunovska, K. et al. A direct comparison of in vitro and in vivo nucleic acid selivery mediated by hundreds of nanoparticles reveals a weak correlation. Nano Lett. 18, 2148–2157 (2018).
Dahlman, J. E. et al. Barcoded nanoparticles for high throughput in vivo discovery of targeted therapeutics. Proc. Natl Acad. Sci. USA 114, 2060–2065 (2017).
Huayamares, S. G. et al. High-throughput screens identify a lipid nanoparticle that preferentially delivers mRNA to human tumors in vivo. J. Control. Release 357, 394–403 (2023).
El-Mayta, R. et al. A nanoparticle platform for accelerated in vivo oral delivery screening of nucleic acids. Adv. Ther. 4, 2000111 (2021).
Love, K. T. et al. Lipid-like materials for low-dose, in vivo gene silencing. Proc. Natl Acad. Sci. USA 107, 1864–1869 (2010).
Semple, S. C. et al. Rational design of cationic lipids for siRNA delivery. Nat. Biotechnol. 28, 172–176 (2010).
Jayaraman, M. et al. Maximizing the potency of siRNA lipid nanoparticles for hepatic gene silencing in vivo. Angew. Chem. 124, 8657–8661 (2012).
Dusse, L. M. et al. Revisiting HELLP syndrome. Clin. Chim. Acta 451, 117–120 (2015).
Fenton, O. S. et al. Synthesis and biological evaluation of ionizable lipid materials for the in vivo delivery of messenger RNA to B lymphocytes. Adv. Mater. 29, 1606944 (2017).
Xue, L. et al. Rational design of bisphosphonate lipid-like materials for mRNA delivery to the bone microenvironment. J. Am. Chem. Soc. 144, 9926–9937 (2022).
Kauffman, K. J. et al. Optimization of lipid nanoparticle formulations for mRNA delivery in vivo with fractional factorial and definitive Sscreening designs. Nano Lett. 15, 7300–7306 (2015).
Billingsley, M. M. et al. Orthogonal design of experiments for optimization of lipid nanoparticles for mRNA engineering of CAR T cells. Nano Lett. 22, 533–542 (2022).
Swingle, K. L. et al. Amniotic fluid stabilized lipid nanoparticles for in utero intra-amniotic mRNA delivery. J. Control. Release 341, 616–633 (2022).
Veiga, N. et al. Cell specific delivery of modified mRNA expressing therapeutic proteins to leukocytes. Nat. Commun. 9, 4493 (2018).
Parhiz, H. et al. PECAM-1 directed re-targeting of exogenous mRNA providing two orders of magnitude enhancement of vascular delivery and expression in lungs independent of apolipoprotein E-mediated uptake. J. Control. Release 291, 106–115 (2018).
Shi, D., Toyonaga, S. & Anderson, D. G. In vivo RNA delivery to hematopoietic stem and progenitor cells via targeted lipid nanoparticles. Nano Lett. 23, 2938–2944 (2023).
Breda, L. et al. In vivo hematopoietic stem cell modification by mRNA delivery. Science 381, 436–443 (2023).
Cullis, P. R. & Hope, M. J. Lipid nanoparticle systems for enabling gene therapies. Mol. Ther. 25, 1467–1475 (2017).
Irvin-Choy, N. S., Nelson, K. M., Dang, M. N., Gleghorn, J. P. & Day, E. S. Gold nanoparticle biodistribution in pregnant mice following intravenous administration varies with gestational age. Nanomed. Nanotechnol. Biol. Med. 36, 102412 (2021).
Raz, T. et al. The hemodynamic basis for positional- and inter-fetal dependent effects in dual arterial supply of mouse pregnancies. PLoS ONE 7, e52273 (2012).
Kertschanska, S., Kosanke, G. & Kaufmann, P. Pressure dependence of so-called transtrophoblastic channels during fetal perfusion of human placental villi. Microsc. Res. Tech. 38, 52–62 (1997).
Kertschanska, S., Stulcová, B., Kaufmann, P. & Stulc, J. Distensible transtrophoblastic channels in the rat placenta. Placenta 21, 670–677 (2000).
Francia, V., Schiffelers, R. M., Cullis, P. R. & Witzigmann, D. The biomolecular corona of lipid nanoparticles for gene therapy. Bioconjug. Chem. 31, 2046–2059 (2020).
Bashiri, G. et al. Nanoparticle protein corona: from structure and function to therapeutic targeting. Lab Chip 23, 1432–1466 (2023).
Akinc, A. et al. Targeted delivery of RNAi therapeutics with endogenous and exogenous ligand-based mechanisms. Mol. Ther. 18, 1357–1364 (2010).
Chamley, L. W., Allen, J. L. & Johnson, P. M. Synthesis of β2 glycoprotein 1 by the human placenta. Placenta 18, 403–410 (1997).
Robertson, S. A. et al. Effect of β2‐glycoprotein I null mutation on reproductive outcome and antiphospholipid antibody‐mediated pregnancy pathology in mice. Mol. Hum. Reprod. 10, 409–416 (2004).
Waker, C. A., Kaufman, M. R. & Brown, T. L. Current state of preeclampsia mouse models: approaches, relevance, and standardization. Front. Physiol. 12, 681632 (2021).
Ding, X., Yang, Z., Han, Y. & Yu, H. Correlation of long-chain fatty acid oxidation with oxidative stress and inflammation in pre-eclampsia-like mouse models. Placenta 36, 1442–1449 (2015).
Huai, J., Yang, Z., Yi, Y.-H. & Wang, G.-J. Different effects of pravastatin on preeclampsia-like symptoms in different mouse models. Chin. Med. J. (Engl.) 131, 461–470 (2018).
Swingle, K. L., Hamilton, A. G. & Mitchell, M. J. Flow cytometric analysis of the murine placenta to evaluate nanoparticle platforms during pregnancy. Placenta https://doi.org/10.1016/j.placenta.2024.08.007 (2024).
Umapathy, A., Chamley, L. W. & James, J. L. Reconciling the distinct roles of angiogenic/anti-angiogenic factors in the placenta and maternal circulation of normal and pathological pregnancies. Angiogenesis 23, 105–117 (2020).
Aneman, I. et al. Mechanisms of key innate immune cells in early- and late-onset preeclampsia. Front. Immunol. 11, 1864 (2020).
Bergmann, A. et al. Reduction of circulating soluble Flt-1 alleviates preeclampsia-like symptoms in a mouse model. J. Cell. Mol. Med. 14, 1857–1867 (2010).
Wu, H. et al. Arginase-1-dependent promotion of TH17 differentiation and disease progression by MDSCs in systemic lupus erythematosus. Sci. Transl. Med. 8, 331ra40 (2016).
Hamilton, A. G., Swingle, K. L. & Mitchell, M. J. Biotechnology: overcoming biological barriers to nucleic acid delivery using lipid nanoparticles. PLoS Biol. 21, e3002105 (2023).
Yan, Y. et al. Non-viral vectors for RNA delivery. J. Control. Release 342, 241–279 (2022).
Stefanovic, V. International Academy of Perinatal Medicine (IAPM) guidelines for screening, prediction, prevention and management of pre-eclampsia to reduce maternal mortality in developing countries. J. Perinat. Med. 51, 164–169 (2023).
Pardi, N., Muramatsu, H., Weissman, D. & Karikó, K. in Synthetic Messenger RNA and Cell Metabolism Modulation: Methods and Protocols Vol. 969 (ed. Rabinovich, P. M.) 29–42 (Humana, 2013).
Karikó, K. et al. Incorporation of pseudouridine into mRNA yields superior nonimmunogenic vector with increased translational capacity and biological stability. Mol. Ther. 16, 1833–1840 (2008).
Weissman, D., Pardi, N., Muramatsu, H. & Karikó, K. in Synthetic Messenger RNA and Cell Metabolism Modulation: Methods and Protocols (ed. Rabinovich, P. M.) 43–54 (Humana, 2013).
Zhang, R. et al. Helper lipid structure influences protein adsorption and delivery of lipid nanoparticles to spleen and liver. Biomater. Sci. 9, 1449–1463 (2021).
Chen, D. et al. Rapid discovery of potent siRNA-containing lipid nanoparticles enabled by controlled microfluidic formulation. J. Am. Chem. Soc. 134, 6948–6951 (2012).
Hajj, K. A. et al. Branched-tail lipid nanoparticles potently deliver mRNA in vivo due to enhanced ionization at endosomal pH. Small 15, 1805097 (2019).
Acknowledgements
M.J.M. acknowledges support from a US National Institutes of Health (NIH) Director’s New Innovator Award (DP2 TR002776), a Burroughs Wellcome Fund Career Award at the Scientific Interface (CASI), a US National Science Foundation CAREER Award (CBET-2145491) and the NIH (NICHD R01 HD115877). K.L.S., A.G.H., H.C.S., H.C.G., A.S.T., A.M.M., E.L.H. and A.J.M. acknowledge support from the US National Science Foundation Graduate Research Fellowship. R.P. was supported by an NIH National Heart, Lung, and Blood Institute Ruth L. Kirschstein Pre-Doctoral National Research Service Award. We thank the Next-Generation Sequencing Core at the University of Pennsylvania (RRID:SCR_022382) for assistance with next-generation sequencing. Data for this manuscript were generated in the Penn Cytomics and Cell Sorting Shared Resource Laboratory at the University of Pennsylvania (RRID:SCR_022376), which was partially supported by an Abramson Cancer Center NCI Grant (P30 016520). We also thank the Cell and Development Biology Microscopy Core at the University of Pennsylvania for access to the confocal laser scanning microscope used in this work (RRID:SCR_022373), and F. Chen from the Histotechnology Facility at the Wistar Institute for preparing histological samples for this work. Funding support for the Wistar Institute core facilities was provided by a Cancer Center Support Grant (P30 CA010815).
Author information
Authors and Affiliations
Contributions
K.L.S. and M.J.M. contributed to conceptualization, writing of the original draft and project administration. K.L.S., A.G.H., and M.J.M. contributed to the methodology and review and editing of the paper. K.L.S. and A.G.H. contributed to software and/or code and formal data analysis. K.L.S., A.G.H., H.C.S., H.C.G., A.S.T., R.P., A.M.M., E.L.H., A.J.M., X.H., R.A.J. and A.A.G. contributed to investigation. M.-G.A. and D.W. contributed resources. M.J.M. was responsible for supervision and funding acquisition.
Corresponding author
Ethics declarations
Competing interests
K.L.S., H.C.S., H.C.G. and M.J.M. have filed a patent application based on this work. M.J.M. is an inventor on a patent related to this work filed by the Trustees of the University of Pennsylvania (PCT/US20/56252). D.W. is an inventor on several patents related to this work filed by the Trustees of the University of Pennsylvania (11/990,646; 13/ 585,517; 13/839,023; 13/839,155; 14/456,302; 15/339,363; 16/299,202). The remaining authors declare no competing interests.
Peer review
Peer review information
Nature thanks Hideyoshi Harashima, Roy van der Meel and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 High-throughput in vivo evaluation of a 98 LNP library using molecular barcoding in non-pregnant and pregnant mice.
a, A large library of 98 LNP formulations was designed by synthesizing 24 unique ionizable lipid structures from 8 polyamine cores and 3 epoxide tails. 12 of these ionizable lipids were then further explored to formulate LNPs of varied excipient composition. b, Each of the 98 LNPs was formulated encapsulating a unique DNA barcode (b-DNA) to enable high-throughput, in vivo screening. The pooled LNPs were administered i.v. to non-pregnant and pregnant mice (n = 6 biological replicates) following which tissues were collected, processed, and prepared for next generation sequencing. Demultiplexing and subsequent data analysis identified a placenta-tropic LNP formulation. c, Heatmap depicting relative accumulation for each LNP/b-DNA in non-pregnant mouse tissues. Ion., ionizable; chol., cholesterol. Illustrations in b were created using BioRender (https://biorender.com).
Extended Data Fig. 2 Enrichment and correlation analysis of b-DNA LNP delivery in non-pregnant and pregnant mice.
a–c, Volcano plots depicting significantly enriched (top right quadrant) and significantly depleted (top left quadrant) LNPs compared with the liver-tropic C12-200 LNP formulation in (a) non-pregnant and (b) pregnant tissues as well as (c) placentas and fetuses. Normalized delivery is reported as the mean (n = 6 biological replicates). For each tissue, two-sided, one-way ANOVAs with post hoc Student’s t tests using the Holm-Bonferroni correction for multiple comparisons with the C12-200 LNP were used to compare normalized delivery across LNP formulations for generating p values. d–g, The squared Pearson’s correlation coefficient for mean normalized delivery (r2) was calculated for each tissue pair and is presented as a heatmap for (d) non-pregnant mouse tissues, (e) pregnant mouse tissues, (f) between non-pregnant and pregnant mouse tissues, and (g) between pregnant mouse tissues and the placentas and fetuses. Dist., distal; Prox., proximal.
Extended Data Fig. 3 Validation of results from high-throughput screening via luciferase mRNA delivery in non-pregnant mice.
LNP 6 (negative control), LNP 55 (placenta-tropic), LNP 97 (C12-200), and LNP 98 (DLin-MC3-DMA) were formulated with luciferase mRNA and administered to non-pregnant mice at a dose of 0.6 mg kg−1 mRNA. a, Six hours after administration, tissues (H: heart, Lu: lungs, Li: liver, K: kidneys, S: spleen) were dissected and imaged using an in vivo imaging system (IVIS). b–e, Luminescence was quantified in the (b) lungs, (c) liver, and (d) spleen which was then used to calculate (e) a spleen-to-liver ratio. Luminescence measurements are reported as the mean ± s.e.m. (n = 4 biological replicates). Ordinary two-sided, one-way ANOVAs with post hoc Student’s t tests using the Holm-Šídák correction for multiple comparisons were used to compare luminescence across treatment groups.
Extended Data Fig. 4 Cellular LNP delivery in the spleen and placenta for industry and clinical standard LNPs in healthy and inflammation-induced pre-eclamptic mice.
To evaluate differences in biodistribution between healthy and pre-eclamptic pregnant mice,inflammation-induced pre-eclampsia was established via i.p. administration of 1 µg kg−1 lipopolysaccharide (LPS). DiD-labelled LNPs 97 and 98 were administered at an mRNA dose of 1 mg kg−1. Twelve hours later, cellular LNP delivery was evaluated in the (a–f) spleen and (g–l) placenta via flow cytometry. The percentage of DiD+ cells is reported as the mean ± s.e.m. (PBS, LNP 97, LNP 98, LPS + LNP 98: n = 4 biological replicates; LPS + LNP 97: n = 3 biological replicates). Either ordinary (a–f) or nested (g–l) two-sided, one-way ANOVAs with post hoc Student’s t tests using the Holm-Šídák correction for multiple comparisons were used to compare the percentage of DiD+ cells across treatment groups. CK7: cytokeratin 7.
Extended Data Fig. 5 VEGF mRNA LNP 55 improves maternal weight and serum concentration of inflammatory cytokines in inflammation-induced pre-eclampsia.
Inflammation-induced pre-eclampsia was established through i.p. administration of 1 µg kg−1 lipopolysaccharide (LPS) on gestational day E7.5. 1 mg kg−1 VEGF mRNA LNP 55 was then administered i.v. on gestational day E11. a–c, Change in maternal weight was measured daily (a), and on gestational day E17 (b,c) fetal and placental weight were recorded. d–j, Serum levels of (d) VEGF, (e) sFlt-1, (f) alanine transaminase (ALT), (g) aspartate aminotransferase (AST), (h) tumour necrosis factor (TNF), (i) interleukin-6 (IL-6), and (j) interferon-γ (IFNγ) were evaluated on gestational days E11.5 and E17. k, Mean blood vessel area in the placental labyrinth was quantified from H&E-stainined placental sections. Maternal weight change and serum protein levels are reported as the mean ± s.e.m. (n = 8 biological replicates). Fetal and placental weight are reported as the median with the 25th and 75th percentiles (n = 8 biological replicates, 6–9 fetuses or placentas per mouse). Mean blood vessel area is reported as the median with the 25th and 75th percentiles (n = 8 biological replicates, 1–2 placentas per mouse, 2 sections per placenta, 2–3 images per section). Ordinary two-sided, two-way (a, d–j) or nested two-sided, one-way (b–c, k) ANOVAs with post hoc Student’s t tests using the Holm-Šídák correction for multiple comparisons were used to compare responses across treatment groups.
Extended Data Fig. 6 VEGF mRNA LNP 55 reduces liver enzyme levels in serum and improves placental blood vessel area in hypoxia-induced pre-eclampsia.
Hypoxia-induced pre-eclampsia was established through i.v. administration of 1 × 109 plaque forming units (PFU) of soluble Fms-like tyrosine kinase-1 adenovirus (Adv-sFlt-1) on gestational day E7.5. 1 mg kg−1 VEGF mRNA LNPs 55 or 98 were then administered i.v. on gestational day E11. a–d, Maternal weight change was recorded daily (a), and on gestational day E17 (b) total litter weight, (c) litter size, and (d) albumin concentration in urine were measured. e–j, Serum levels of (e) VEGF, (f) alanine transaminase (ALT), (g) aspartate aminotransferase (AST), (h) tumour necrosis factor (TNF), (i) interleukin-6 (IL-6), and (j) interferon-γ (IFNγ) were evaluated on gestational days E11.5 and E17. k, Placental vasculature in the labyrinth was visualized with H&E staining; stained sections were used to quantify mean blood vessel area. l, Similarly, renal histology was visualized using H&E staining with arrows indicating glomeruli. Maternal weight change, total litter weight, litter size, urine albumin concentration, and serum protein levels are reported as the mean ± s.e.m. (Adv-sFlt-1 + VEGF mRNA LNP 55: n = 5 biological replicates; PBS, Adv-sFlt-1: n = 4 biological replicates; Adv-sFlt-1 + VEGF mRNA LNP 98: n = 3 biological replicates). Mean blood vessel area is reported as the median with the 25th and 75th percentiles (1–2 placentas per mouse, 2 sections per placenta, 2–3 images per section). Ordinary (b–d) or nested (k) two-sided, one-way ANOVAs or ordinary two-sided, two-way ANOVAs (a, e–j) with post hoc Student’s t tests using the Holm-Šídák correction for multiple comparisons were used to compare responses across treatment groups.
Extended Data Fig. 7 In inflammation-induced pre-eclampsia, VEGF mRNA LNP 55 partially restores a healthy immune landscape in the blood and placenta.
a–c, Immunophenotyping was performed to evaluate differences in the proportion of immune cell populations in the (a) blood, (b) spleen, and (c) placenta in inflammation-induced pre-eclampsia following administration of the VEGF mRNA LNP 55 therapeutic. The proportion of immune cells are reported as mean ± s.e.m. (n = 8 biological replicates). Ordinary two-sided, one-way (a–b) or nested two-sided, one-way (c) ANOVAs with post hoc Student’s t tests using the Holm-Šídák correction for multiple comparisons were used to compare responses across treatment groups.
Supplementary information
Supplementary Information
Supplementary Figs. 1–29 and Tables 1–13.
Supplementary Data
Source data for Supplementary Figs. 3, 9, 11, 12, 14–18, 20, 23, 24 and 26–28.
Source data
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Swingle, K.L., Hamilton, A.G., Safford, H.C. et al. Placenta-tropic VEGF mRNA lipid nanoparticles ameliorate murine pre-eclampsia. Nature 637, 412–421 (2025). https://doi.org/10.1038/s41586-024-08291-2
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41586-024-08291-2
This article is cited by
-
Peptide codes for organ-selective mRNA delivery
Nature Materials (2026)
-
Design principles of lipid nanoparticles for RNA delivery
Nature Reviews Bioengineering (2026)
-
Spatiotemporal targeting of messenger RNA lipid nanoparticles to the endometrium for the treatment of reproductive disorders
Nature Nanotechnology (2026)
-
The RNA delivery dilemma—lipid versus polymer nanoparticle platforms
Drug Delivery and Translational Research (2026)
-
Protein corona formed on lipid nanoparticles compromises delivery efficiency of mRNA cargo
Nature Communications (2025)