Abstract
Psilocybin is a serotonergic psychedelic with therapeutic potential for treating mental illnesses1,2,3,4. At the cellular level, psychedelics induce structural neural plasticity5,6, exemplified by the drug-evoked growth and remodelling of dendritic spines in cortical pyramidal cells7,8,9. A key question is how these cellular modifications map onto cell-type-specific circuits to produce the psychedelics’ behavioural actions10. Here we use in vivo optical imaging, chemogenetic perturbation and cell-type-specific electrophysiology to investigate the impact of psilocybin on the two main types of pyramidal cells in the mouse medial frontal cortex. We find that a single dose of psilocybin increases the density of dendritic spines in both the subcortical-projecting, pyramidal tract (PT) and intratelencephalic (IT) cell types. Behaviourally, silencing the PT neurons eliminates psilocybin’s ability to ameliorate stress-related phenotypes, whereas silencing IT neurons has no detectable effect. In PT neurons only, psilocybin boosts synaptic calcium transients and elevates firing rates acutely after administration. Targeted knockout of 5-HT2A receptors abolishes psilocybin’s effects on stress-related behaviour and structural plasticity. Collectively, these results identify that a pyramidal cell type and the 5-HT2A receptor in the medial frontal cortex have essential roles in psilocybin’s long-term drug action.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
Details for sample sizes and statistical tests for all experiments are provided in Supplementary Table 1. Data associated with the study are available at GitHub (https://github.com/Kwan-Lab/shaoliao2025). The RNA-seq dataset was obtained from publicly available sources at the Allen Institute (https://doi.org/10.1016/j.cell.2021.04.021). Source data are provided with this paper.
Code availability
Code for data analysis associated with the study are available at GitHub (https://github.com/Kwan-Lab/shaoliao2025), and from the corresponding author on request.
Change history
29 September 2025
A Correction to this paper has been published: https://doi.org/10.1038/s41586-025-09671-y
References
Goodwin, G. M. et al. Single-dose psilocybin for a treatment-resistant episode of major depression. N. Engl. J. Med. 387, 1637–1648 (2022).
Davis, A. K. et al. Effects of psilocybin-assisted therapy on major depressive disorder: a randomized clinical trial. JAMA Psychiatry 78, 481–489 (2021).
Carhart-Harris, R. et al. Trial of psilocybin versus escitalopram for depression. N. Engl. J. Med. 384, 1402–1411 (2021).
Bogenschutz, M. P. et al. Percentage of heavy drinking days following psilocybin-assisted psychotherapy vs placebo in the treatment of adult patients with alcohol use disorder: a randomized clinical trial. JAMA Psychiatry 79, 953–962 (2022).
Ly, C. et al. Psychedelics promote structural and functional neural plasticity. Cell Rep. 23, 3170–3182 (2018).
Jones, K. A. et al. Rapid modulation of spine morphology by the 5-HT2A serotonin receptor through kalirin-7 signaling. Proc. Natl Acad. Sci. USA 106, 19575–19580 (2009).
Shao, L. X. et al. Psilocybin induces rapid and persistent growth of dendritic spines in frontal cortex in vivo. Neuron 109, 2535–2544 (2021).
de la Fuente Revenga, M. et al. Prolonged epigenomic and synaptic plasticity alterations following single exposure to a psychedelic in mice. Cell Rep. 37, 109836 (2021).
Jefferson, S. J. et al. 5-MeO-DMT modifies innate behaviors and promotes structural neural plasticity in mice. Neuropsychopharmacology 48, 1257–1266 (2023).
Kwan, A. C., Olson, D. E., Preller, K. H. & Roth, B. L. The neural basis of psychedelic action. Nat. Neurosci. 25, 1407–1419 (2022).
Duman, R. S. & Aghajanian, G. K. Synaptic dysfunction in depression: potential therapeutic targets. Science 338, 68–72 (2012).
Cameron, L. P. et al. A non-hallucinogenic psychedelic analogue with therapeutic potential. Nature 589, 474–479 (2021).
Lu, J. et al. An analog of psychedelics restores functional neural circuits disrupted by unpredictable stress. Mol. Psychiatry 26, 6237–6252 (2021).
Anastasiades, P. G. & Carter, A. G. Circuit organization of the rodent medial prefrontal cortex. Trends Neurosci. 44, 550–563 (2021).
Baker, A. et al. Specialized subpopulations of deep-layer pyramidal neurons in the neocortex: bridging cellular properties to functional consequences. J. Neurosci. 38, 5441–5455 (2018).
Shepherd, G. M. Corticostriatal connectivity and its role in disease. Nat. Rev. Neurosci. 14, 278–291 (2013).
Li, N., Chen, T. W., Guo, Z. V., Gerfen, C. R. & Svoboda, K. A motor cortex circuit for motor planning and movement. Nature 519, 51–56 (2015).
Musall, S. et al. Pyramidal cell types drive functionally distinct cortical activity patterns during decision-making. Nat. Neurosci. 26, 495–505 (2023).
Tang, L. & Higley, M. J. Layer 5 circuits in V1 differentially control visuomotor behavior. Neuron 105, 346–354 (2020).
Garcia, A. F., Crummy, E. A., Webb, I. G., Nooney, M. N. & Ferguson, S. M. Distinct populations of cortical pyramidal neurons mediate drug reward and aversion. Nat. Commun. 12, 182 (2021).
Davies, M. F., Deisz, R. A., Prince, D. A. & Peroutka, S. J. Two distinct effects of 5-hydroxytryptamine on single cortical neurons. Brain Res. 423, 347–352 (1987).
Araneda, R. & Andrade, R. 5-Hydroxytryptamine2 and 5-hydroxytryptamine1A receptors mediate opposing responses on membrane excitability in rat association cortex. Neuroscience 40, 199–412 (1991).
Avesar, D. & Gulledge, A. T. Selective serotonergic excitation of callosal projection neurons. Front. Neural Circuits 6, 12 (2012).
Elliott, M. C., Tanaka, P. M., Schwark, R. W. & Andrade, R. Serotonin differentially regulates L5 pyramidal cell classes of the medial prefrontal cortex in rats and mice. eNeuro https://doi.org/10.1523/ENEURO.0305-17.2018 (2018).
Amargos-Bosch, M. et al. Co-expression and in vivo interaction of serotonin1A and serotonin2A receptors in pyramidal neurons of prefrontal cortex. Cereb. Cortex 14, 281–299 (2004).
Savalia, N. K., Shao, L. X. & Kwan, A. C. A dendrite-focused framework for understanding the actions of ketamine and psychedelics. Trends Neurosci. 44, 260–275 (2021).
Puig, M. V., Celada, P., Diaz-Mataix, L. & Artigas, F. In vivo modulation of the activity of pyramidal neurons in the rat medial prefrontal cortex by 5-HT2A receptors: relationship to thalamocortical afferents. Cereb. Cortex 13, 870–882 (2003).
Kim, Y. et al. Whole-brain mapping of neuronal activity in the learned helplessness model of depression. Front. Neural Circuits 10, 3 (2016).
Davoudian, P. A., Shao, L. X. & Kwan, A. C. Shared and distinct brain regions targeted for immediate early gene expression by ketamine and psilocybin. ACS Chem. Neurosci. 14, 468–480 (2023).
Armbruster, B. N., Li, X., Pausch, M. H., Herlitze, S. & Roth, B. L. Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand. Proc. Natl Acad. Sci. USA 104, 5163–5168 (2007).
Matho, K. S. et al. Genetic dissection of the glutamatergic neuron system in cerebral cortex. Nature 598, 182–187 (2021).
Nagai, Y. et al. Deschloroclozapine, a potent and selective chemogenetic actuator enables rapid neuronal and behavioral modulations in mice and monkeys. Nat. Neurosci. 23, 1157–1167 (2020).
Halberstadt, A. L., Chatha, M., Klein, A. K., Wallach, J. & Brandt, S. D. Correlation between the potency of hallucinogens in the mouse head-twitch response assay and their behavioral and subjective effects in other species. Neuropharmacology 167, 107933 (2020).
Malenka, R. C., Lancaster, B. & Zucker, R. S. Temporal limits on the rise in postsynaptic calcium required for the induction of long-term potentiation. Neuron 9, 121–128 (1992).
Bittner, K. C., Milstein, A. D., Grienberger, C., Romani, S. & Magee, J. C. Behavioral time scale synaptic plasticity underlies CA1 place fields. Science 357, 1033–1036 (2017).
Lee, S. J., Escobedo-Lozoya, Y., Szatmari, E. M. & Yasuda, R. Activation of CaMKII in single dendritic spines during long-term potentiation. Nature 458, 299–304 (2009).
Friedrich, J., Zhou, P. & Paninski, L. Fast online deconvolution of calcium imaging data. PLoS Comput. Biol. 13, e1005423 (2017).
Ali, F. et al. Ketamine disinhibits dendrites and enhances calcium signals in prefrontal dendritic spines. Nat. Commun. 11, 72 (2020).
Chen, T. W. et al. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature 499, 295–300 (2013).
DeFelipe, J., Arellano, J. I., Gomez, A., Azmitia, E. C. & Munoz, A. Pyramidal cell axons show a local specialization for GABA and 5-HT inputs in monkey and human cerebral cortex. J. Comp. Neurol. 433, 148–155 (2001).
Jun, J. J. et al. Fully integrated silicon probes for high-density recording of neural activity. Nature 551, 232–236 (2017).
Cameron, L. P. et al. 5-HT2ARs mediate therapeutic behavioral effects of psychedelic tryptamines. ACS Chem. Neurosci. 14, 351–358 (2023).
Hesselgrave, N., Troppoli, T. A., Wulff, A. B., Cole, A. B. & Thompson, S. M. Harnessing psilocybin: antidepressant-like behavioral and synaptic actions of psilocybin are independent of 5-HT2R activation in mice. Proc. Natl Acad. Sci. USA 118, e2022489118 (2021).
Sekssaoui, M., Bockaert, J., Marin, P. & Bécamel, C. Antidepressant-like effects of psychedelics in a chronic despair mouse model: is the 5-HT2A receptor the unique player? Neuropsychopharmacology 49, 747–756 (2024).
Choi, W. et al. Serotonin signals through a gut-liver axis to regulate hepatic steatosis. Nat. Commun. 9, 4824 (2018).
Yao, Z. et al. A taxonomy of transcriptomic cell types across the isocortex and hippocampal formation. Cell 184, 3222–3241 (2021).
Aghajanian, G. K. & Marek, G. J. Serotonin induces excitatory postsynaptic potentials in apical dendrites of neocortical pyramidal cells. Neuropharmacology 36, 589–599 (1997).
Kaplan, A. L. et al. Bespoke library docking for 5-HT2A receptor agonists with antidepressant activity. Nature 610, 582–591 (2022).
Cao, D. et al. Structure-based discovery of nonhallucinogenic psychedelic analogs. Science 375, 403–411 (2022).
Dembrow, N. C., Zemelman, B. V. & Johnston, D. Temporal dynamics of L5 dendrites in medial prefrontal cortex regulate integration versus coincidence detection of afferent inputs. J. Neurosci. 35, 4501–4514 (2015).
Anastasiades, P. G., Collins, D. P. & Carter, A. G. Mediodorsal and ventromedial thalamus engage distinct L1 circuits in the prefrontal cortex. Neuron 109, 314–330 (2021).
Silberberg, G. & Markram, H. Disynaptic inhibition between neocortical pyramidal cells mediated by Martinotti cells. Neuron 53, 735–746 (2007).
Wu, S. J. et al. Cortical somatostatin interneuron subtypes form cell-type-specific circuits. Neuron 111, 2675–2692 (2023).
Suzuki, M. & Larkum, M. E. General anesthesia decouples cortical pyramidal neurons. Cell 180, 666–676 (2020).
Bharioke, A. et al. General anesthesia globally synchronizes activity selectively in layer 5 cortical pyramidal neurons. Neuron 110, 2024–2040 (2022).
Wilkinson, S. T., Holtzheimer, P. E., Gao, S., Kirwin, D. S. & Price, R. B. Leveraging neuroplasticity to enhance adaptive learning: the potential for synergistic somatic-behavioral treatment combinations to improve clinical outcomes in depression. Biol. Psychiatry 85, 454–465 (2019).
Feng, G. et al. Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of GFP. Neuron 28, 41–51 (2000).
Tervo, D. G. et al. A designer AAV variant permits efficient retrograde access to projection. Neuron 92, 372–382 (2016).
Shamash, P., Carandini, M., Harris, K. D. & Steinmetz, N. A. A tool for analyzing electrode tracks from slice histology. Preprint at bioRxiv https://doi.org/10.1101/447995 (2018).
Wang, Q. et al. The Allen Mouse Brain Common Coordinate Framework: a 3D reference atlas. Cell 181, 936–953 (2020).
Claudi, F. et al. Visualizing anatomically registered data with brainrender. eLife 10, e65751 (2021).
Pologruto, T. A., Sabatini, B. L. & Svoboda, K. ScanImage: flexible software for operating laser scanning microscopes. Biomed. Eng. Online 2, 13 (2003).
Thevenaz, P., Ruttimann, U. E. & Unser, M. A pyramid approach to subpixel registration based on intensity. IEEE Trans. Image Process. 7, 27–41 (1998).
Holtmaat, A. et al. Long-term, high-resolution imaging in the mouse neocortex through a chronic cranial window. Nat. Protoc. 4, 1128–1144 (2009).
Mitrić, M. et al. Layer- and subregion-specific electrophysiological and morphological changes of the medial prefrontal cortex in a mouse model of neuropathic pain. Sci. Rep. 9, 9479 (2019).
Radnikow, G. & Feldmeyer, D. Layer- and cell type-specific modulation of excitatory neuronal activity in the neocortex. Front. Neuroanat. 12, 1 (2018).
Pnevmatikakis, E. A. & Giovannucci, A. NoRMCorre: an online algorithm for piecewise rigid motion correction of calcium imaging data. J. Neurosci. Methods 291, 83–94 (2017).
Ali, F. et al. Inhibitory regulation of calcium transients in prefrontal dendritic spines is compromised by a nonsense Shank3 mutation. Mol. Psychiatry 26, 1945–1966 (2021).
Lutcke, H., Gerhard, F., Zenke, F., Gerstner, W. & Helmchen, F. Inference of neuronal network spike dynamics and topology from calcium imaging data. Front. Neural Circuits 7, 201 (2013).
Friedman, A. et al. A corticostriatal path targeting striosomes controls decision-making under conflict. Cell 161, 1320–1333 (2015).
Boudreau, E. et al. Intraperitoneal catheter placement for pharmacological imaging studies in conscious mice. Lab. Anim. 39, 23–25 (2010).
Siegle, J. H. et al. Open Ephys: an open-source, plugin-based platform for multichannel electrophysiology. J. Neural Eng. 14, 045003 (2017).
Buccino, A. P. et al. SpikeInterface, a unified framework for spike sorting. eLife 9, e61834 (2020).
Pachitariu, M. et al. Kilosort: realtime spike-sorting for extracellular electrophysiology with hundreds of channels. Preprint at bioRxiv https://doi.org/10.1101/061481 (2016).
Liu, R. J. & Aghajanian, G. K. Stress blunts serotonin- and hypocretin-evoked EPSCs in prefrontal cortex: role of corticosterone-mediated apical dendritic atrophy. Proc. Natl Acad. Sci. USA 105, 359–364 (2008).
Acknowledgements
We thank L. Sun for help with analysing the slice electrophysiology data. Psilocybin was provided by Usona Institute’s Investigational Drug & Material Supply Program. The Usona Institute IDMSP is supported by A. Sherwood, R. Kargbo and K. Kaylo. This work was supported by NIH grants R01MH121848, R01MH128217, R01MH137047, U01NS128660, One Mind–COMPASS Rising Star Award (A.C.K.); NIH training grants T32GM007205 (P.A.D. and N.K.S.), T32NS041228 (C.L.); NIH fellowships F30DA059437 (P.A.D.) and F30MH129085 (N.K.S.); Source Research Foundation student grant (P.A.D.); NIH instrumentation grants S10RR025502 and S10OD032251 (Cornell Biotechnology Resource Center Imaging Facility); NIH grants R00NS114166, R01NS133434 and R01DA059378 (A.C.); State of Connecticut, Department of Mental Health and Addiction Services (A.C. and R.-J.L.).
Author information
Authors and Affiliations
Contributions
L.-X.S., C.L. and A.C.K. planned the study. L.-X.S. and C.L. conducted and analysed the imaging and behavioural experiments. P.A.D. and Q.J. conducted and analysed the electrophysiological experiments. N.K.S. and O.M.B. analysed the dendritic calcium imaging data. R.-J.L. and A.C. conducted slice electrophysiology experiments. Q.J. assisted in animal surgery. Q.J., D.T., C.W. and J.D.N. assisted in behavioural experiments and histology. S.C.W. and C.W. conducted pilot studies to validate the protocols for the behavioural assays. H.K. generated and provided the Htr2af/f mice. L.-X.S., C.L. and A.C.K. drafted the manuscript. All of the authors reviewed the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
A.C.K. has been a scientific advisor or consultant for Boehringer Ingelheim, Empyrean Neuroscience, Freedom Biosciences and Xylo Bio. A.C.K. has received research support from Intra-Cellular Therapies. The other authors declare no competing interests.
Peer review
Peer review information
Nature thanks Adema Ribic, Bryan Roth and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Distinct projection targets and morphological differences between PT and IT neurons.
a, To express GFP in IT neurons, we injected AAV-CAG-FLEX-eGFP in the ACAd and medial MOs portion of medial frontal cortex and low titre of the retrogradely transported AAVretro-hSyn-Cre in the contralateral striatum of adult C57BL/6J mice. Post hoc histology and imaging of the GFP fluorescence in coronal sections shows ipsilateral and contralateral projections to various striatal and cortical regions. CP, caudoputamen. b, To express GFP in PT neurons, we injected AAV-CAG-FLEX-eGFP in the ACAd and medial MOs portion of medial frontal cortex and low titre of the retrogradely transported AAVretro-hSyn-Cre in the ipsilateral pons of adult C57BL/6J mice. Post hoc histology and imaging of the GFP fluorescence in coronal sections shows ipsilateral projections to striatum and subcortical regions including the pons (lower rightmost image). c, In vivo two-photon images of apical dendrites from PT and IT neurons targeted to express GFP using retrogradely transported viruses. d, Baseline spine density for all imaged dendrites on Day -3 prior to any psilocybin or saline administration. PT neurons have lower spine density than IT neurons (P < 0.001, two-sample t-test). Yellow, PT neurons. Purple, IT neurons. PT: n = 160 branches from 17 mice. IT: n = 142 branches from 16 mice. e, Similar to (d) for spine head width. PT: n = 1071 spines from 6 mice. IT: n = 615 spines from 5 mice. PT neurons have larger spine head width than IT neurons (P < 0.001, two-sample t-test). ***, p < 0.001. Detailed sample size n values are provided in Methods. Statistical analyses are provided in Supplementary Table 1.
Extended Data Fig. 2 Effects of psilocybin on structural plasticity in frontal cortical PT and IT neurons.
a, b, Potential differential effect of psilocybin on dendritic spine head width in frontal cortical PT and IT neurons. Spine head width in the apical tuft of PT neurons (a) after psilocybin (yellow; 1 mg/kg, i.p.) or saline (grey) across days, expressed as fold-change from baseline in first imaging session (day -3). b, Similar to (a) for IT neurons after psilocybin (purple) or saline (light purple). There was cell-type difference in psilocybin’s effect on spine head width (interaction effect of treatment × time × cell type: P = 0.007, mixed effects model). These results show that PT neurons have a more pronounced increase in spine head width than IT neurons, indicative of a strengthening of excitatory connections in addition to gaining new inputs for PT neurons. This enlargement in spine head for PT neurons was less durable than the changes in spine density, returning to baseline after 35 days. We note the results should be interpreted with the consideration that the spine head width is near the spatial resolution limit for in vivo two-photon microscopy. c, Density of dendritic spines in the apical tuft of PT neurons after psilocybin (yellow; 1 mg/kg, i.p.) or saline (grey) across days. d-f, Similar to (c) for spine head width, formation rate, and elimination rate. g-j, Similar to (c-f) for IT neurons. These figure panels correspond to Fig. 1i–n and panel (a,b in this figure), except here across-dendrite values are shown, without taking advantage of the longitudinal data for within-dendrite baseline normalization. n = 8 mice (PT neurons, saline), n = 9 mice (PT neurons, psilocybin) in (a, c-f). n = 8 mice (IT neurons, saline), n = 8 mice (IT neurons, psilocybin) in (b, g-j). k-r, Psilocybin effects on structural plasticity in PT and IT neurons by sex of the animals. k, Density of dendritic spines in apical tuft of PT neurons in female (top row) and male mice (bottom row) after psilocybin (1 mg/kg, i.p.) or saline across days, expressed as fold-change from baseline in first imaging session (day -3). l, Similar to (k) for spine head width. m, Spine formation rate determined by number of new and existing spines in consecutive imaging sessions across two-day interval, expressed as difference from baseline in first interval (day -3 to day -1). n, Similar to (m) for elimination rate. n = 4 mice (PT neurons, saline, female), n = 4 (PT neurons, psilocybin, female), n = 4 (PT neurons, saline, male), n = 5 (PT neurons, psilocybin, male) in (k-n). o-r, Similar to (k-n) for IT neurons in female (top row) and male mice (bottom row). n = 5 mice (IT neurons, saline, female), n = 5 (IT neurons, psilocybin, female), n = 3 (IT neurons, saline, male), n = 3 (IT neurons, psilocybin, male). We did not detect effect of sex for any of the measures (interaction effect of treatment × sex × cell type, indicated in plots, mixed effects model). s-v, Psilocybin has no effect on spine protrusion length. s, Protrusion length of dendritic spines in apical tuft of PT neurons for all mice (left), or separately plotted for females (middle) and males (right), after psilocybin (1 mg/kg, i.p.) or saline across days, expressed as fold-change from baseline in first imaging session (day -3). t, Similar to (s) for IT neurons. Psilocybin had no detectable effect on spine protrusion length (main effect of treatment: P = 0.309, mixed effects model). u, Protrusion length of dendritic spines in apical tuft of PT neurons for all mice after psilocybin (1 mg/kg, i.p.) or saline across days, without taking advantage of the longitudinal data for within-dendrite baseline normalization. v, Similar to (u) for IT neurons. n = 8 mice (PT neurons, saline, 4 females, 4 males), n = 9 (PT neurons, psilocybin, 4 females, 5 males) in (s, u). n = 8 (IT neurons, saline, 5 females, 3 males), n = 8 (IT neurons, psilocybin, 5 females, 3 males) in (t, v). Data are mean and s.e.m. across dendrites. *, p < 0.05. ***, p < 0.001, post hoc with Bonferroni correction for multiple comparisons. Detailed sample size n values are provided in Methods. Statistical analyses are provided in Supplementary Table 1.
Extended Data Fig. 3 Effects of psilocybin on structural plasticity of IT neurons residing in layer 2/3 or layer 5.
a, Volumetric reconstruction from z-stack images of GFP-expressing IT neurons. IT neurons can be divided into two groups based on the laminar position of their cell body (200–400 μm or 450–600 μm). For the deep-lying IT neurons, sometimes the cell body could not be imaged due to the depth limitation of two-photon microscopy, but nonetheless the apical trunk was observed at >450 μm. b, Density of dendritic spines (left), spine head width (middle), and spine protrusion length (right) in apical tuft of IT neurons residing in layer 2/3 (top row) or layer 5 (bottom row) after psilocybin (1 mg/kg, i.p.) or saline across days, expressed as fold-change from baseline in first imaging session (day -3). The same mice were used for both depth ranges (200–400 μm and 450–600 μm). c, Left: spine formation rate determined by number of new and existing spines in consecutive imaging sessions across two-day interval, expressed as difference from baseline in first interval (day -3 to day -1). Right: similar to left for elimination rate. d-e, Similar to (b) for density of dendritic spines and spine head width but further divided the data based on the sex of the animal. n = 8 mice (IT neurons, saline, 5 females, 3 males), n = 8 (IT neurons, psilocybin, 5 females, 3 males) in (b-e). The analysis was motivated by the question: is psilocybin-evoked increase in spine size specific to cell type (IT versus PT), or specific to laminar position (layer 2/3 versus layer 5)? This is because IT neurons can be both superficial and deep, but PT is only found in deep layer. We detected no significant depth dependence for spine size for layer 2/3 and deep layer 5 IT neurons (interaction effect of treatment × soma depth, indicated in plots, mixed effects model). Data are mean and s.e.m. across dendrites. Detailed sample size n values are provided in Methods. Statistical analyses are provided in Supplementary Table 1.
Extended Data Fig. 4 Effects of psilocybin and chemogenetic manipulation of PT and IT neurons on behaviour by sex of the animals.
a, For head-twitch response assessed 10 min after drug administration, effect of chemogenetic inactivation in male (left) and female (right) Fezf2-creER mice during psilocybin (1 mg/kg, i.p.) or saline administration. Circle, individual animal. n = 14 (mCherry_psilocybin, 6 males and 8 females), n = 13 (mCherry_saline, 6 males and 7 females), n = 20 (hM4DGi_psilocybin, 12 males and 8 females), and n = 15 (hM4DGi_saline, 7 males and 8 females). b, Similar to (a) for Plxnd1-creER mice. n = 15 (mCherry_psilocybin, 8 males and 7 females), n = 12 (mCherry_saline, 6 males and 6 females), n = 11 (hM4DGi_psilocybin, 6 males and 5 females), and n = 13 (hM4DGi_saline, 7 males and 6 females). c-d, Similar to (a-b) for learned helplessness assessed 24 h after drug administration. For both male and female mice, there were significant effect of PT inactivation interfering with psilocybin’s impact on learned helplessness (interaction effect of treatment and DREADD: P = 0.001 for males and P = 0.009 for females, two-factor ANOVA). Fezf2-creER mice: n = 13 (mCherry_psilocybin, 8 males and 5 females), n = 15 (mCherry_saline, 8 males and 7 females), n = 16 (hM4DGi_psilocybin, 9 males and 7 females), and n = 13 (hM4DGi_saline, 5 males and 8 females). Plxnd1-creER mice: n = 14 (mCherry_psilocybin, 7 males and 7 females), n = 11 (mCherry_saline, 5 males and 6 females), n = 11 (hM4DGi_psilocybin, 6 males and 5 females), and n = 11 (hM4DGi_saline, 5 males and 6 females). e-f, Similar to (a-b) for tail suspension test assessed 24 h after drug administration. For male mice, there was significant effect of PT inactivation interfering with psilocybin’s impact on tail suspension (interaction effect of treatment and DREADD: P = 0.002 for males and P = 0.08 for females, two-factor ANOVA). Fezf2-creER mice: n = 14 (mCherry_psilocybin, 7 males and 7 females), n = 10 (mCherry_saline, 5 males and 5 females), n = 13 (hM4DGi_psilocybin, 6 males and 7 females), and n = 12 (hM4DGi_saline, 8 males and 4 females). Plxnd1-creER mice: n = 14 (mCherry_psilocybin, 7 males and 7 females), n = 9 (mCherry_saline, 5 males and 4 females), n = 9 (hM4DGi_psilocybin, 5 males and 4 females), and n = 9 (hM4DGi_saline, 5 males and 4 females). *, p < 0.05. ** p < 0.01. ***, p < 0.001, post hoc with Bonferroni correction for multiple comparisons. Data are mean and s.e.m. across mice. Detailed sample size n values are provided in Methods. Statistical analyses are provided in Supplementary Table 1.
Extended Data Fig. 5 Frontal cortical PT neurons are essential for psilocybin’s facilitating effects on fear extinction.
a, Chronic stress-induced resistance to fear extinction. b, Time spent freezing during conditioning. c, Time spent freezing during extinction session. Chemogenetic inactivation of frontal cortical PT neurons significantly diminished psilocybin’s facilitating effect (interaction effect of treatment × DREADD: P = 0.003, two-factor ANOVA). d-e, Similar to (c) for extinction retention sessions. n = 13 (mCherry, saline), n = 17 (mCherry, psilocybin), n = 15 (hM4DGi, saline), n = 13 (hM4DGi, psilocybin) in (b-d). n = 13 (mCherry, saline), n = 16 (mCherry, psilocybin), n = 15 (hM4DGi, saline), n = 13 (hM4DGi, psilocybin) in (e). Data are mean and s.e.m. across mice. *, p < 0.05. ** p < 0.01. ***, p < 0.001, post hoc with Bonferroni correction for multiple comparisons. Detailed sample size n values are provided in Methods. Statistical analyses are provided in Supplementary Table 1.
Extended Data Fig. 6 Example ΔF/F0 traces from the dendritic branches and spines of PT and IT cells.
a, An example in vivo two-photon image of GCaMP6f-expressing apical dendrite and spines from a PT neuron. Dashed lines, the dendritic branch. Arrows, spines attached to the branch. b, Top, ΔF/F0 trace of a PT dendritic branch (ΔF/Fbranch). Middle, ΔF/F0 trace recorded from a dendritic spine attached to that branch (ΔF/Fspine). Bottom, the branch-subtracted spine calcium signal (ΔF/Fsynaptic), subtracting the scaled version of ΔF/Fbranch signal from the ΔF/Fspine signals. The synaptic calcium signals of dendritic spines shown and analysed in the paper are branch-subtracted ΔF/Fspine transients. Note that negative values can appear due to the subtraction procedure; however, negative transients are not detected by the peeling algorithm and therefore do not impact the subsequent analyses. Left, ΔF/F0 signals before psilocybin. Right, ΔF/F0 signals after psilocybin. c, Similar to (b) for ΔF/F0 traces in an IT neuron. d, Left, ΔF/F0 from two different dendritic branches of PT neurons before and after saline. Right, ΔF/F0 from two different dendritic branches of PT neurons, before and after psilocybin (1 mg/kg, i.p.). e, Similar to (d) for dendritic branches of IT neurons. f-g, Similar to (d-e) for dendritic spines.
Extended Data Fig. 7 Two-photon imaging quantification in PT and IT cells.
a, Schematic illustrating how fluorescent transients are processed and analysed to derive event rate, amplitude, and frequency. b, Fractional change in frequency detected in dendritic branches of PT neurons (top row) and dendritic branches of IT neurons (bottom row) after psilocybin (PT, yellow; IT, purple) or saline (PT, grey; IT, light purple). c, Similar to (b) for amplitude. d, e, Similar to (b, c) for dendritic spines. PT: n = 140 branches from 4 mice (Saline), n = 149 branches from 4 mice (Psilocybin). IT: n = 90 branches from 3 mice (Saline), n = 95 branches from 3 mice (Psilocybin). ** p < 0.01. ***, p < 0.001, post hoc with Bonferroni correction for multiple comparisons. Detailed sample size n values are provided in Methods. Statistical analyses are provided in Supplementary Table 1.
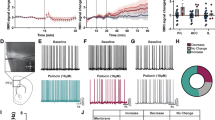
Extended Data Fig. 8 Quality inspection and waveform analysis of recorded cells.
a, The three quality metrics, including the empirical distribution of recorded units and thresholds used for curation of single units to include for the analysis of the electrophysiology data. b, Mean spike waveform features for all opto-tagged neurons and other untagged cells in Fezf2-2A-creER and Plxnd1-2A-creER mice. There is no single feature of the spike waveform that can reliably classify the two types of opto-tagged neurons or from the untagged cells. c-g, More examples of opto-tagged neurons and untagged neurons. c, d, Spike raster of neurons classified as opto-tagged in Fezf2-2A-creER (left) and Plxnd1-2A-creER (right) mice. e-g, Spike raster of neurons classified as untagged in Fezf2-2A-creER (left) and Plxnd1-2A-creER (right) mice.
Extended Data Fig. 9 Validation of 5-HT2A receptor knockout in experimental models.
a, Example sEPSC traces from 5 GFP+ layer 5 pyramidal neurons, with each set of 3 traces coming from recording of the same cell, including baseline (black), after bath application of 20 μM 5-HT (red), and after bath application of 20 μM 5-HT with 100 nM MDL100,907 (grey). Cells 1 and 2 are from control animals. Cells 3, 4, and 5 are animals with local 5-HT2A receptor knockout. b, Mean sEPSC amplitude from GFP+ layer 5 pyramidal neurons for baseline, 20 μM 5-HT, and 20 μM 5-HT + 100 nM MDL100,907 conditions, for control mice (grey) and local 5-HT2A receptor knockout mice (blue). Circle, individual cell. Mean and s.e.m. across cells. n = 22 cells (Baseline), n = 22 cells (+5-HT), n = 6 cells (+5-HT + MDL) from 4 mice in the Control condition, n = 23 cells (Baseline), n = 23 cells (+5-HT), n = 7 cells (+5-HT + MDL) from 4 mice in the 5-HT2AR local KO condition. c-e, Constitutive Camk2acre;Htr2af/f mice had reduced Htr2a transcripts and fewer psilocybin-induced head-twitch response. c, Breeding scheme to generate Camk2acre;Htr2af/f mice. d, Transcript expression via qPCR from whole-brain tissue. n = 2 (Control), n = 3 (Camk2acre;Htr2af/f). e, Head-twitch response induced by psilocybin (1 mg/kg, i.p.). Mean and s.e.m. across mice. n = 11 (Control), n = 12 (Camk2acre;Htr2af/f). ** p < 0.01. Detailed sample size n values are provided in Methods. Statistical analyses are provided in Supplementary Table 1.
Extended Data Fig. 10 5-HT2A receptor is required for psilocybin-induced structural plasticity in pyramidal neurons in Thy1GFP mice and in PT neurons.
a, HTR2A antibody staining shows colocalization of 5-HT2A receptors and GFP-expressing cell bodies and neurites in the medial frontal cortex of a Thy1GFP line M mouse. b, To image dendrites from neurons without 5-HT2A receptors, we injected a low titre of AAV-hSyn-Cre-P2A-dTomato into the medial frontal cortex of Thy1GFP; Htr2af/f mouse. The subset of neurons with viral-mediated transgene expression would have Cre recombinase for knockout of 5-HT2A receptors and have dTomato for identification. Only dendrites that expressed both dTomato and GFP were scored. Control animals were Thy1GFP; Htr2a+/+. c, Density of dendritic spines in the apical tuft of dTomato- and GFP-expressing neurons after psilocybin (green) or saline (grey) in Thy1GFP; Htr2af/f mice or after psilocybin in Thy1GFP; Htr2a+/+ mice (orange), expressed as fold-change from baseline in first imaging session (day -3). n = 38 dendrites from 2 mice (Thy1GFP; Htr2a+/+, psilocybin), n = 49 dendrites from 5 mice (Thy1GFP; Htr2af/f, psilocybin), n = 98 dendrites from 3 mice (Thy1GFP; Htr2af/f, saline). d, HTR2A antibody staining shows colocalization of 5-HT2A receptors and PT neurons targeted to express GFP using retrogradely transported virus in a C57BL/6J mouse (left). For PT neuron-targeted 5-HT2A receptor knockout, 5-HT2A receptors were absent in PT neurons targeted using retrogradely transported virus in a Htr2af/f mouse (right). e, From two-photon microscopy, spine head width across days, expressed as fold-change from baseline in first imaging session (day -3), in wild type mice after saline (light grey) or psilocybin (yellow) and in mice with PT neuron-targeted 5-HT2A receptor knockout after saline (grey) or psilocybin (blue). Post hoc test compared WT:saline and WT:psilocybin groups. f, Similar to (e) for spine protrusion length. g, Density of dendritic spines, spine head width, and spine protrusion length in the apical tuft of PT neurons across days, in wild type mice after saline (light grey) or psilocybin (yellow) and in mice with PT neuron-targeted 5-HT2A receptor knockout after saline (grey) or psilocybin (blue), without taking advantage of the longitudinal data for within-dendrite baseline normalization. h, Similar to (g) for formation rate and elimination rate. n = 7 mice (WT, saline), n = 5 (WT, psilocybin), n = 5 (PT_5-HT2AR KO, saline), n = 6 (PT_5-HT2AR KO, psilocybin) in (e-h). i, Spine head width on day 1 plotted separately for pre-existing versus newly formed spines in different conditions. n = 7 mice (WT, saline), n = 5 (WT, psilocybin), n = 4 (PT_5-HT2AR KO, saline), n = 5 (PT_5-HT2AR KO, psilocybin). j, From confocal microscopy, spine head width in the apical tuft of PT neurons in wild type mice after saline (light grey) or psilocybin (yellow) and in mice with PT neuron-targeted 5-HT2A receptor knockout after saline (grey) or psilocybin (blue, interaction effect of treatment × genotype: P = 0.025, two-factor ANOVA). Circle, individual dendritic segment. n = 7 mice (WT, saline), n = 5 (WT, psilocybin), n = 3 (PT_5-HT2AR KO, saline), n = 5 (PT_5-HT2AR KO, psilocybin). k, Similar to (j) protrusion length of dendritic spines. n = same as in (j). Data are mean and s.e.m. across dendrites. * p < 0.05. **, p < 0.01. ***, p < 0.001, post hoc with Bonferroni correction for multiple comparisons. Detailed sample size n values are provided in Methods. Statistical analyses are provided in Supplementary Table 1.
Supplementary information
Supplementary Table 1
Detailed statistics for all data. This table provides comprehensive statistical details for all datasets reported in the Article, including statistical analyses, P values, error estimates and sample sizes.
Source data
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shao, LX., Liao, C., Davoudian, P.A. et al. Psilocybin’s lasting action requires pyramidal cell types and 5-HT2A receptors. Nature 642, 411–420 (2025). https://doi.org/10.1038/s41586-025-08813-6
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41586-025-08813-6
This article is cited by
-
The transformational power of psychedelics: catalysts for creativity, consciousness, and mental health
Molecular Psychiatry (2025)
-
Exploring the therapeutic potential of psychedelics in treating substance use disorders
Molecular Psychiatry (2025)
-
Short- and long-term modulation of rat prefrontal cortical activity following single doses of psilocybin
Molecular Psychiatry (2025)
-
Psychedelic neuroplasticity of cortical neurons lacking 5-HT2A receptors
Molecular Psychiatry (2025)
-
Psychedelics target neuroimmune interactions to limit fear
Cell Research (2025)