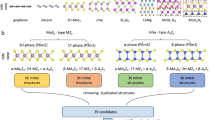
Abstract
Tailoring magnetic ordering in solid-state materials is essential for emerging spintronics1,2. However, substitutional lattice doping in magnetic semiconductors is often constrained by the low solubility of magnetic elements3,4,5, limiting the maximum achievable doping concentration (for example, less than 5%) and ferromagnetic ordering temperature6. The intercalation of magnetic elements in layered two-dimensional atomic crystals (2DACs) without breaking in-plane covalent bonds offers an alternative approach to incorporate a much higher concentration of magnetic atoms (for example, up to 50%) beyond the typical solubility limit. However, commonly used chemical and electrochemical intercalation methods are largely confined to a few isolated examples so far. Here we report a general two-step intercalation and cation-exchange strategy to produce a library of highly ordered magnetic intercalation superlattices (MISLs) with tunable magnetic ordering. Monovalent transition-metal cations Cu+ and Ag+, divalent magnetic cations Mn2+, Fe2+, Co2+ and Ni2+, and trivalent rare-earth cations Eu3+ and Gd3+ have been successfully incorporated into group-VIB 2DACs, including MoS2, MoSe2, MoTe2, WS2, WSe2 and WTe2, and group-IVB, -VB, -IIIA, -IVA and -VA 2DACs, including TiS2, NbS2, NbSe2, TaS2, In2Se3, SnSe2, Bi2Se3 and Bi2Te3. We show that these MISLs can be prepared with tunable concentrations of magnetic intercalants, enabling tailored magnetic ordering across a diverse array of functional 2DACs, including semiconductors, topological insulators, and superconductors. This work establishes a versatile material platform for both fundamental investigations and spintronics applications.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The original data files that support the findings of this study are available on the figshare public database at https://doi.org/10.6084/m9.figshare.28908146 (ref. 50) and from X.D. upon request.
References
Picozzi, S. Engineering ferromagnetism. Nat. Mater. 3, 349–350 (2004).
Ohno, H. A window on the future of spintronics. Nat. Mater. 9, 952–954 (2010).
Ohno, H. Making nonmagnetic semiconductors ferromagnetic. Science 281, 951–956 (1998).
Ohno, Y. et al. Electrical spin injection in a ferromagnetic semiconductor heterostructure. Nature 402, 790–792 (1999).
Csontos, M. et al. Pressure-induced ferromagnetism in (In,Mn)Sb dilute magnetic semiconductor. Nat. Mater. 4, 447–449 (2005).
Dietl, T. A ten-year perspective on dilute magnetic semiconductors and oxides. Nat. Mater. 9, 965–974 (2010).
Deng, H. et al. High-temperature quantum anomalous Hall regime in a MnBi2Te4/Bi2Te3 superlattice. Nat. Phys. 17, 36–42 (2021).
Yi, D. et al. Emergent electric field control of phase transformation in oxide superlattices. Nat. Commun. 11, 902 (2020).
Liu, S. et al. Two-dimensional ferromagnetic superlattices. Natl Sci. Rev. 7, 745–754 (2020).
Ren, H., Wan, Z. & Duan, X. Van der Waals superlattices. Natl Sci. Rev. 9, nwab166 (2021).
Wan, Z., Qian, Q., Huang, Y. & Duan, X. Layered hybrid superlattices as designable quantum solids. Nature 635, 49–60 (2024).
Li, Z. et al. Molecule-confined engineering toward superconductivity and ferromagnetism in two-dimensional superlattice. J. Am. Chem. Soc. 139, 16398–16404 (2017).
Husremović, S. et al. Hard ferromagnetism down to the thinnest limit of iron-intercalated tantalum disulfide. J. Am. Chem. Soc. 144, 12167–12176 (2022).
Wang, C. et al. Monolayer atomic crystal molecular superlattices. Nature 555, 231–236 (2018).
Whittingham, M. S. & Gamble, F. R. The lithium intercalates of the transition metal dichalcogenides. Mater. Res. Bull. 10, 363–371 (1975).
Zheng, J. et al. High yield exfoliation of two-dimensional chalcogenides using sodium naphthalenide. Nat. Commun. 5, 2995 (2014).
Qian, Q. et al. Chiral molecular intercalation superlattices. Nature 606, 902–908 (2022).
Li, Z. et al. Imprinting ferromagnetism and superconductivity in single atomic layers of molecular superlattices. Adv. Mater. 32, 1907645 (2020).
Koski, K. J. et al. Chemical intercalation of zerovalent metals into 2D layered Bi2Se3 nanoribbons. J. Am. Chem. Soc. 134, 13773–13779 (2012).
Gong, Y. et al. Spatially controlled doping of two-dimensional SnS2 through intercalation for electronics. Nat. Nanotechnol. 13, 294–299 (2018).
Ren, H. et al. Precision control of amphoteric doping in CuxBi2Se3 nanoplates. Precis. Chem. 2, 421–427 (2024).
Zeng, Z. et al. An effective method for the fabrication of few-layer-thick inorganic nanosheets. Angew. Chem. Int. Ed. 51, 9052–9056 (2012).
Lin, Z. et al. Solution-processable 2D semiconductors for high-performance large-area electronics. Nature 562, 254–258 (2018).
He, Q. et al. In situ probing molecular intercalation in two-dimensional layered semiconductors. Nano Lett. 19, 6819–6826 (2019).
Wang, G. et al. Revisiting the structural evolution of MoS2 during alkali metal (Li, Na, and K) intercalation. ACS Appl. Energy Mater. 4, 14180–14190 (2021).
Acerce, M., Voiry, D. & Chhowalla, M. Metallic 1T phase MoS2 nanosheets as supercapacitor electrode materials. Nat. Nanotechnol. 10, 313–318 (2015).
Guo, Y. et al. Probing the dynamics of the metallic-to-semiconducting structural phase transformation in MoS2 crystals. Nano Lett. 15, 5081–5088 (2015).
Molina-Sánchez, A. & Wirtz, L. Phonons in single-layer and few-layer MoS2 and WS2. Phys. Rev. B 84, 155413 (2011).
Zou, J., Li, F., Bissett, M. A., Kim, F. & Hardwick, L. J. Intercalation behaviour of Li and Na into 3-layer and multilayer MoS2 flakes. Electrochim. Acta 331, 135284 (2020).
Li, H. et al. From bulk to monolayer MoS2: evolution of Raman scattering. Adv. Funct. Mater. 22, 1385–1390 (2012).
Zhu, L. et al. Investigation of CoS2-based thin films as model catalysts for the oxygen reduction reaction. J. Catal. 258, 235–242 (2008).
Kappera, R. et al. Phase-engineered low-resistance contacts for ultrathin MoS2 transistors. Nat. Mater. 13, 1128–1134 (2014).
Flory, M. A., McLamarrah, S. K. & Ziurys, L. M. High-resolution spectroscopy of CoS (X4Δi): examining 3d transition-metal sulfide bonds. J. Chem. Phys. 123, 164312 (2005).
Yu, Z. X. et al. The structure of the CoS2 (100)-(1×1) surface. J. Condens. Matter Phys. 19, 156223 (2007).
Luo, Y. et al. Two-dimensional MoS2 confined Co(OH)2 electrocatalysts for hydrogen evolution in alkaline electrolytes. ACS Nano 12, 4565–4573 (2018).
Schlapp, R. & Penney, W. G. Influence of crystalline fields on the susceptibilities of salts of paramagnetic ions. II. The iron group, especially Ni, Cr and Co. Phys. Rev. 42, 666–686 (1932).
Greaney, M., Huan, G., Ramanujachary, K. V., Teweldemedhin, Z. & Greenblatt, M. Antiferro-to-ferromagnetic transition in metallic TlCo2SxSe2−x (0 ≤ x ≤ 2.0) with the ThCr2Si2 type structure. Solid State Commun. 79, 803–810 (1991).
Griffith, J. S. & Orgel, L. E. Ligand-field theory. Q. Rev. Chem. Soc. 11, 381–393 (1957).
Deng, W. et al. Constructing matched sub-nanometric cobalt clusters with multiple oxidation and metallic states for efficient propane dehydrogenation. Commun. Mater. 5, 215 (2024).
Ko, K. T. et al. RKKY ferromagnetism with Ising-like spin states in intercalated Fe1/4TaS2. Phys. Rev. Lett. 107, 247201 (2011).
Ruderman, M. A. & Kittel, C. Indirect exchange coupling of nuclear magnetic moments by conduction electrons. Phys. Rev. 96, 99–102 (1954).
Yosida, K. Magnetic properties of Cu–Mn alloys. Phys. Rev. 106, 893–898 (1957).
Priour, D. J. & Das Sarma, S. Phase diagram of the disordered RKKY model in dilute magnetic semiconductors. Phys. Rev. Lett. 97, 127201 (2006).
Lei, S. et al. High mobility in a van der Waals layered antiferromagnetic metal. Sci. Adv. 6, eaay6407 (2020).
Mugiraneza, S. & Hallas, A. M. Tutorial: a beginner’s guide to interpreting magnetic susceptibility data with the Curie-Weiss law. Commun. Phys. 5, 95 (2022).
Xie, L. S., Husremović, S., Gonzalez, O., Craig, I. M. & Bediako, D. K. Structure and magnetism of iron- and chromium-intercalated niobium and tantalum disulfides. J. Am. Chem. Soc. 144, 9525–9542 (2022).
Pfleiderer, C. et al. Coexistence of superconductivity and ferromagnetism in the d-band metal ZrZn2. Nature 412, 58–61 (2001).
Shermadini, Z. et al. Coexistence of magnetism and superconductivity in the iron-based compound Cs0.8(FeSe0.98)2. Phys. Rev. Lett. 106, 117602 (2011).
Rahmanian, E. et al. 1T-phase tungsten chalcogenides (WS2, WSe2, WTe2) decorated with TiO2 nanoplatelets with enhanced electron transfer activity for biosensing applications. ACS Appl. Nano Mater. 1, 7006–7015 (2018).
Zhou, J., Zhou. J. & Duan, X. Replication data for: A cation-exchange approach to tunable magnetic intercalation superlattices. figshare https://doi.org/10.6084/m9.figshare.28908146 (2025).
Acknowledgements
We acknowledge discussions and support from S. Zheng and C. Zhang on structural analysis. We thank N. Bodzin for the support with the focused-ion-beam sample preparations. We are grateful for the support from the Office of Naval Research through grant number N00014-22-1-2631. We acknowledge the Electron Imaging Center for NanoMachines (EICN) at California NanoSystem Institute (CNSI) and Nanoelectronic Research Facility (NRF) at UCLA for technical support. The cross-sectional STEM experiments were partly conducted using facilities and instrumentation at the UC Irvine Materials Research Institute (IMRI), which is supported in part by the National Science Foundation through the UC Irvine Materials Research Science and Engineering Center (DMR-2011967). W.F. and Y.P. acknowledge the support by NSF through the University of Wisconsin Materials Research Science and Engineering Center (DMR-2309000). Z.S. was supported by the ERC-CZ programme (project LL2101) and by project LUAUS23049 from the Ministry of Education Youth and Sports and by large infrastructure from project registration number CZ.02.1.01/0.0/0.0/15_003/0000444 financed by the European Funding for Regional Development (EFRR).
Author information
Authors and Affiliations
Contributions
X.D. and Y.H. conceived of the research. Jingxuan Zhou and Jingyuan Zhou designed the experiments, developed the cation-exchange procedure, performed material characterizations and analysed the data together. Z.W., Q.Q. and H.R. contributed to the discussions and data analysis. X.Y. and X.P. contributed STEM characterizations. B.Z. performed Raman measurement and data analysis A.Z. performed XPS measurements. W.F. and Y.P. contributed to discussion and data analysis in theoretical modelling. Z.S. provided TaS2, TiS2, NbS2, NbSe2, SnSe2, WTe2, WS2 and MoTe2 crystals. Y.H. and X.D. supervised the research. Jingxuan Zhou, Jingyuan Zhou and X.D. co-wrote the paper with input from all the authors. All authors discussed the results and commented on the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks Yongji Gong, Su-Yang Xu and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Additional characterizations of Li-intercalation/cation-exchange process.
a, XRD of unprotected LiMoS2, protected LiMoS2, and Co1/2MoS2. The LiMoS2 without hermetic sealing exhibited a significantly expanded interlayer spacing of 11.6 Å, likely due to the formation of Li(H2O)xMoS2 or (LiOH)xMoS2 upon exposure to environmental moisture. b, Zoom-in Raman spectra of pristine MoS2, LiMoS2 and Co1/2MoS2 superlattice. c, EDS mapping of Co, Mo, and S in Co1/2MoS2 nanosheets confirming the homogeneous distribution of Co atoms in Co1/2MoS2 superlattice. Scale bar, 500 nm.
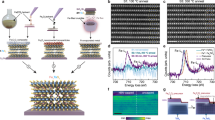
Extended Data Fig. 2 Structural model and structural analyses of Co1/2MoS2 superlattice.
a,b,c, Proposed Co1/2MoS2 supercell (1×2×1) (a), schematic structure along [001] direction (b) and [110] direction (c). d,e, Experimental SAED (d) and simulated SAED patterns (e) from the proposed structural model of Co1/2MoS2 superlattice, exhibiting consistent diffraction patterns with some intensity deviation. Scale bars: 5 nm–1; f, Simulated SAED of Co1/2MoS2 superlattice with 5% vacancies in Co sublattice gives highly consistent diffraction patterns. Scale bar, 5 nm–1. g,k, HAADF-STEM images of Li-exfoliated MoS2 and Co1/2MoS2 superlattice, respectively. Scale bars, 1 nm. h,l, Simulated HAADF-STEM images of Li-exfoliated MoS2 and Co1/2MoS2 superlattice, respectively. Scale bars, 1 nm. i,m, Cross-sectional HAADF-STEM images of few layers Li-exfoliated MoS2 and Co1/2MoS2 superlattice. Scale bars, 1 nm. j,n, Simulated cross-sectional STEM images of few layers MoS2 and Co1/2MoS2 superlattice. Scale bars, 1 nm. It is noted that that XPS studies suggest a partial restoration of 2H-phase in Co1/2MoS2 superlattice, thus, identification of other possible local structural configurations will be an interesting topic in future studies. Green, Mo; yellow, S; red, Co.
Extended Data Fig. 3 Supplementary atomistic structural analyses of Co1/2MoS2 superlattice.
a-f, In-plane HAADF-STEM images of additional Co1/2MoS2 superlattice samples. Scale bars, 1 nm. g-i, Cross-sectional HAADF-STEM images of additional Co1/2MoS2 superlattice samples. Scale bars, 1 nm.
Extended Data Fig. 4 Photographs of cation-exchange intercalation in other 2DACs.
a, LiMoSe2 and CoxMoSe2 soaked in water. b, LiMoTe2 and CoxMoTe2 soaked in water. c, LiWS2 and CoxWS2 soaked in water. d, LiWSe2 and CoxWSe2 soaked in water. e, LiWTe2 and CoxWTe2 soaked in water. f, LiIn2Se3 and CoxIn2Se3 soaked in water. g, LiSnSe2 and CoxSnSe2 soaked in water. h, LiBi2Se3 and CoxBi2Se3 soaked in water. Li-intercalated materials react strongly with water, causing significant hydrogen bubble formation and rapid exfoliation. In contrast, Co-exchanged materials remain stable in water, indicating their distinct chemical reactivities and visually confirming the successful replacement of Li+ with Co2+.
Extended Data Fig. 5 Structural analyses of RE+-intercalated superlattices.
Cross-sectional HAADF-STEM images and corresponding structural models of Eu1/3MoS2 superlattice along (a,b) “zigzag” direction and (c,d) “armchair” direction. Cross-sectional HAADF-STEM images of Eu1/3MoS2 superlattice revealed that the Eu cations are centered within the vdW gap, occupying the octahedral interstitial sites, which is consistent with the larger ionic radius of Eu3+ (95 pm) compared to Co2+ (75 pm) and Li+ (76 pm). Scale bars, 0.5 nm. e,f, Cross-sectional HADDF-STEM images of GdxNbSe2 superlattice (e) and EuxWTe2 superlattice (f). Scale bars, 0.5 nm. Blue, Mo; yellow, S; red, Eu.
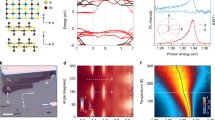
Extended Data Fig. 6 Temperature dependent magnetic properties of Co1/2MoS2 superlattice.
a, Temperature dependent (2–400 K) M-H loops of Co1/2MoS2 superlattice under magnetic field H⊥ab. b, Temperature dependent (2–1000 K) M-H of Co1/2MoS2 superlattice under magnetic field H//ab. The inset zoom-in plots show persistent hysteresis as high temperature. c, Coercivity Hc vs temperature of Co1/2MoS2 superlattice, magnetic field H⊥ab. d, M-H loops of Co1/2MoS2 superlattice powder before and after exposure in ambient conditions (~25 °C, ~30–60% humidity) for two months, exhibiting nearly the same magnetic properties with little change. e, M-H loops of CoxMoS2 superlattices with different Co2+ concentrations at room temperature (under magnetic field H⊥ab).
Extended Data Fig. 7 Comparison of metallic Co0.5MoS2 and semiconducting Co0.16MoS2 with lower Co concentration.
a,b, XPS spectra of Mo 3d in Co0.5MoS2 and Co0.16MoS2, demonstrating partial 1T-phase transition in Co1/2MoS2 (a) and preservation of 2H-phase in Co0.16MoS2 (b). The Co concentration was controlled by reducing the initial Li-intercalation concentration. c,d, PL spectra of Co0.5MoS2 and Co0.16MoS2 nanoflakes before (black) and after (red) Li-intercalation/cation-exchange process. In comparison with notable PL degradation (due to 1T-phase transition) in Co0.5MoS2, little PL degradation is observed in Co0.16MoS2, confirming the preservation of 2H-semiconducting phase. Inset of d shows the OM image of Co0.16MoS2 flake and corresponding PL intensity mapping at 680 nm. e,f, Corresponding M-H loops of Co0.5MoS2 and Co0.16MoS2 at 300 K, respectively. The S-shaped M-H curve confirms ferromagnetic ordering in Co0.16MoS2 at 300 K.
Extended Data Fig. 8 Ferromagnetic and antiferromagnetic characteristics of CoxMoS2 with variable Co concentrations.
a, Magnetic hysteresis (M-H) curves of CoxMoS2 with different Co concentrations at 300 K. b, Temperature-dependent magnetic susceptibility measurement of CoxMoS2 with various Co concentration. In Co0.03MoS2 and Co0.05MoS2, the sharp turning around 5 K (Neel temperature) manifests an antiferromagnetic ground state of CoxMoS2 with diluted Co concentrations.
Extended Data Fig. 9 Magnetization measurements (M-H loops) of MoS2 intercalated with different magnetic cations.
a, MnxMoS2. b, FexMoS2. c, NixMoS2. d, EuxMoS2. e, GdxMoS2.
Extended Data Fig. 10 Magnetization measurements of Co-intercalated 2DACs.
a-k, Magnetic hysteresis (M-H) loops of CoxMoSe2 (a), CoxMoTe2 (b), CoxWSe2 (c), CoxWTe2 (d), CoxTiS2 (e), CoxNbS2 (f), CoxTaS2 (g), CoxSnSe2 (h), CoxBi2Se3 (i), CoxBi2Te3 (j), and CoxGraphite (k).
Supplementary information
Supplementary Information
Supplementary Tables 1–4 and References.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhou, J., Zhou, J., Wan, Z. et al. A cation-exchange approach to tunable magnetic intercalation superlattices. Nature 643, 683–690 (2025). https://doi.org/10.1038/s41586-025-09147-z
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41586-025-09147-z